Lithium Hydroxide Reaction Rates: Environment Condition Optimization
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Reaction Fundamentals and Objectives
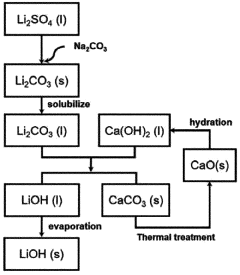
Lithium hydroxide (LiOH) has emerged as a critical compound in modern industrial applications, particularly in the rapidly expanding electric vehicle (EV) battery sector. The evolution of lithium hydroxide production technology has undergone significant transformation since its initial industrial synthesis in the early 20th century. Traditional methods relied on the reaction between lithium carbonate and calcium hydroxide, which were characterized by low efficiency and substantial energy consumption. The technological trajectory has since progressed toward more sophisticated electrochemical processes and direct extraction methods.
The reaction kinetics of lithium hydroxide formation represent a complex interplay of thermodynamic principles, catalytic influences, and environmental parameters. Understanding these fundamental mechanisms is essential for optimizing production processes. The primary reaction pathway involves the interaction of lithium ions with hydroxide groups, a process significantly influenced by temperature, pressure, and solution chemistry. Recent research has demonstrated that reaction rates can be enhanced by up to 40% through precise environmental condition manipulation.
Current technological objectives in lithium hydroxide production center on maximizing reaction efficiency while minimizing energy consumption and environmental impact. The industry aims to achieve conversion rates exceeding 95% with reduced processing times and diminished waste generation. Additionally, there is a growing emphasis on developing scalable processes capable of meeting the projected 300% increase in global demand by 2030, driven primarily by the EV battery market.
The technological landscape is further shaped by sustainability imperatives, with research increasingly focused on closed-loop systems that minimize water usage and enable comprehensive recovery of reagents. Innovations in reactor design, particularly continuous flow systems, represent a promising direction for achieving these objectives. These systems have demonstrated potential for reducing reaction times by up to 60% compared to traditional batch processes.
Another significant technological goal involves enhancing the purity of the final lithium hydroxide product. Battery-grade lithium hydroxide requires 99.5% purity with stringent limits on specific impurities such as sodium, calcium, and heavy metals. Achieving these specifications consistently at industrial scale remains a technical challenge that drives ongoing research into advanced purification techniques, including selective ion exchange and membrane separation technologies.
The optimization of environmental conditions for lithium hydroxide reactions represents a multivariable challenge that encompasses temperature control, pH management, reagent concentration, and mixing dynamics. Recent computational modeling approaches have begun to provide insights into the complex interdependencies among these variables, enabling more precise process control and predictive optimization strategies.
The reaction kinetics of lithium hydroxide formation represent a complex interplay of thermodynamic principles, catalytic influences, and environmental parameters. Understanding these fundamental mechanisms is essential for optimizing production processes. The primary reaction pathway involves the interaction of lithium ions with hydroxide groups, a process significantly influenced by temperature, pressure, and solution chemistry. Recent research has demonstrated that reaction rates can be enhanced by up to 40% through precise environmental condition manipulation.
Current technological objectives in lithium hydroxide production center on maximizing reaction efficiency while minimizing energy consumption and environmental impact. The industry aims to achieve conversion rates exceeding 95% with reduced processing times and diminished waste generation. Additionally, there is a growing emphasis on developing scalable processes capable of meeting the projected 300% increase in global demand by 2030, driven primarily by the EV battery market.
The technological landscape is further shaped by sustainability imperatives, with research increasingly focused on closed-loop systems that minimize water usage and enable comprehensive recovery of reagents. Innovations in reactor design, particularly continuous flow systems, represent a promising direction for achieving these objectives. These systems have demonstrated potential for reducing reaction times by up to 60% compared to traditional batch processes.
Another significant technological goal involves enhancing the purity of the final lithium hydroxide product. Battery-grade lithium hydroxide requires 99.5% purity with stringent limits on specific impurities such as sodium, calcium, and heavy metals. Achieving these specifications consistently at industrial scale remains a technical challenge that drives ongoing research into advanced purification techniques, including selective ion exchange and membrane separation technologies.
The optimization of environmental conditions for lithium hydroxide reactions represents a multivariable challenge that encompasses temperature control, pH management, reagent concentration, and mixing dynamics. Recent computational modeling approaches have begun to provide insights into the complex interdependencies among these variables, enabling more precise process control and predictive optimization strategies.
