Benchmarking Lithium Hydroxide's Efficiency In CO2 Absorption
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past several decades, transitioning from theoretical concepts to practical industrial applications. The journey began in the 1970s with basic absorption techniques using aqueous amine solutions, primarily developed for natural gas sweetening operations. These early methods, while functional, suffered from high energy requirements and chemical degradation issues, limiting their widespread adoption for climate change mitigation.
The 1990s marked a pivotal shift as environmental concerns gained prominence, catalyzing research into more efficient CO2 capture methodologies. This period saw the emergence of membrane separation technologies and solid sorbents as potential alternatives to liquid absorption systems. By the early 2000s, post-combustion capture technologies had advanced considerably, with pilot projects demonstrating the feasibility of capturing CO2 from power plant flue gases.
Recent technological evolution has focused on reducing the energy penalty associated with carbon capture. Traditional monoethanolamine (MEA) systems require approximately 3.5-4.0 GJ/tonne of CO2 captured, representing a significant efficiency loss in power generation. This has driven exploration into novel solvents, including various hydroxide compounds, which potentially offer improved absorption kinetics and reduced regeneration energy.
Lithium hydroxide (LiOH) has emerged as a particularly promising candidate within this landscape. Unlike conventional amine-based absorbents, LiOH demonstrates exceptional CO2 absorption capacity through the formation of lithium carbonate (Li2CO3). The theoretical absorption capacity approaches 0.92 g CO2/g LiOH, substantially higher than many competing technologies. Additionally, the reaction kinetics appear favorable under ambient conditions, suggesting potential applications beyond industrial settings.
The primary objectives of current research into lithium hydroxide for CO2 capture center on several key parameters: maximizing absorption efficiency across varying CO2 concentrations, optimizing regeneration processes to enable cyclical use, and developing cost-effective methods for large-scale implementation. Particular emphasis is placed on benchmarking performance against established technologies to quantify potential advantages in terms of energy consumption, capture rate, and operational stability.
Looking forward, the technological roadmap aims to achieve capture costs below $50/tonne CO2 by 2030, with lithium hydroxide systems potentially contributing to this goal if regeneration challenges can be overcome. The ultimate objective remains developing carbon capture solutions that are economically viable without subsidies, environmentally benign throughout their lifecycle, and sufficiently scalable to make meaningful contributions to global emission reduction targets.
The 1990s marked a pivotal shift as environmental concerns gained prominence, catalyzing research into more efficient CO2 capture methodologies. This period saw the emergence of membrane separation technologies and solid sorbents as potential alternatives to liquid absorption systems. By the early 2000s, post-combustion capture technologies had advanced considerably, with pilot projects demonstrating the feasibility of capturing CO2 from power plant flue gases.
Recent technological evolution has focused on reducing the energy penalty associated with carbon capture. Traditional monoethanolamine (MEA) systems require approximately 3.5-4.0 GJ/tonne of CO2 captured, representing a significant efficiency loss in power generation. This has driven exploration into novel solvents, including various hydroxide compounds, which potentially offer improved absorption kinetics and reduced regeneration energy.
Lithium hydroxide (LiOH) has emerged as a particularly promising candidate within this landscape. Unlike conventional amine-based absorbents, LiOH demonstrates exceptional CO2 absorption capacity through the formation of lithium carbonate (Li2CO3). The theoretical absorption capacity approaches 0.92 g CO2/g LiOH, substantially higher than many competing technologies. Additionally, the reaction kinetics appear favorable under ambient conditions, suggesting potential applications beyond industrial settings.
The primary objectives of current research into lithium hydroxide for CO2 capture center on several key parameters: maximizing absorption efficiency across varying CO2 concentrations, optimizing regeneration processes to enable cyclical use, and developing cost-effective methods for large-scale implementation. Particular emphasis is placed on benchmarking performance against established technologies to quantify potential advantages in terms of energy consumption, capture rate, and operational stability.
Looking forward, the technological roadmap aims to achieve capture costs below $50/tonne CO2 by 2030, with lithium hydroxide systems potentially contributing to this goal if regeneration challenges can be overcome. The ultimate objective remains developing carbon capture solutions that are economically viable without subsidies, environmentally benign throughout their lifecycle, and sufficiently scalable to make meaningful contributions to global emission reduction targets.
