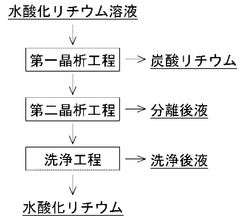
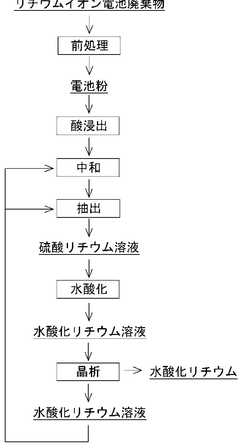
Optimizing Lithium Hydroxide Processing For Higher Purity Levels
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Processing Evolution and Objectives
Lithium hydroxide has emerged as a critical material in the global energy transition, particularly for high-performance lithium-ion batteries used in electric vehicles and renewable energy storage systems. The evolution of lithium hydroxide processing technology spans several decades, with significant advancements occurring in the past 20 years as demand for higher purity materials has intensified.
Initially, lithium hydroxide was produced primarily through the lime soda process, where lithium carbonate reacted with calcium hydroxide. This traditional method typically yielded purities of 95-98%, which was sufficient for industrial applications of the time. However, as battery technology advanced, particularly with the development of nickel-rich cathode materials (NMC811, NCA), the requirements for lithium hydroxide purity increased dramatically.
The mid-2000s marked a turning point with the introduction of electrochemical processing methods, which improved purity levels to approximately 99%. This advancement coincided with the early commercialization of electric vehicles, creating a symbiotic relationship between processing technology development and market demand for higher-performance batteries.
Between 2010 and 2020, significant innovations in membrane filtration, selective ion exchange, and crystallization techniques pushed purity levels to 99.5% and beyond. These improvements directly translated to battery performance enhancements, including increased energy density, improved cycle life, and better thermal stability.
Current state-of-the-art processing aims to achieve 99.9% purity (battery grade) with minimal metallic impurities, particularly sodium, calcium, magnesium, and transition metals that can severely impact battery performance and safety. The primary objective now is to develop scalable, energy-efficient processes that can consistently deliver these high-purity levels while reducing production costs and environmental impact.
Looking forward, the technical objectives for lithium hydroxide processing center around four key areas: increasing purity to ultra-high levels (>99.95%), reducing energy consumption in the purification process, minimizing water usage through closed-loop systems, and developing direct extraction methods that bypass the lithium carbonate intermediate step.
Another critical objective is the development of more sustainable processing routes that reduce chemical waste and enable effective recycling of process materials. This includes innovations in green chemistry approaches that utilize bio-based reagents and ambient processing conditions rather than harsh chemicals and energy-intensive thermal treatments.
The achievement of these objectives will be essential to support the projected growth in electric vehicle adoption and grid-scale energy storage deployment, which is expected to increase lithium hydroxide demand by over 400% by 2030.
Initially, lithium hydroxide was produced primarily through the lime soda process, where lithium carbonate reacted with calcium hydroxide. This traditional method typically yielded purities of 95-98%, which was sufficient for industrial applications of the time. However, as battery technology advanced, particularly with the development of nickel-rich cathode materials (NMC811, NCA), the requirements for lithium hydroxide purity increased dramatically.
The mid-2000s marked a turning point with the introduction of electrochemical processing methods, which improved purity levels to approximately 99%. This advancement coincided with the early commercialization of electric vehicles, creating a symbiotic relationship between processing technology development and market demand for higher-performance batteries.
Between 2010 and 2020, significant innovations in membrane filtration, selective ion exchange, and crystallization techniques pushed purity levels to 99.5% and beyond. These improvements directly translated to battery performance enhancements, including increased energy density, improved cycle life, and better thermal stability.
Current state-of-the-art processing aims to achieve 99.9% purity (battery grade) with minimal metallic impurities, particularly sodium, calcium, magnesium, and transition metals that can severely impact battery performance and safety. The primary objective now is to develop scalable, energy-efficient processes that can consistently deliver these high-purity levels while reducing production costs and environmental impact.
Looking forward, the technical objectives for lithium hydroxide processing center around four key areas: increasing purity to ultra-high levels (>99.95%), reducing energy consumption in the purification process, minimizing water usage through closed-loop systems, and developing direct extraction methods that bypass the lithium carbonate intermediate step.