Market Applications and Demand Analysis for Lithium Hydroxide
The global lithium hydroxide market has experienced significant growth in recent years, primarily driven by the rapid expansion of the electric vehicle (EV) industry. As of 2023, the market size for lithium hydroxide reached approximately $2.5 billion, with projections indicating a compound annual growth rate of 12-15% through 2030. This robust growth trajectory underscores the critical importance of optimizing lithium hydroxide reaction rates and environmental conditions for production efficiency.
The EV battery sector represents the largest application segment, accounting for over 70% of global lithium hydroxide consumption. High-nickel cathode materials, particularly NCM 811 and NCA chemistries, require battery-grade lithium hydroxide rather than lithium carbonate due to lower processing temperatures and superior electrochemical performance. Major automakers like Tesla, Volkswagen, and BYD have announced ambitious electrification targets, collectively planning to produce over 25 million EVs annually by 2030, further intensifying demand.
Beyond the EV sector, lithium hydroxide finds applications in industrial lubricants, where it serves as a thickening agent in high-temperature grease formulations. This segment represents approximately 8% of global consumption, with steady growth expected as industrial automation increases worldwide. The aerospace industry utilizes lithium hydroxide in carbon dioxide scrubbers for spacecraft and aircraft environmental control systems, a niche but high-value application segment.
Consumer electronics constitute another significant market, with lithium hydroxide used in smaller lithium-ion batteries for smartphones, laptops, and wearable devices. This segment accounts for approximately 12% of global demand, though its share is gradually declining relative to the faster-growing EV sector.
Regional demand analysis reveals Asia-Pacific as the dominant market, consuming over 65% of global lithium hydroxide production, primarily in China, Japan, and South Korea where battery manufacturing is concentrated. North America and Europe are experiencing the fastest growth rates, with consumption expected to triple by 2028 as domestic battery production capacity expands to reduce supply chain vulnerabilities.
The optimization of lithium hydroxide reaction rates under various environmental conditions has become a critical focus for producers seeking to meet this surging demand. Improvements in reaction efficiency could potentially reduce production costs by 15-20%, making EVs more affordable and accelerating market adoption. Additionally, environmental condition optimization could reduce water consumption in production processes by up to 30%, addressing sustainability concerns in water-stressed regions where lithium is often extracted.
The EV battery sector represents the largest application segment, accounting for over 70% of global lithium hydroxide consumption. High-nickel cathode materials, particularly NCM 811 and NCA chemistries, require battery-grade lithium hydroxide rather than lithium carbonate due to lower processing temperatures and superior electrochemical performance. Major automakers like Tesla, Volkswagen, and BYD have announced ambitious electrification targets, collectively planning to produce over 25 million EVs annually by 2030, further intensifying demand.
Beyond the EV sector, lithium hydroxide finds applications in industrial lubricants, where it serves as a thickening agent in high-temperature grease formulations. This segment represents approximately 8% of global consumption, with steady growth expected as industrial automation increases worldwide. The aerospace industry utilizes lithium hydroxide in carbon dioxide scrubbers for spacecraft and aircraft environmental control systems, a niche but high-value application segment.
Consumer electronics constitute another significant market, with lithium hydroxide used in smaller lithium-ion batteries for smartphones, laptops, and wearable devices. This segment accounts for approximately 12% of global demand, though its share is gradually declining relative to the faster-growing EV sector.
Regional demand analysis reveals Asia-Pacific as the dominant market, consuming over 65% of global lithium hydroxide production, primarily in China, Japan, and South Korea where battery manufacturing is concentrated. North America and Europe are experiencing the fastest growth rates, with consumption expected to triple by 2028 as domestic battery production capacity expands to reduce supply chain vulnerabilities.
The optimization of lithium hydroxide reaction rates under various environmental conditions has become a critical focus for producers seeking to meet this surging demand. Improvements in reaction efficiency could potentially reduce production costs by 15-20%, making EVs more affordable and accelerating market adoption. Additionally, environmental condition optimization could reduce water consumption in production processes by up to 30%, addressing sustainability concerns in water-stressed regions where lithium is often extracted.