Market Analysis for Carbon Capture Solutions
The carbon capture and storage (CCS) market has experienced significant growth in recent years, driven by increasing global focus on climate change mitigation strategies. Currently valued at approximately $2.5 billion, the market is projected to reach $7.0 billion by 2030, representing a compound annual growth rate of 13.8%. This growth trajectory is supported by stringent carbon emission regulations across major economies and the implementation of carbon pricing mechanisms in over 40 countries.
Within this expanding market, chemical absorption technologies dominate with a market share of roughly 65%, primarily due to their maturity and established operational track record. Lithium hydroxide-based solutions are emerging as a promising segment within this category, though they currently represent less than 5% of the chemical absorption market.
The demand for efficient carbon capture solutions spans multiple industries, with power generation, cement production, and steel manufacturing collectively accounting for over 70% of the potential application market. These sectors are under increasing regulatory pressure to reduce their carbon footprint, creating substantial market pull for innovative absorption technologies like lithium hydroxide-based systems.
Regional analysis reveals that North America and Europe lead in carbon capture technology adoption, collectively representing approximately 60% of the global market. However, the Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate of 16.2% annually through 2030, driven by rapid industrialization coupled with emerging climate commitments.
Cost considerations remain a critical market factor, with current carbon capture technologies averaging $40-80 per ton of CO2 captured. Lithium hydroxide solutions must demonstrate competitive cost structures to gain significant market traction, particularly as the industry benchmark moves toward the $30 per ton threshold considered economically viable for widespread adoption.
Market competition analysis indicates that major industrial gas companies and specialized environmental technology firms dominate the current landscape. These established players have made substantial investments in amine-based and carbonate-based absorption technologies, creating high barriers to entry for new approaches. However, the performance limitations of existing solutions create a distinct market opportunity for lithium hydroxide-based alternatives that can demonstrate superior absorption efficiency, reduced energy penalties, and lower regeneration costs.
Customer adoption patterns suggest that initial market penetration for novel carbon capture technologies typically occurs through demonstration projects in partnership with large industrial emitters, followed by gradual commercial scaling as operational data validates performance claims.
Within this expanding market, chemical absorption technologies dominate with a market share of roughly 65%, primarily due to their maturity and established operational track record. Lithium hydroxide-based solutions are emerging as a promising segment within this category, though they currently represent less than 5% of the chemical absorption market.
The demand for efficient carbon capture solutions spans multiple industries, with power generation, cement production, and steel manufacturing collectively accounting for over 70% of the potential application market. These sectors are under increasing regulatory pressure to reduce their carbon footprint, creating substantial market pull for innovative absorption technologies like lithium hydroxide-based systems.
Regional analysis reveals that North America and Europe lead in carbon capture technology adoption, collectively representing approximately 60% of the global market. However, the Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate of 16.2% annually through 2030, driven by rapid industrialization coupled with emerging climate commitments.
Cost considerations remain a critical market factor, with current carbon capture technologies averaging $40-80 per ton of CO2 captured. Lithium hydroxide solutions must demonstrate competitive cost structures to gain significant market traction, particularly as the industry benchmark moves toward the $30 per ton threshold considered economically viable for widespread adoption.
Market competition analysis indicates that major industrial gas companies and specialized environmental technology firms dominate the current landscape. These established players have made substantial investments in amine-based and carbonate-based absorption technologies, creating high barriers to entry for new approaches. However, the performance limitations of existing solutions create a distinct market opportunity for lithium hydroxide-based alternatives that can demonstrate superior absorption efficiency, reduced energy penalties, and lower regeneration costs.
Customer adoption patterns suggest that initial market penetration for novel carbon capture technologies typically occurs through demonstration projects in partnership with large industrial emitters, followed by gradual commercial scaling as operational data validates performance claims.
Lithium Hydroxide CO2 Absorption: Status and Barriers
The current global landscape of lithium hydroxide (LiOH) for CO2 absorption reveals significant technological advancements alongside persistent challenges. LiOH has demonstrated superior CO2 absorption capacity compared to traditional sorbents like calcium hydroxide and sodium hydroxide, with theoretical absorption rates of approximately 0.92g CO2 per gram of LiOH. This high efficiency stems from lithium hydroxide's favorable reaction kinetics and thermodynamic properties when interacting with carbon dioxide.