Another critical objective is the development of more sustainable processing routes that reduce chemical waste and enable effective recycling of process materials. This includes innovations in green chemistry approaches that utilize bio-based reagents and ambient processing conditions rather than harsh chemicals and energy-intensive thermal treatments.
The achievement of these objectives will be essential to support the projected growth in electric vehicle adoption and grid-scale energy storage deployment, which is expected to increase lithium hydroxide demand by over 400% by 2030.
Market Demand Analysis for High-Purity Lithium Hydroxide
The global market for high-purity lithium hydroxide has experienced exponential growth in recent years, primarily driven by the rapid expansion of the electric vehicle (EV) industry. As battery manufacturers continue to develop higher energy density batteries with longer lifespans, the demand for battery-grade lithium hydroxide with purity levels exceeding 99.5% has become increasingly critical.
Market research indicates that the global lithium hydroxide market was valued at approximately $2.3 billion in 2022 and is projected to reach $6.8 billion by 2028, representing a compound annual growth rate (CAGR) of 19.7%. This growth trajectory significantly outpaces that of other lithium compounds, highlighting the strategic importance of high-purity lithium hydroxide in advanced battery technologies.
The EV sector remains the primary demand driver, accounting for over 80% of high-purity lithium hydroxide consumption. Major automotive manufacturers have announced ambitious electrification targets, with companies like Volkswagen, GM, and Ford committing to invest billions in EV development over the next decade. These commitments translate directly to increased demand for high-purity lithium hydroxide, as it is essential for producing nickel-rich cathode materials used in high-performance EV batteries.
Beyond automotive applications, emerging markets for high-purity lithium hydroxide include grid-scale energy storage systems and consumer electronics. The energy storage sector, in particular, is expected to grow at a CAGR of 24% through 2030, creating additional demand pressure on high-purity lithium hydroxide supplies.
Regional analysis reveals that Asia-Pacific, particularly China, South Korea, and Japan, dominates the consumption landscape due to their established battery manufacturing ecosystems. However, significant growth is anticipated in North America and Europe as these regions develop domestic battery production capabilities to reduce supply chain vulnerabilities.
Supply-side constraints present significant challenges to meeting this growing demand. Current production methods struggle to consistently achieve purity levels above 99.8% at commercial scale, creating a premium market segment for ultra-high-purity products. Price differentials between standard battery-grade (99.5%) and ultra-high-purity (99.9%) lithium hydroxide can exceed 30%, highlighting the economic incentive for technological innovation in purification processes.
Industry stakeholders report that impurity profiles are becoming increasingly important, with specific focus on reducing sodium, calcium, and heavy metal contaminants that negatively impact battery performance and longevity. Battery manufacturers are implementing increasingly stringent specifications, creating market segmentation based on purity levels and specific impurity profiles.
Market research indicates that the global lithium hydroxide market was valued at approximately $2.3 billion in 2022 and is projected to reach $6.8 billion by 2028, representing a compound annual growth rate (CAGR) of 19.7%. This growth trajectory significantly outpaces that of other lithium compounds, highlighting the strategic importance of high-purity lithium hydroxide in advanced battery technologies.
The EV sector remains the primary demand driver, accounting for over 80% of high-purity lithium hydroxide consumption. Major automotive manufacturers have announced ambitious electrification targets, with companies like Volkswagen, GM, and Ford committing to invest billions in EV development over the next decade. These commitments translate directly to increased demand for high-purity lithium hydroxide, as it is essential for producing nickel-rich cathode materials used in high-performance EV batteries.
Beyond automotive applications, emerging markets for high-purity lithium hydroxide include grid-scale energy storage systems and consumer electronics. The energy storage sector, in particular, is expected to grow at a CAGR of 24% through 2030, creating additional demand pressure on high-purity lithium hydroxide supplies.
Regional analysis reveals that Asia-Pacific, particularly China, South Korea, and Japan, dominates the consumption landscape due to their established battery manufacturing ecosystems. However, significant growth is anticipated in North America and Europe as these regions develop domestic battery production capabilities to reduce supply chain vulnerabilities.