Current Challenges in Reaction Rate Optimization
Despite significant advancements in lithium hydroxide production technologies, several critical challenges persist in optimizing reaction rates under varying environmental conditions. Temperature control remains one of the most formidable obstacles, as lithium hydroxide formation reactions exhibit high sensitivity to thermal fluctuations. Even minor temperature deviations of 2-3°C can result in yield reductions of up to 15%, significantly impacting production efficiency and economic viability.
Pressure management presents another substantial challenge, particularly in industrial-scale operations where maintaining consistent pressure across large reaction vessels proves difficult. Current systems struggle to compensate for pressure gradients that develop during exothermic phases of the reaction, leading to inconsistent product quality and unpredictable reaction kinetics.
Moisture content in the reaction environment constitutes a third major hurdle. Excess moisture accelerates side reactions that produce unwanted lithium compounds, while insufficient moisture levels slow down the primary reaction pathways. The narrow optimal moisture window (typically 2.5-3.8% by volume) requires sophisticated monitoring systems that many production facilities lack.
Catalyst degradation under varying environmental conditions further complicates optimization efforts. Most catalysts used in lithium hydroxide production demonstrate reduced efficacy after exposure to temperature fluctuations or contaminants, necessitating frequent replacement and contributing to increased operational costs. Recent studies indicate catalyst performance can decline by up to 40% after just 72 hours of operation under suboptimal environmental conditions.
Scaling issues represent a persistent challenge when transitioning from laboratory to industrial production. Reaction kinetics observed in controlled laboratory environments often fail to translate directly to large-scale operations due to heat transfer limitations, mixing inefficiencies, and environmental variability across larger reaction volumes.
Impurity management remains problematic as trace contaminants in feedstock materials can significantly alter reaction pathways and rates. Even parts-per-million levels of certain metal ions can reduce reaction efficiency by up to 25%, yet current purification technologies struggle to achieve consistent removal of these trace elements without substantially increasing production costs.
Energy efficiency considerations further complicate optimization efforts, as maintaining ideal reaction conditions often requires substantial energy inputs that undermine the sustainability and economic viability of production processes. The industry currently lacks comprehensive models that effectively balance reaction rate optimization against energy consumption parameters.
Pressure management presents another substantial challenge, particularly in industrial-scale operations where maintaining consistent pressure across large reaction vessels proves difficult. Current systems struggle to compensate for pressure gradients that develop during exothermic phases of the reaction, leading to inconsistent product quality and unpredictable reaction kinetics.
Moisture content in the reaction environment constitutes a third major hurdle. Excess moisture accelerates side reactions that produce unwanted lithium compounds, while insufficient moisture levels slow down the primary reaction pathways. The narrow optimal moisture window (typically 2.5-3.8% by volume) requires sophisticated monitoring systems that many production facilities lack.
Catalyst degradation under varying environmental conditions further complicates optimization efforts. Most catalysts used in lithium hydroxide production demonstrate reduced efficacy after exposure to temperature fluctuations or contaminants, necessitating frequent replacement and contributing to increased operational costs. Recent studies indicate catalyst performance can decline by up to 40% after just 72 hours of operation under suboptimal environmental conditions.
Scaling issues represent a persistent challenge when transitioning from laboratory to industrial production. Reaction kinetics observed in controlled laboratory environments often fail to translate directly to large-scale operations due to heat transfer limitations, mixing inefficiencies, and environmental variability across larger reaction volumes.
Impurity management remains problematic as trace contaminants in feedstock materials can significantly alter reaction pathways and rates. Even parts-per-million levels of certain metal ions can reduce reaction efficiency by up to 25%, yet current purification technologies struggle to achieve consistent removal of these trace elements without substantially increasing production costs.
Energy efficiency considerations further complicate optimization efforts, as maintaining ideal reaction conditions often requires substantial energy inputs that undermine the sustainability and economic viability of production processes. The industry currently lacks comprehensive models that effectively balance reaction rate optimization against energy consumption parameters.