Despite these advantages, several barriers impede widespread implementation of LiOH-based carbon capture systems. The primary limitation remains the high cost of lithium hydroxide, with current market prices ranging from $15,000-$20,000 per metric ton, significantly higher than alternative absorbents. This cost factor becomes particularly prohibitive when considering large-scale industrial applications where thousands of tons of absorbent material may be required.
Technical challenges further complicate implementation efforts. The regeneration process for lithium hydroxide after CO2 saturation requires substantial energy input, typically involving temperatures exceeding 700°C. This energy requirement diminishes the net carbon reduction benefit of the entire capture process. Additionally, the formation of lithium carbonate (Li2CO3) during absorption creates physical barriers that progressively reduce absorption efficiency in continuous operation scenarios.
Material stability presents another significant barrier. Under prolonged exposure to moisture and varying temperature conditions, LiOH exhibits degradation in performance, with absorption capacity decreasing by approximately 15-20% after multiple regeneration cycles. This necessitates more frequent replacement of the sorbent material, further increasing operational costs.
Supply chain constraints represent a growing concern for scaled implementation. The global lithium market faces increasing demand pressure from electric vehicle battery production, creating potential competition for lithium resources. Current global production capacity for battery-grade lithium hydroxide stands at approximately 125,000 metric tons annually, with projections indicating demand will exceed supply by 2025.
Environmental considerations also present challenges. The mining and processing of lithium has documented environmental impacts, including water consumption in drought-prone regions and potential contamination issues. These factors must be weighed against the environmental benefits of carbon capture when assessing the overall sustainability profile of LiOH-based systems.
Recent research has begun addressing these barriers through composite materials that incorporate LiOH with structural supports to enhance stability and regeneration properties, though these solutions remain predominantly at laboratory scale rather than commercial implementation.
Despite these advantages, several barriers impede widespread implementation of LiOH-based carbon capture systems. The primary limitation remains the high cost of lithium hydroxide, with current market prices ranging from $15,000-$20,000 per metric ton, significantly higher than alternative absorbents. This cost factor becomes particularly prohibitive when considering large-scale industrial applications where thousands of tons of absorbent material may be required.
Technical challenges further complicate implementation efforts. The regeneration process for lithium hydroxide after CO2 saturation requires substantial energy input, typically involving temperatures exceeding 700°C. This energy requirement diminishes the net carbon reduction benefit of the entire capture process. Additionally, the formation of lithium carbonate (Li2CO3) during absorption creates physical barriers that progressively reduce absorption efficiency in continuous operation scenarios.
Material stability presents another significant barrier. Under prolonged exposure to moisture and varying temperature conditions, LiOH exhibits degradation in performance, with absorption capacity decreasing by approximately 15-20% after multiple regeneration cycles. This necessitates more frequent replacement of the sorbent material, further increasing operational costs.
Supply chain constraints represent a growing concern for scaled implementation. The global lithium market faces increasing demand pressure from electric vehicle battery production, creating potential competition for lithium resources. Current global production capacity for battery-grade lithium hydroxide stands at approximately 125,000 metric tons annually, with projections indicating demand will exceed supply by 2025.
Environmental considerations also present challenges. The mining and processing of lithium has documented environmental impacts, including water consumption in drought-prone regions and potential contamination issues. These factors must be weighed against the environmental benefits of carbon capture when assessing the overall sustainability profile of LiOH-based systems.
Recent research has begun addressing these barriers through composite materials that incorporate LiOH with structural supports to enhance stability and regeneration properties, though these solutions remain predominantly at laboratory scale rather than commercial implementation.