Supply-side constraints present significant challenges to meeting this growing demand. Current production methods struggle to consistently achieve purity levels above 99.8% at commercial scale, creating a premium market segment for ultra-high-purity products. Price differentials between standard battery-grade (99.5%) and ultra-high-purity (99.9%) lithium hydroxide can exceed 30%, highlighting the economic incentive for technological innovation in purification processes.
Industry stakeholders report that impurity profiles are becoming increasingly important, with specific focus on reducing sodium, calcium, and heavy metal contaminants that negatively impact battery performance and longevity. Battery manufacturers are implementing increasingly stringent specifications, creating market segmentation based on purity levels and specific impurity profiles.
Current Purification Technologies and Challenges
The lithium hydroxide purification landscape is currently dominated by several established technologies, each with specific advantages and limitations. The conventional purification process typically involves precipitation, crystallization, and filtration steps. In this process, lithium-containing solutions undergo chemical treatment to precipitate impurities, followed by crystallization to form lithium hydroxide monohydrate crystals, which are then filtered and dried. While effective for achieving commercial-grade purity (99.0-99.5%), this method struggles to consistently deliver battery-grade purity (≥99.8%) without additional processing steps.
Ion exchange technology has emerged as a significant advancement in lithium hydroxide purification. This technique utilizes specialized resins to selectively remove metal impurities such as calcium, magnesium, sodium, and potassium. The process demonstrates high efficiency for certain impurities but may require multiple cycles for comprehensive purification, increasing operational costs and processing time. Additionally, resin degradation over time necessitates periodic replacement, adding to maintenance expenses.
Membrane-based separation technologies, particularly nanofiltration and reverse osmosis, have gained traction in recent years. These methods leverage semi-permeable membranes to separate lithium hydroxide from impurities based on molecular size and charge differences. While offering advantages in continuous processing capabilities and reduced chemical consumption, membrane technologies face challenges with fouling and scaling, especially when processing solutions with high concentrations of divalent ions.
Solvent extraction represents another purification approach, utilizing organic extractants to selectively separate lithium from impurities. This method offers high selectivity for certain impurity profiles but introduces complexity through the need for multiple extraction stages and organic solvent management. Environmental and safety concerns regarding solvent handling have limited widespread industrial adoption.
The primary technical challenges across all current purification technologies center on several key factors. First, achieving consistent ultra-high purity levels (>99.9%) remains difficult, particularly when dealing with variable feedstock quality. Second, the removal of specific trace impurities, especially transition metals like iron and aluminum at parts-per-million levels, presents persistent challenges. Third, current processes typically involve significant energy consumption, water usage, and chemical inputs, raising sustainability concerns.
Scale-up challenges also persist, with laboratory-proven technologies often encountering efficiency losses when implemented at industrial scale. Additionally, most purification technologies face economic constraints when targeting higher purity levels, as marginal improvements in purity often require disproportionate increases in processing costs and complexity.
Ion exchange technology has emerged as a significant advancement in lithium hydroxide purification. This technique utilizes specialized resins to selectively remove metal impurities such as calcium, magnesium, sodium, and potassium. The process demonstrates high efficiency for certain impurities but may require multiple cycles for comprehensive purification, increasing operational costs and processing time. Additionally, resin degradation over time necessitates periodic replacement, adding to maintenance expenses.
Membrane-based separation technologies, particularly nanofiltration and reverse osmosis, have gained traction in recent years. These methods leverage semi-permeable membranes to separate lithium hydroxide from impurities based on molecular size and charge differences. While offering advantages in continuous processing capabilities and reduced chemical consumption, membrane technologies face challenges with fouling and scaling, especially when processing solutions with high concentrations of divalent ions.
Solvent extraction represents another purification approach, utilizing organic extractants to selectively separate lithium from impurities. This method offers high selectivity for certain impurity profiles but introduces complexity through the need for multiple extraction stages and organic solvent management. Environmental and safety concerns regarding solvent handling have limited widespread industrial adoption.
The primary technical challenges across all current purification technologies center on several key factors. First, achieving consistent ultra-high purity levels (>99.9%) remains difficult, particularly when dealing with variable feedstock quality. Second, the removal of specific trace impurities, especially transition metals like iron and aluminum at parts-per-million levels, presents persistent challenges. Third, current processes typically involve significant energy consumption, water usage, and chemical inputs, raising sustainability concerns.