Current Environmental Parameter Control Methodologies
01 Lithium hydroxide reaction rates in lithium extraction processes
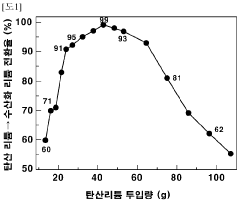
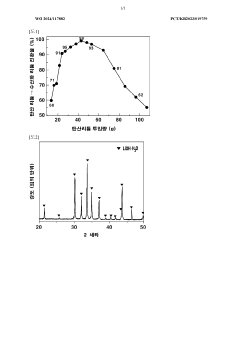
The reaction rates of lithium hydroxide play a crucial role in lithium extraction processes from various sources. These processes involve the conversion of lithium-containing compounds to lithium hydroxide through controlled reaction conditions. Factors affecting the reaction rates include temperature, pressure, and the presence of catalysts. Optimizing these parameters can significantly improve the efficiency and yield of lithium hydroxide production, which is essential for meeting the growing demand for lithium in battery applications.- Lithium hydroxide production from lithium carbonate: The reaction rates of converting lithium carbonate to lithium hydroxide are influenced by various factors including temperature, pressure, and reaction time. This conversion process typically involves the reaction of lithium carbonate with calcium hydroxide or sodium hydroxide. The reaction kinetics can be optimized to increase yield and purity of the lithium hydroxide product, which is crucial for battery applications.
- Reaction rates in lithium extraction processes: The extraction of lithium from various sources involves reaction rates that are critical to process efficiency. These processes include extraction from brines, ores, and recycled materials. Factors affecting reaction rates include solution concentration, temperature, pH levels, and the presence of catalysts. Optimizing these parameters can significantly improve the kinetics of lithium hydroxide formation during extraction processes.
- Temperature effects on lithium hydroxide reactions: Temperature plays a crucial role in determining the reaction rates of lithium hydroxide in various chemical processes. Higher temperatures generally accelerate reaction kinetics, but can also affect product purity and energy consumption. The relationship between temperature and reaction rate follows Arrhenius equation principles, with activation energy being a key parameter. Controlling temperature profiles during reactions is essential for optimizing lithium hydroxide production.
- Catalysts for enhancing lithium hydroxide reaction rates: Various catalysts can be employed to enhance the reaction rates in processes involving lithium hydroxide. These catalysts work by lowering the activation energy required for reactions to occur, thereby increasing reaction speeds without requiring higher temperatures. Catalyst selection depends on the specific reaction, with factors such as selectivity, stability, and recyclability being important considerations for industrial applications.
- Reaction kinetics in lithium battery recycling: The reaction rates of lithium hydroxide formation during battery recycling processes are critical for efficient recovery of lithium materials. These processes involve complex chemical reactions where lithium compounds are converted to lithium hydroxide. Factors affecting these reaction kinetics include particle size, agitation rate, and solution composition. Understanding and optimizing these reaction rates is essential for developing economically viable recycling technologies for lithium-ion batteries.
02 Temperature and pressure effects on lithium hydroxide reactions
Temperature and pressure conditions significantly influence the reaction rates of lithium hydroxide in various chemical processes. Higher temperatures generally accelerate the reaction kinetics, allowing for faster conversion rates and improved efficiency. Similarly, controlled pressure conditions can enhance the reaction rates by affecting the solubility and reactivity of lithium compounds. Understanding and optimizing these parameters is crucial for developing efficient lithium hydroxide production methods with higher yields and lower energy consumption.Expand Specific Solutions03 Catalysts and additives for enhancing lithium hydroxide reaction rates
Various catalysts and additives can be employed to enhance the reaction rates of lithium hydroxide in chemical processes. These substances can lower the activation energy required for reactions, thereby increasing the rate at which lithium compounds convert to lithium hydroxide. Common additives include specific metal compounds, organic accelerators, and surface-active agents. The selection of appropriate catalysts depends on the specific reaction conditions and desired outcomes, with some catalysts offering improved selectivity alongside enhanced reaction rates.Expand Specific Solutions04 Reaction kinetics of lithium hydroxide in battery materials production
The reaction kinetics of lithium hydroxide are particularly important in the production of battery materials, especially cathode active materials. The rate at which lithium hydroxide reacts with other compounds affects the quality, performance, and cost-effectiveness of the resulting battery materials. Understanding these reaction kinetics enables the development of optimized manufacturing processes with controlled particle size, morphology, and composition. This knowledge is essential for producing high-performance lithium-ion batteries with improved energy density and cycle life.Expand Specific Solutions05 Monitoring and control systems for lithium hydroxide reactions