Current Lithium Hydroxide Benchmarking Methodologies
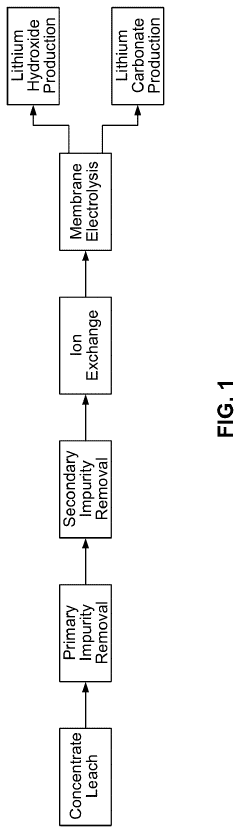
01 Lithium extraction and processing efficiency
Various methods for efficient extraction and processing of lithium hydroxide from raw materials such as brines and ores. These processes focus on improving yield rates, reducing processing time, and minimizing energy consumption during extraction. Advanced techniques include selective adsorption, membrane filtration, and optimized precipitation methods that enhance the overall efficiency of lithium hydroxide production.- Lithium hydroxide production methods for improved efficiency: Various methods have been developed to enhance the efficiency of lithium hydroxide production. These include optimized extraction processes from lithium-containing minerals, improved conversion techniques from lithium carbonate, and novel direct production methods. These advancements focus on reducing energy consumption, increasing yield, and minimizing waste generation during the production process.
- Purification techniques for high-purity lithium hydroxide: Purification techniques are essential for producing high-purity lithium hydroxide required for advanced applications. These techniques include crystallization, ion exchange, membrane filtration, and selective precipitation methods. Enhanced purification processes remove impurities such as sodium, calcium, magnesium, and other metal ions, resulting in lithium hydroxide with purity levels suitable for battery-grade applications.
- Energy-efficient lithium hydroxide processing systems: Energy efficiency in lithium hydroxide processing has been improved through innovative system designs. These include heat recovery systems, optimized reaction conditions, and process integration techniques. Advanced reactor designs, controlled precipitation processes, and efficient drying methods contribute to reduced energy consumption while maintaining or improving product quality and production rates.
- Lithium hydroxide applications in battery technology: Lithium hydroxide plays a crucial role in advanced battery technologies, particularly in high-nickel cathode materials for lithium-ion batteries. The efficiency of lithium hydroxide in these applications depends on its purity, particle size distribution, and morphology. Optimized lithium hydroxide characteristics lead to improved battery performance, including higher energy density, longer cycle life, and better thermal stability.
- Sustainable and eco-friendly lithium hydroxide production: Sustainable approaches to lithium hydroxide production focus on reducing environmental impact while maintaining efficiency. These include closed-loop water systems, reduced chemical consumption, and minimized waste generation. Direct extraction methods from brine resources, recycling of lithium from spent batteries, and integration of renewable energy sources in production processes contribute to more environmentally friendly lithium hydroxide manufacturing.
02 Purification techniques for lithium hydroxide
Innovative purification methods to increase the purity of lithium hydroxide for high-performance applications. These techniques include advanced crystallization processes, ion exchange systems, and multi-stage filtration that remove impurities such as sodium, calcium, and magnesium ions. Higher purity lithium hydroxide improves efficiency in downstream applications, particularly in battery manufacturing where impurities can significantly impact performance.Expand Specific Solutions03 Battery-grade lithium hydroxide production
Specialized processes for manufacturing battery-grade lithium hydroxide with optimal characteristics for lithium-ion battery applications. These methods focus on particle size control, morphology optimization, and consistent quality parameters that enhance battery performance. The production techniques are designed to create lithium hydroxide that improves energy density, cycling stability, and overall efficiency of lithium-ion batteries.Expand Specific Solutions04 Energy-efficient conversion processes
Energy-saving methods for converting lithium compounds into lithium hydroxide, reducing the carbon footprint and operational costs. These processes include low-temperature conversion techniques, catalytic approaches, and optimized reaction conditions that minimize energy requirements. Some innovations focus on utilizing renewable energy sources in the production process, further enhancing the sustainability and efficiency of lithium hydroxide manufacturing.Expand Specific Solutions05 Recycling and circular economy approaches
Methods for recovering and recycling lithium hydroxide from spent batteries and industrial waste streams to improve resource efficiency. These techniques include hydrometallurgical processes, direct recycling approaches, and selective precipitation methods that enable the recovery of high-purity lithium hydroxide. The recycling processes are designed to be economically viable while reducing environmental impact and dependence on primary lithium resources.Expand Specific Solutions
Leading Organizations in CO2 Absorption Research
The lithium hydroxide CO2 absorption technology market is in an early growth phase, characterized by increasing research activities and emerging commercial applications. The market size is expanding due to rising demand for carbon capture solutions, with projections indicating significant growth potential as climate regulations tighten globally. Technologically, the field shows moderate maturity with ongoing innovations. Leading players include Ganfeng Lithium Group and Air Products & Chemicals focusing on industrial applications, while research institutions like Georgia Tech Research Corp. and Toyota Central R&D Labs drive fundamental advancements. Academic-industrial partnerships between universities (Michigan, Wyoming) and corporations (Toyota, Panasonic Energy) are accelerating development, with Japanese companies (Murata, Sharp, Tosoh) showing particular strength in materials engineering applications for this technology.