Scale-up challenges also persist, with laboratory-proven technologies often encountering efficiency losses when implemented at industrial scale. Additionally, most purification technologies face economic constraints when targeting higher purity levels, as marginal improvements in purity often require disproportionate increases in processing costs and complexity.
Advanced Purification Methodologies and Techniques
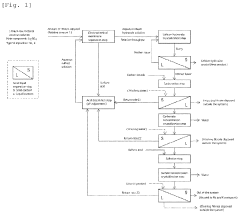
01 Purification methods for high-purity lithium hydroxide
Various purification methods are employed to achieve high-purity lithium hydroxide, including crystallization, ion exchange, and membrane filtration. These processes remove impurities such as sodium, potassium, calcium, and magnesium ions. Advanced purification techniques can achieve battery-grade lithium hydroxide with purity levels exceeding 99.5%, which is essential for high-performance lithium-ion batteries.- Purification methods for high-purity lithium hydroxide: Various purification methods are employed to achieve high-purity lithium hydroxide, including crystallization, ion exchange, and membrane separation techniques. These processes remove impurities such as sodium, potassium, calcium, and magnesium ions to meet battery-grade specifications. Advanced purification methods can achieve purity levels exceeding 99.9%, which is essential for high-performance lithium-ion batteries.
- Battery-grade lithium hydroxide specifications: Battery-grade lithium hydroxide requires specific purity levels to ensure optimal performance in lithium-ion batteries. Typically, battery-grade specifications demand purity levels of 99.5% to 99.95%, with strict limits on metallic impurities such as sodium, calcium, and iron. These specifications are critical for battery performance, as impurities can negatively impact battery efficiency, capacity, and cycle life.
- Production processes affecting purity levels: The production process significantly influences the final purity of lithium hydroxide. Different extraction methods from various lithium sources (brine, spodumene, clay) result in different impurity profiles. Direct lithium extraction technologies, calcination parameters, and reaction conditions all affect the purity of the final product. Optimized production processes can minimize contamination and achieve higher purity levels without extensive secondary purification.
- Analytical methods for purity determination: Accurate determination of lithium hydroxide purity requires sophisticated analytical techniques. Methods such as inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy (AAS), and X-ray fluorescence (XRF) are commonly used to quantify both the lithium content and trace impurities. These analytical methods ensure that the lithium hydroxide meets the required specifications for various applications, particularly in the battery industry.
- Industrial-scale purification systems: Industrial-scale systems for lithium hydroxide purification involve specialized equipment and continuous processing techniques. These systems often incorporate multiple purification stages, including filtration, crystallization, and washing processes. Advanced industrial purification systems can efficiently process large volumes of lithium hydroxide while maintaining consistent high purity levels. Recent innovations focus on reducing energy consumption and water usage while improving recovery rates.
02 Production of battery-grade lithium hydroxide
Battery-grade lithium hydroxide requires specific purity levels to ensure optimal performance in lithium-ion batteries. The production process typically involves multiple purification steps to remove metallic impurities and other contaminants. Battery-grade lithium hydroxide generally requires purity levels of 99.5% or higher, with strict limits on impurities such as sodium, calcium, magnesium, and heavy metals to prevent adverse effects on battery performance and lifespan.Expand Specific Solutions03 Extraction and processing of lithium from brines and minerals
Lithium hydroxide can be produced from various sources, including brines and hard rock minerals like spodumene. The extraction process involves concentration, conversion, and purification steps. For brine sources, solar evaporation is often used to concentrate lithium, followed by chemical processing to convert it to lithium hydroxide. For mineral sources, acid leaching, roasting, and other techniques are employed to extract lithium before conversion to lithium hydroxide of varying purity levels.Expand Specific Solutions04 Quality control and purity standards for lithium hydroxide
Quality control measures and standardized testing methods are essential for ensuring consistent purity levels in lithium hydroxide production. These include analytical techniques such as ICP-MS, atomic absorption spectroscopy, and titration to determine the exact purity and impurity profiles. Industry standards define different grades of lithium hydroxide, with technical grade typically at 56.5-57.5% LiOH and battery grade at >56.5% LiOH with much stricter limits on specific impurities.Expand Specific Solutions05 Innovative processes for enhancing lithium hydroxide purity
Recent innovations focus on developing more efficient and environmentally friendly processes for producing high-purity lithium hydroxide. These include direct lithium extraction technologies, electrochemical purification methods, and novel sorbents for selective lithium recovery. These advanced processes aim to reduce energy consumption, minimize waste generation, and achieve higher purity levels while lowering production costs, enabling the production of ultra-high purity lithium hydroxide exceeding 99.9%.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The lithium hydroxide processing market is currently in a growth phase, driven by increasing demand for high-purity materials for advanced battery applications. The global market size is expanding rapidly, projected to reach several billion dollars by 2025 with a CAGR exceeding 10%. Technologically, the field is advancing from established processes toward more efficient, environmentally sustainable methods. Leading players include Albemarle and Ganfeng Lithium with mature commercial-scale production capabilities, while BASF, Sumitomo Metal Mining, and POSCO Holdings are advancing innovative purification technologies. Emerging companies like Nemaska Lithium and Chengdu Chemphys are developing proprietary processes for ultra-high purity production. The competitive landscape features both established chemical conglomerates and specialized lithium producers competing to achieve higher purity levels while reducing environmental impact and production costs.