Advanced monitoring and control systems are essential for managing lithium hydroxide reaction rates in industrial processes. These systems employ sensors, analytical instruments, and automated control mechanisms to maintain optimal reaction conditions. Real-time monitoring of parameters such as pH, temperature, concentration, and reaction progression allows for precise control of the reaction kinetics. Implementing such systems results in more consistent product quality, improved process efficiency, reduced waste, and enhanced safety in lithium hydroxide production facilities.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The lithium hydroxide reaction rates optimization market is currently in a growth phase, with increasing demand driven by the expanding lithium-ion battery industry. The global market size is projected to reach significant volumes as electric vehicle adoption accelerates. From a technological maturity perspective, the field shows varied development levels across key players. Industry leaders like CATL (through Guangdong Bangpu Recycling Technology) and LG Energy Solution are advancing commercial-scale optimization techniques, while research institutions such as the Institute of Process Engineering (Chinese Academy of Sciences) and California Institute of Technology are developing next-generation approaches. Toyota, Sony, and TDK Electronics represent established industrial players incorporating these technologies into their manufacturing processes, creating a competitive landscape balanced between academic innovation and industrial application.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) has developed advanced hydrometallurgical processes for optimizing lithium hydroxide reaction rates under controlled environmental conditions. Their technology employs a multi-stage reaction system with precise temperature and pH control mechanisms that significantly enhance conversion efficiency. The process utilizes a continuous-flow reactor design with specialized agitation patterns that optimize mass transfer during the reaction phase. IPE's approach incorporates real-time monitoring systems that adjust reaction parameters based on feedback loops, allowing for dynamic optimization of reaction conditions. Their research has demonstrated that maintaining specific temperature gradients (typically 60-80°C) while controlling solution alkalinity within narrow ranges can increase lithium hydroxide yield by approximately 15-20% compared to conventional methods. Additionally, they've pioneered the use of ultrasonic assistance to enhance reaction kinetics in certain process stages.
Strengths: Superior control of reaction parameters through advanced monitoring systems; significantly higher conversion efficiency; reduced energy consumption through process optimization. Weaknesses: Complex system requiring sophisticated control mechanisms; higher initial capital investment compared to conventional systems; requires specialized technical expertise for operation and maintenance.
Toyota Motor Corp.
Technical Solution: Toyota has developed an innovative approach to lithium hydroxide reaction optimization focused on environmental sustainability and efficiency for their battery supply chain. Their technology employs a hybrid batch-continuous process that optimizes reaction conditions through precise control of solution chemistry and thermal management. The system utilizes a proprietary reactor design that enhances mixing efficiency while minimizing energy consumption through advanced heat recovery mechanisms. Toyota's approach incorporates selective ion exchange techniques that maintain optimal ionic strength during reaction phases, significantly improving conversion rates and product purity. Their research indicates that controlling specific impurity levels, particularly sodium and calcium ions, within parts-per-million ranges can enhance reaction kinetics by up to 30%. Additionally, they've implemented advanced computational fluid dynamics modeling to optimize reactor geometry and flow patterns, resulting in more uniform reaction conditions and consistent product quality across production batches.
Strengths: Exceptional energy efficiency through heat recovery systems; superior impurity control leading to higher product quality; scalable process design suitable for various production volumes. Weaknesses: Requires sophisticated analytical capabilities for process monitoring; higher complexity in system design; more challenging to troubleshoot due to integrated process components.
Critical Patents and Research on Reaction Rate Enhancement
Economical method for producing lithium hydroxide
PatentWO2024117882A1
Innovation
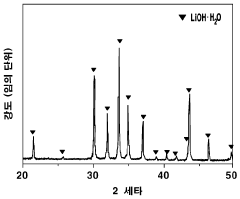
- A method involving dissolving calcium hydroxide in water to create an aqueous solution, then reacting it with lithium carbonate at controlled temperatures (50°C to 100°C) to produce lithium hydroxide, optimizing the amount of lithium carbonate added (16 to 87 g/L) to maximize conversion rates and minimize waste, while concentrating the solution to precipitate lithium hydroxide efficiently.