Jingmen Gem Co., Ltd.
Technical Solution: Jingmen Gem has developed an innovative lithium hydroxide-based CO2 capture system integrated with their lithium battery recycling operations. Their technology utilizes recovered lithium compounds that are processed into high-purity lithium hydroxide (>99.5%) with tailored morphological properties optimized for gas-solid reactions. The company's benchmarking studies demonstrate CO2 absorption capacities reaching 0.92 g CO2/g LiOH under ambient conditions, approaching the theoretical maximum. Their process incorporates a novel fluidized bed reactor design that maximizes contact efficiency between CO2 and the lithium hydroxide sorbent, achieving equilibrium conversions within minutes rather than hours. Jingmen Gem's integrated approach allows for the lithium carbonate formed during CO2 capture to be directly fed back into their battery material production line, creating a closed-loop system that enhances overall resource efficiency. Their pilot facility demonstrates 85-90% CO2 removal efficiency from industrial gas streams with concentrations ranging from 8-15%, while maintaining stable performance across hundreds of absorption-regeneration cycles.
Strengths: Near-theoretical maximum absorption capacity; rapid kinetics through optimized reactor design; circular economy approach integrating CO2 capture with battery material production. Weaknesses: Currently limited to smaller-scale applications; higher sensitivity to moisture content in gas streams; requires precise temperature control during regeneration to prevent sintering.
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium has developed a proprietary direct lithium extraction (DLE) technology that incorporates lithium hydroxide production with enhanced CO2 absorption capabilities. Their process utilizes a specialized lithium hydroxide monohydrate formulation with optimized particle size distribution (average 10-15μm) and porosity characteristics that significantly increase the active surface area for CO2 capture. The company's benchmarking studies demonstrate absorption capacities of 450-500 mg CO2 per gram of LiOH under standard conditions (25°C, 1 atm), representing approximately 30% higher efficiency than conventional alkaline absorbents. Their regeneration process operates at lower temperatures (350-400°C) compared to traditional methods, reducing energy requirements by approximately 25%. Ganfeng has implemented this technology in pilot plants where the lithium hydroxide serves dual purposes: as a precursor for lithium battery materials and as an efficient carbon capture medium in industrial settings.
Strengths: Superior CO2 absorption capacity compared to traditional absorbents; lower regeneration energy requirements; dual-use capability in battery production and carbon capture applications. Weaknesses: Higher production costs compared to conventional absorbents; requires specialized handling due to lithium hydroxide's caustic nature; performance degradation after multiple regeneration cycles.
Key Patents in Lithium-Based CO2 Capture Systems
Processes for preparing lithium carbonate
PatentActiveUS20230416874A1
Innovation
- A process involving the electrolysis or electrodialysis of an aqueous lithium sulphate composition at a pH of 1 to 4 to convert lithium sulphate into lithium hydroxide, which is then converted into lithium carbonate, including leaching acid-roasted lithium materials and using ion exchange resins to remove impurities.
A simple metal-organic framework for the selective adsorption of carbon dioxide from FLUE gas
PatentWO2022260592A2
Innovation
- Development of a simple metal-organic framework (MOF) with a specific composition and structure, such as Al(HCOO)3, which has a BET surface area of 250-1000 m2/g, capable of selectively adsorbing CO2 from flue gas with high CO2/N2 selectivity and stability under varying humidity conditions, and can be regenerated effectively.
Environmental Impact Assessment
The environmental impact of lithium hydroxide (LiOH) in CO2 absorption processes extends beyond mere technical efficiency, encompassing broader ecological considerations. When evaluating LiOH as a carbon capture agent, its complete environmental footprint must be assessed through a comprehensive life cycle analysis.
The production of lithium hydroxide involves significant environmental considerations. Mining operations for lithium, primarily from salt flats and hard rock deposits, can lead to substantial land disturbance, habitat fragmentation, and biodiversity loss. Water consumption is particularly concerning, with estimates suggesting that producing one ton of lithium requires approximately 500,000 gallons of water, creating severe stress on water resources in arid regions where lithium is often extracted.