BASF Corp.
Technical Solution: BASF has developed an advanced lithium hydroxide purification technology called CAT-Pure that specifically targets the removal of transition metal impurities critical for battery performance. Their process employs a combination of selective precipitation and advanced chromatographic separation techniques. The CAT-Pure system begins with conventional lithium hydroxide feedstock and subjects it to a proprietary multi-stage purification process that selectively targets transition metals like iron, copper, nickel and cobalt - elements particularly detrimental to battery performance even at parts-per-million levels. BASF's innovation includes specialized chelating agents that selectively bind to these metals, followed by a chromatographic separation system that can reduce these impurities to below 1ppm each. The process also incorporates advanced crystallization technology that controls nucleation and crystal growth parameters to produce lithium hydroxide with consistent particle size distribution and morphology. Their technology has demonstrated capability to achieve total metallic impurities below 20ppm while maintaining lithium hydroxide purity above 99.9%. The system also features integrated quality control with real-time monitoring of critical impurities, allowing for process adjustments to maintain consistent purity levels across production batches.
Strengths: Exceptional removal of transition metal impurities critical for battery performance, consistent particle morphology control, and integrated real-time quality monitoring systems. Weaknesses: The specialized chelating agents and chromatographic materials add cost to the purification process, and the system requires more complex operation compared to conventional precipitation methods.
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium has developed an advanced direct lithium extraction (DLE) process specifically optimized for high-purity lithium hydroxide production. Their proprietary technology employs a multi-stage purification system that combines selective adsorption with advanced membrane filtration to remove impurities like sodium, calcium, magnesium and heavy metals. The process incorporates a crystallization technique that utilizes controlled temperature gradients and seeding protocols to produce battery-grade lithium hydroxide with purity levels exceeding 99.95%. Ganfeng's system also features an innovative closed-loop water recycling component that reduces water consumption by approximately 70% compared to traditional evaporation methods, while simultaneously minimizing environmental impact. Their process has demonstrated capability to reduce metallic impurities to below 10ppm, making their lithium hydroxide particularly suitable for high-nickel cathode materials used in next-generation electric vehicle batteries.
Strengths: Achieves exceptionally high purity levels (>99.95%) with minimal metallic impurities, significantly reduced water consumption, and lower environmental footprint. Weaknesses: The multi-stage purification system requires higher initial capital investment and more complex operational controls compared to conventional methods, potentially limiting scalability for smaller operations.
Critical Patents and Innovations in Lithium Hydroxide Purification
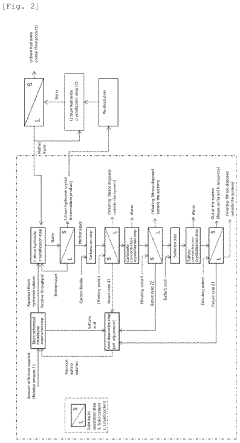
Process for producing lithium hydroxide
PatentPendingUS20240240330A1
Innovation
- A process involving electrochemical membrane separation, crystallization, carbonation, and sulfation steps is employed to efficiently remove alkali metal impurities, recycle lithium, and optimize the yield of high-purity lithium hydroxide, utilizing carbon dioxide and sulfuric acid to convert lithium-containing carbonate compounds back into a sulfate solution for reuse, thereby minimizing waste and increasing lithium recovery.