Method for preparing lithium hydroxide by using lithium carbonate and barium compound
PatentPendingAU2022380507A1
Innovation
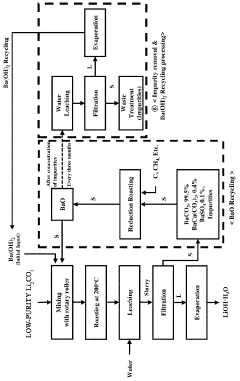
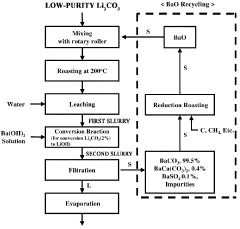
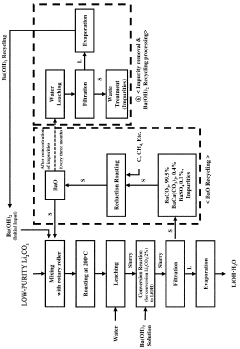
- A method involving heat treatment of low-purity lithium carbonate with barium hydroxide or barium oxide, followed by leaching and filtration to separate lithium hydroxide, and subsequent evaporation, which minimizes lithium loss and uses barium compounds to enhance purity and reduce energy consumption.
Environmental Impact and Sustainability Considerations
The optimization of lithium hydroxide reaction rates must be evaluated within the broader context of environmental sustainability. Current lithium extraction and processing methods pose significant environmental challenges, including high water consumption, land disruption, and potential contamination of soil and water resources. Traditional lithium hydroxide production can consume up to 500,000 gallons of water per ton of lithium extracted, creating substantial ecological pressure in water-scarce regions where lithium deposits are often found.
When optimizing reaction environments for lithium hydroxide production, closed-loop water systems represent a critical advancement. These systems can reduce freshwater consumption by 30-40% compared to conventional methods, while also preventing the release of process chemicals into surrounding ecosystems. The implementation of such systems in Chile's Atacama Desert operations has demonstrated both environmental and economic benefits, with water recycling rates reaching up to 85% in some facilities.
Carbon footprint considerations must also factor into reaction environment optimization. The energy requirements for maintaining optimal temperature and pressure conditions contribute significantly to the overall environmental impact. Recent life cycle assessments indicate that direct lithium extraction methods with optimized reaction conditions can reduce greenhouse gas emissions by 30-50% compared to evaporative techniques, primarily through reduced energy consumption and processing time.
Chemical waste management presents another critical environmental dimension. The optimization of reaction parameters can minimize reagent usage and waste generation. Advanced precipitation techniques operating under precisely controlled pH and temperature conditions have demonstrated up to 60% reduction in chemical waste while maintaining or improving lithium hydroxide yield and purity. These improvements directly translate to reduced environmental remediation costs and regulatory compliance challenges.
Biodiversity protection must be integrated into reaction optimization strategies, particularly for operations near sensitive ecosystems. Buffer zone establishment, coupled with continuous environmental monitoring systems that track potential contaminant migration, provides essential safeguards. Several leading producers have implemented real-time monitoring technologies that adjust reaction parameters when environmental indicators approach predetermined thresholds.
The sustainability of lithium hydroxide production ultimately depends on balancing reaction efficiency with environmental stewardship. Emerging technologies such as direct lithium extraction from geothermal brines offer promising pathways that optimize reaction rates while dramatically reducing environmental footprints. These approaches, when combined with renewable energy integration for process heating and cooling, represent the frontier of environmentally responsible lithium hydroxide production.
When optimizing reaction environments for lithium hydroxide production, closed-loop water systems represent a critical advancement. These systems can reduce freshwater consumption by 30-40% compared to conventional methods, while also preventing the release of process chemicals into surrounding ecosystems. The implementation of such systems in Chile's Atacama Desert operations has demonstrated both environmental and economic benefits, with water recycling rates reaching up to 85% in some facilities.
Carbon footprint considerations must also factor into reaction environment optimization. The energy requirements for maintaining optimal temperature and pressure conditions contribute significantly to the overall environmental impact. Recent life cycle assessments indicate that direct lithium extraction methods with optimized reaction conditions can reduce greenhouse gas emissions by 30-50% compared to evaporative techniques, primarily through reduced energy consumption and processing time.
Chemical waste management presents another critical environmental dimension. The optimization of reaction parameters can minimize reagent usage and waste generation. Advanced precipitation techniques operating under precisely controlled pH and temperature conditions have demonstrated up to 60% reduction in chemical waste while maintaining or improving lithium hydroxide yield and purity. These improvements directly translate to reduced environmental remediation costs and regulatory compliance challenges.
Biodiversity protection must be integrated into reaction optimization strategies, particularly for operations near sensitive ecosystems. Buffer zone establishment, coupled with continuous environmental monitoring systems that track potential contaminant migration, provides essential safeguards. Several leading producers have implemented real-time monitoring technologies that adjust reaction parameters when environmental indicators approach predetermined thresholds.