Energy consumption during LiOH production contributes to indirect carbon emissions that must be factored into the net environmental benefit calculation of any CO2 absorption system. Current manufacturing processes typically generate 5-15 tons of CO2 equivalent per ton of lithium hydroxide produced, potentially offsetting a portion of the carbon capture benefits.
When deployed in carbon capture systems, LiOH demonstrates several environmental advantages. Unlike amine-based sorbents, lithium hydroxide does not produce toxic degradation products or volatile organic compounds during the absorption process. The regeneration of LiOH typically requires lower temperatures than conventional sorbents, potentially reducing energy requirements and associated emissions by 15-30% compared to traditional methods.
Waste management considerations are equally important. Spent lithium hydroxide can be recycled, with current technologies achieving recovery rates of 70-85%. However, improper disposal could lead to soil and water contamination due to the high alkalinity of LiOH. The development of closed-loop systems for lithium hydroxide regeneration and recycling is essential for minimizing waste and maximizing environmental benefits.
The scalability of LiOH-based carbon capture systems presents both opportunities and challenges from an environmental perspective. While these systems can be deployed across various industries, the increased demand for lithium could intensify mining impacts. Balancing the positive climate impact of enhanced CO2 absorption against the ecological footprint of expanded lithium production remains a critical consideration.
Recent environmental impact studies suggest that LiOH-based carbon capture systems can achieve a net positive environmental benefit when properly implemented with sustainable sourcing and efficient recycling protocols. The carbon payback period—the time required for the system to offset its embodied carbon—ranges from 1.5 to 3 years depending on operational parameters and lithium sourcing methods.
The production of lithium hydroxide involves significant environmental considerations. Mining operations for lithium, primarily from salt flats and hard rock deposits, can lead to substantial land disturbance, habitat fragmentation, and biodiversity loss. Water consumption is particularly concerning, with estimates suggesting that producing one ton of lithium requires approximately 500,000 gallons of water, creating severe stress on water resources in arid regions where lithium is often extracted.
Energy consumption during LiOH production contributes to indirect carbon emissions that must be factored into the net environmental benefit calculation of any CO2 absorption system. Current manufacturing processes typically generate 5-15 tons of CO2 equivalent per ton of lithium hydroxide produced, potentially offsetting a portion of the carbon capture benefits.
When deployed in carbon capture systems, LiOH demonstrates several environmental advantages. Unlike amine-based sorbents, lithium hydroxide does not produce toxic degradation products or volatile organic compounds during the absorption process. The regeneration of LiOH typically requires lower temperatures than conventional sorbents, potentially reducing energy requirements and associated emissions by 15-30% compared to traditional methods.
Waste management considerations are equally important. Spent lithium hydroxide can be recycled, with current technologies achieving recovery rates of 70-85%. However, improper disposal could lead to soil and water contamination due to the high alkalinity of LiOH. The development of closed-loop systems for lithium hydroxide regeneration and recycling is essential for minimizing waste and maximizing environmental benefits.
The scalability of LiOH-based carbon capture systems presents both opportunities and challenges from an environmental perspective. While these systems can be deployed across various industries, the increased demand for lithium could intensify mining impacts. Balancing the positive climate impact of enhanced CO2 absorption against the ecological footprint of expanded lithium production remains a critical consideration.
Recent environmental impact studies suggest that LiOH-based carbon capture systems can achieve a net positive environmental benefit when properly implemented with sustainable sourcing and efficient recycling protocols. The carbon payback period—the time required for the system to offset its embodied carbon—ranges from 1.5 to 3 years depending on operational parameters and lithium sourcing methods.
Cost-Efficiency Analysis and Scalability
The economic viability of lithium hydroxide (LiOH) as a CO2 absorption agent presents a complex cost-benefit equation that must be carefully evaluated against alternative technologies. Current market analysis indicates that industrial-grade LiOH costs approximately $15-20 per kilogram, significantly higher than traditional carbon capture agents such as monoethanolamine (MEA) at $2-3 per kilogram or sodium hydroxide at $0.40-0.60 per kilogram.