Method for producing lithium hydroxide
PatentWO2025013610A1
Innovation
- A two-step crystallization process is employed, where the first step involves concentrating a lithium hydroxide solution containing carbonate ions to precipitate and separate lithium carbonate crystals, followed by a second step under reduced pressure to further remove carbonate ions, ensuring high purity lithium hydroxide is obtained.
Environmental Impact and Sustainability Considerations
The optimization of lithium hydroxide processing for higher purity levels carries significant environmental implications that must be carefully considered. Traditional lithium processing methods often involve extensive water usage, chemical consumption, and energy-intensive operations that generate substantial waste streams and carbon emissions. As the demand for high-purity lithium hydroxide continues to grow, particularly for advanced battery applications, the environmental footprint of production processes becomes increasingly important.
Water management represents one of the most critical environmental challenges in lithium hydroxide processing. Conventional methods can require up to 500,000 gallons of water per ton of lithium produced, placing immense pressure on local water resources, especially in arid regions where many lithium operations are located. Advanced purification techniques that incorporate closed-loop water systems and efficient filtration technologies can reduce freshwater consumption by up to 70%, significantly mitigating impacts on local ecosystems and communities.
Energy consumption during lithium hydroxide processing contributes substantially to the carbon footprint of battery supply chains. The calcination and crystallization stages are particularly energy-intensive, often relying on fossil fuel sources. Transitioning to renewable energy sources for processing operations can reduce greenhouse gas emissions by 40-60%, while implementing heat recovery systems and optimizing reaction conditions can further improve energy efficiency.
Chemical usage in purification processes presents additional environmental concerns. Traditional methods employ caustic reagents and solvents that can generate hazardous waste streams requiring specialized disposal. Emerging green chemistry approaches utilize biodegradable reagents, catalytic processes, and selective ion exchange technologies that minimize harmful chemical inputs while achieving higher purity levels. These innovations can reduce hazardous waste generation by up to 80% compared to conventional methods.
Waste management strategies are evolving to address the solid residues and brine solutions generated during processing. Advanced techniques now focus on extracting valuable by-products from waste streams, including magnesium, boron, and potassium compounds. This circular economy approach not only reduces waste volume but creates additional revenue streams that improve the overall sustainability profile of operations.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in lithium production. Companies pursuing higher purity lithium hydroxide must navigate complex compliance requirements while meeting growing market demands for environmentally responsible sourcing. Those implementing best practices in water conservation, emissions reduction, and waste minimization gain competitive advantages through improved stakeholder relations and access to premium markets that prioritize sustainability credentials.
Water management represents one of the most critical environmental challenges in lithium hydroxide processing. Conventional methods can require up to 500,000 gallons of water per ton of lithium produced, placing immense pressure on local water resources, especially in arid regions where many lithium operations are located. Advanced purification techniques that incorporate closed-loop water systems and efficient filtration technologies can reduce freshwater consumption by up to 70%, significantly mitigating impacts on local ecosystems and communities.
Energy consumption during lithium hydroxide processing contributes substantially to the carbon footprint of battery supply chains. The calcination and crystallization stages are particularly energy-intensive, often relying on fossil fuel sources. Transitioning to renewable energy sources for processing operations can reduce greenhouse gas emissions by 40-60%, while implementing heat recovery systems and optimizing reaction conditions can further improve energy efficiency.
Chemical usage in purification processes presents additional environmental concerns. Traditional methods employ caustic reagents and solvents that can generate hazardous waste streams requiring specialized disposal. Emerging green chemistry approaches utilize biodegradable reagents, catalytic processes, and selective ion exchange technologies that minimize harmful chemical inputs while achieving higher purity levels. These innovations can reduce hazardous waste generation by up to 80% compared to conventional methods.
Waste management strategies are evolving to address the solid residues and brine solutions generated during processing. Advanced techniques now focus on extracting valuable by-products from waste streams, including magnesium, boron, and potassium compounds. This circular economy approach not only reduces waste volume but creates additional revenue streams that improve the overall sustainability profile of operations.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in lithium production. Companies pursuing higher purity lithium hydroxide must navigate complex compliance requirements while meeting growing market demands for environmentally responsible sourcing. Those implementing best practices in water conservation, emissions reduction, and waste minimization gain competitive advantages through improved stakeholder relations and access to premium markets that prioritize sustainability credentials.