The sustainability of lithium hydroxide production ultimately depends on balancing reaction efficiency with environmental stewardship. Emerging technologies such as direct lithium extraction from geothermal brines offer promising pathways that optimize reaction rates while dramatically reducing environmental footprints. These approaches, when combined with renewable energy integration for process heating and cooling, represent the frontier of environmentally responsible lithium hydroxide production.
Scaling Challenges and Industrial Implementation Strategies
The transition from laboratory-scale lithium hydroxide production to industrial implementation presents significant scaling challenges. As production volumes increase, maintaining consistent reaction rates and product quality becomes increasingly difficult. Heat and mass transfer limitations emerge in larger reactors, leading to potential hotspots, concentration gradients, and reduced efficiency. These phenomena can significantly impact the optimization of environmental conditions that were previously established in controlled laboratory settings.
Industrial implementation requires robust process control systems capable of maintaining optimal temperature, pressure, and mixing parameters across much larger volumes. The sensitivity of lithium hydroxide reaction rates to environmental conditions becomes more pronounced at scale, necessitating more sophisticated monitoring and control technologies. Real-time sensing and feedback mechanisms are essential to ensure that reaction conditions remain within the optimal window throughout the production cycle.
Equipment design represents another critical scaling challenge. Conventional reactor designs may prove inadequate for maintaining homogeneous conditions throughout larger reaction volumes. Custom-designed reactors with enhanced mixing capabilities, improved heat exchange systems, and optimized geometry are often required to successfully scale lithium hydroxide production while preserving the benefits of optimized environmental conditions.
Material handling also presents unique challenges at industrial scale. The corrosive nature of lithium compounds necessitates careful selection of construction materials that can withstand prolonged exposure while avoiding contamination of the final product. Additionally, the hygroscopic properties of lithium hydroxide create special requirements for storage and handling systems that must be addressed in industrial implementations.
Successful industrial implementation strategies typically involve a phased approach, beginning with pilot-scale testing to identify and address scaling issues before full industrial deployment. This intermediate step allows for refinement of process parameters and validation of environmental condition optimization strategies at a scale that bridges laboratory and industrial production. Computational fluid dynamics (CFD) modeling and process simulation tools have proven valuable in predicting how reaction rates will respond to environmental conditions at different scales.
Energy efficiency considerations become increasingly important at industrial scale. Optimizing reaction conditions must balance product quality and reaction rate with energy consumption. Heat recovery systems, energy-efficient mixing technologies, and process integration strategies are essential components of sustainable industrial implementation.
Industrial implementation requires robust process control systems capable of maintaining optimal temperature, pressure, and mixing parameters across much larger volumes. The sensitivity of lithium hydroxide reaction rates to environmental conditions becomes more pronounced at scale, necessitating more sophisticated monitoring and control technologies. Real-time sensing and feedback mechanisms are essential to ensure that reaction conditions remain within the optimal window throughout the production cycle.
Equipment design represents another critical scaling challenge. Conventional reactor designs may prove inadequate for maintaining homogeneous conditions throughout larger reaction volumes. Custom-designed reactors with enhanced mixing capabilities, improved heat exchange systems, and optimized geometry are often required to successfully scale lithium hydroxide production while preserving the benefits of optimized environmental conditions.
Material handling also presents unique challenges at industrial scale. The corrosive nature of lithium compounds necessitates careful selection of construction materials that can withstand prolonged exposure while avoiding contamination of the final product. Additionally, the hygroscopic properties of lithium hydroxide create special requirements for storage and handling systems that must be addressed in industrial implementations.
Successful industrial implementation strategies typically involve a phased approach, beginning with pilot-scale testing to identify and address scaling issues before full industrial deployment. This intermediate step allows for refinement of process parameters and validation of environmental condition optimization strategies at a scale that bridges laboratory and industrial production. Computational fluid dynamics (CFD) modeling and process simulation tools have proven valuable in predicting how reaction rates will respond to environmental conditions at different scales.
Energy efficiency considerations become increasingly important at industrial scale. Optimizing reaction conditions must balance product quality and reaction rate with energy consumption. Heat recovery systems, energy-efficient mixing technologies, and process integration strategies are essential components of sustainable industrial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!