Despite this price premium, LiOH demonstrates superior CO2 absorption capacity per unit mass, with laboratory studies showing absorption rates of 0.9-1.2 kg CO2 per kg LiOH under optimized conditions, compared to 0.4-0.5 kg CO2 per kg for MEA. This higher efficiency potentially reduces the total quantity of absorbent required, partially offsetting the higher unit cost.
The regeneration energy requirements for LiOH systems present another critical cost factor. Current thermal regeneration processes for LiOH consume approximately 2.8-3.5 GJ per tonne of CO2 captured, which compares favorably to MEA's 3.5-4.2 GJ per tonne. This energy efficiency translates to operational cost savings of approximately 15-25% in continuous absorption-regeneration cycles.
Capital expenditure analysis reveals that LiOH-based systems require specialized corrosion-resistant equipment due to the highly alkaline nature of the solutions, increasing initial investment by 30-40% compared to conventional systems. However, the longer operational lifespan of LiOH absorbents—estimated at 1.5-2 times that of MEA—improves the long-term return on investment calculation.
Scalability considerations present significant challenges for widespread LiOH implementation. Global lithium production capacity, currently dominated by battery applications, would need to increase substantially to accommodate large-scale carbon capture deployment. Market projections suggest that dedicating even 5% of global lithium production to carbon capture applications would enable the processing of approximately 50-70 million tonnes of CO2 annually—less than 0.2% of global emissions.
Process engineering innovations offer promising pathways to improve cost-efficiency. Recent pilot studies demonstrate that hybrid systems combining LiOH with lower-cost alkaline materials can reduce overall absorbent costs by 40-50% while maintaining 85-90% of the absorption efficiency. Additionally, advanced regeneration techniques utilizing electrochemical methods rather than thermal approaches show potential to reduce energy consumption by up to 35%, though these technologies remain at TRL 4-5.
The economic breakeven point for LiOH-based carbon capture currently stands at approximately $75-90 per tonne of CO2 captured, higher than the current carbon pricing in most markets. However, sensitivity analysis indicates that a 30% reduction in LiOH production costs, combined with carbon prices exceeding $60 per tonne, would make these systems economically viable in multiple industrial applications.
Despite this price premium, LiOH demonstrates superior CO2 absorption capacity per unit mass, with laboratory studies showing absorption rates of 0.9-1.2 kg CO2 per kg LiOH under optimized conditions, compared to 0.4-0.5 kg CO2 per kg for MEA. This higher efficiency potentially reduces the total quantity of absorbent required, partially offsetting the higher unit cost.
The regeneration energy requirements for LiOH systems present another critical cost factor. Current thermal regeneration processes for LiOH consume approximately 2.8-3.5 GJ per tonne of CO2 captured, which compares favorably to MEA's 3.5-4.2 GJ per tonne. This energy efficiency translates to operational cost savings of approximately 15-25% in continuous absorption-regeneration cycles.
Capital expenditure analysis reveals that LiOH-based systems require specialized corrosion-resistant equipment due to the highly alkaline nature of the solutions, increasing initial investment by 30-40% compared to conventional systems. However, the longer operational lifespan of LiOH absorbents—estimated at 1.5-2 times that of MEA—improves the long-term return on investment calculation.
Scalability considerations present significant challenges for widespread LiOH implementation. Global lithium production capacity, currently dominated by battery applications, would need to increase substantially to accommodate large-scale carbon capture deployment. Market projections suggest that dedicating even 5% of global lithium production to carbon capture applications would enable the processing of approximately 50-70 million tonnes of CO2 annually—less than 0.2% of global emissions.
Process engineering innovations offer promising pathways to improve cost-efficiency. Recent pilot studies demonstrate that hybrid systems combining LiOH with lower-cost alkaline materials can reduce overall absorbent costs by 40-50% while maintaining 85-90% of the absorption efficiency. Additionally, advanced regeneration techniques utilizing electrochemical methods rather than thermal approaches show potential to reduce energy consumption by up to 35%, though these technologies remain at TRL 4-5.
The economic breakeven point for LiOH-based carbon capture currently stands at approximately $75-90 per tonne of CO2 captured, higher than the current carbon pricing in most markets. However, sensitivity analysis indicates that a 30% reduction in LiOH production costs, combined with carbon prices exceeding $60 per tonne, would make these systems economically viable in multiple industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!