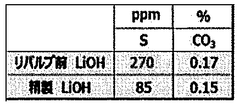
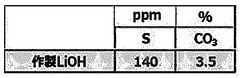
Quality Control and Analytical Testing Methods
Quality control and analytical testing methods are critical components in the optimization of lithium hydroxide processing for achieving higher purity levels. The industry has developed sophisticated techniques to ensure consistent product quality and detect impurities at increasingly lower concentrations.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) represent the gold standard for elemental analysis in lithium hydroxide production. These techniques can detect metallic impurities at parts-per-billion (ppb) levels, which is essential for battery-grade materials where even trace contaminants can significantly impact performance.
X-ray Fluorescence (XRF) spectroscopy provides rapid, non-destructive analysis of lithium hydroxide samples, allowing for real-time monitoring during production. This technique has become increasingly important in continuous processing environments where immediate feedback on composition is necessary for process adjustments.
Particle size distribution analysis using laser diffraction techniques has emerged as a crucial quality control parameter. The uniformity of particle size directly impacts dissolution rates and reactivity in battery applications, with modern systems capable of measuring particles from nanometers to millimeters with high precision.
Automated sampling systems have revolutionized quality control in lithium hydroxide production. These systems can collect representative samples throughout the production process without human intervention, reducing contamination risks and improving statistical reliability through increased sampling frequency.
Statistical Process Control (SPC) methodologies have been widely implemented to monitor process stability and detect deviations before they result in quality issues. Advanced SPC software integrates data from multiple analytical instruments to provide comprehensive quality dashboards and predictive capabilities.
Near-Infrared (NIR) and Raman spectroscopy have gained prominence for their ability to provide real-time, in-line monitoring of chemical composition and crystalline structure. These non-destructive techniques allow for 100% product inspection rather than relying solely on batch sampling.
Moisture analysis using Karl Fischer titration remains essential for lithium hydroxide quality control, as water content significantly impacts product stability and performance in battery applications. Modern automated titrators can achieve precision of ±0.01% water content.
Emerging technologies such as Nuclear Magnetic Resonance (NMR) spectroscopy are being explored for more detailed structural analysis of lithium compounds, potentially offering insights into crystalline defects and molecular arrangements that affect electrochemical performance in battery applications.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) represent the gold standard for elemental analysis in lithium hydroxide production. These techniques can detect metallic impurities at parts-per-billion (ppb) levels, which is essential for battery-grade materials where even trace contaminants can significantly impact performance.
X-ray Fluorescence (XRF) spectroscopy provides rapid, non-destructive analysis of lithium hydroxide samples, allowing for real-time monitoring during production. This technique has become increasingly important in continuous processing environments where immediate feedback on composition is necessary for process adjustments.
Particle size distribution analysis using laser diffraction techniques has emerged as a crucial quality control parameter. The uniformity of particle size directly impacts dissolution rates and reactivity in battery applications, with modern systems capable of measuring particles from nanometers to millimeters with high precision.
Automated sampling systems have revolutionized quality control in lithium hydroxide production. These systems can collect representative samples throughout the production process without human intervention, reducing contamination risks and improving statistical reliability through increased sampling frequency.
Statistical Process Control (SPC) methodologies have been widely implemented to monitor process stability and detect deviations before they result in quality issues. Advanced SPC software integrates data from multiple analytical instruments to provide comprehensive quality dashboards and predictive capabilities.
Near-Infrared (NIR) and Raman spectroscopy have gained prominence for their ability to provide real-time, in-line monitoring of chemical composition and crystalline structure. These non-destructive techniques allow for 100% product inspection rather than relying solely on batch sampling.
Moisture analysis using Karl Fischer titration remains essential for lithium hydroxide quality control, as water content significantly impacts product stability and performance in battery applications. Modern automated titrators can achieve precision of ±0.01% water content.
Emerging technologies such as Nuclear Magnetic Resonance (NMR) spectroscopy are being explored for more detailed structural analysis of lithium compounds, potentially offering insights into crystalline defects and molecular arrangements that affect electrochemical performance in battery applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!