How To Optimize Lithium Hydroxide Electrolyte Formulations
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Electrolyte Development History and Objectives
Lithium hydroxide electrolyte formulations have evolved significantly since the early development of lithium-ion battery technology in the 1970s. Initially, researchers focused on simple salt solutions, primarily using LiPF6 in organic carbonate solvents. The fundamental understanding of lithium-ion transport mechanisms was limited, resulting in electrolytes with modest performance characteristics and stability issues.
The 1990s marked a pivotal shift with the commercial introduction of lithium-ion batteries, driving intensive research into electrolyte optimization. During this period, scientists began exploring the role of lithium hydroxide as an additive to conventional electrolytes, recognizing its potential to neutralize acidic impurities and enhance the formation of stable solid-electrolyte interphase (SEI) layers.
By the early 2000s, research expanded to investigate more complex multi-component electrolyte systems. The introduction of high-voltage cathode materials necessitated electrolytes with improved oxidative stability, leading to experiments with lithium hydroxide modifications to extend the electrochemical stability window. Concurrently, safety concerns prompted investigations into flame-retardant additives and ionic liquid combinations with lithium hydroxide.
The 2010s witnessed accelerated development driven by electric vehicle applications, where energy density, fast charging capability, and cycle life became paramount concerns. Researchers began systematically studying the impact of lithium hydroxide concentration on electrolyte conductivity, viscosity, and interfacial resistance. Advanced characterization techniques, including in-situ spectroscopy and computational modeling, enabled deeper understanding of lithium hydroxide interactions with electrode surfaces.
Recent technological advancements have focused on tailoring lithium hydroxide electrolyte formulations for specific battery chemistries, particularly high-nickel cathodes and silicon-based anodes. These materials present unique challenges requiring precisely optimized electrolyte compositions to mitigate degradation mechanisms and enhance performance.
The primary objectives of current lithium hydroxide electrolyte optimization research include: enhancing ionic conductivity across wide temperature ranges (-40°C to 60°C); improving electrochemical stability at high voltages (>4.5V); minimizing interfacial resistance for faster charging capabilities; extending cycle life through superior SEI formation; and addressing safety concerns by reducing flammability and thermal runaway risks.
Future development aims to establish comprehensive design principles for lithium hydroxide electrolyte formulations that can be tailored to specific battery applications, from high-energy density electric vehicles to fast-charging consumer electronics and grid-scale energy storage systems. This requires fundamental understanding of structure-property relationships and development of predictive models to accelerate formulation optimization.
The 1990s marked a pivotal shift with the commercial introduction of lithium-ion batteries, driving intensive research into electrolyte optimization. During this period, scientists began exploring the role of lithium hydroxide as an additive to conventional electrolytes, recognizing its potential to neutralize acidic impurities and enhance the formation of stable solid-electrolyte interphase (SEI) layers.
By the early 2000s, research expanded to investigate more complex multi-component electrolyte systems. The introduction of high-voltage cathode materials necessitated electrolytes with improved oxidative stability, leading to experiments with lithium hydroxide modifications to extend the electrochemical stability window. Concurrently, safety concerns prompted investigations into flame-retardant additives and ionic liquid combinations with lithium hydroxide.
The 2010s witnessed accelerated development driven by electric vehicle applications, where energy density, fast charging capability, and cycle life became paramount concerns. Researchers began systematically studying the impact of lithium hydroxide concentration on electrolyte conductivity, viscosity, and interfacial resistance. Advanced characterization techniques, including in-situ spectroscopy and computational modeling, enabled deeper understanding of lithium hydroxide interactions with electrode surfaces.
Recent technological advancements have focused on tailoring lithium hydroxide electrolyte formulations for specific battery chemistries, particularly high-nickel cathodes and silicon-based anodes. These materials present unique challenges requiring precisely optimized electrolyte compositions to mitigate degradation mechanisms and enhance performance.
The primary objectives of current lithium hydroxide electrolyte optimization research include: enhancing ionic conductivity across wide temperature ranges (-40°C to 60°C); improving electrochemical stability at high voltages (>4.5V); minimizing interfacial resistance for faster charging capabilities; extending cycle life through superior SEI formation; and addressing safety concerns by reducing flammability and thermal runaway risks.
Future development aims to establish comprehensive design principles for lithium hydroxide electrolyte formulations that can be tailored to specific battery applications, from high-energy density electric vehicles to fast-charging consumer electronics and grid-scale energy storage systems. This requires fundamental understanding of structure-property relationships and development of predictive models to accelerate formulation optimization.
Market Analysis for Advanced Battery Electrolyte Solutions
The global market for advanced battery electrolyte solutions is experiencing unprecedented growth, primarily driven by the rapid expansion of electric vehicle (EV) adoption worldwide. Current market valuations indicate that the lithium-ion battery electrolyte market reached approximately 4.2 billion USD in 2022 and is projected to grow at a compound annual growth rate of 15-18% through 2030. This growth trajectory is significantly outpacing previous forecasts, reflecting accelerated EV adoption rates across major automotive markets.
Lithium hydroxide-based electrolyte formulations represent a particularly promising segment within this market, with demand increasing by over 25% annually due to their superior performance characteristics in high-energy density applications. Major automotive manufacturers are increasingly specifying advanced electrolyte solutions in their battery supply chains, creating substantial pull for innovation in this space.
Regional analysis reveals distinct market dynamics across key territories. Asia-Pacific currently dominates production capacity, with China controlling approximately 75% of global electrolyte manufacturing. However, recent policy shifts in North America and Europe are rapidly changing the landscape, with significant investments in domestic battery supply chains creating new market opportunities for advanced electrolyte technologies.
Consumer demand patterns indicate growing preference for fast-charging capabilities and extended battery life, both areas where optimized electrolyte formulations provide competitive advantages. Market research shows consumers are willing to pay premium prices for EVs offering 20% faster charging times and demonstrably longer battery lifespans, directly correlating to electrolyte performance characteristics.
Industry segmentation analysis reveals automotive applications currently represent the largest market share at 65%, followed by consumer electronics at 18% and grid storage solutions at 12%. The remaining market share is distributed across various industrial applications. The grid storage segment is demonstrating the fastest growth rate, expanding at over 22% annually as renewable energy integration drives demand for efficient energy storage solutions.
Market barriers include supply chain constraints for key electrolyte additives, regulatory hurdles related to transportation of certain chemical components, and price sensitivity in emerging markets. These challenges create strategic opportunities for companies that can develop innovative formulations using more readily available materials or that can demonstrate substantial performance improvements justifying premium pricing.
Forecasting models predict particularly strong growth in electrolyte solutions optimized for extreme temperature operation, silicon-anode compatibility, and reduced flammability - all areas where lithium hydroxide electrolyte optimization shows significant promise for market differentiation.
Lithium hydroxide-based electrolyte formulations represent a particularly promising segment within this market, with demand increasing by over 25% annually due to their superior performance characteristics in high-energy density applications. Major automotive manufacturers are increasingly specifying advanced electrolyte solutions in their battery supply chains, creating substantial pull for innovation in this space.
Regional analysis reveals distinct market dynamics across key territories. Asia-Pacific currently dominates production capacity, with China controlling approximately 75% of global electrolyte manufacturing. However, recent policy shifts in North America and Europe are rapidly changing the landscape, with significant investments in domestic battery supply chains creating new market opportunities for advanced electrolyte technologies.
Consumer demand patterns indicate growing preference for fast-charging capabilities and extended battery life, both areas where optimized electrolyte formulations provide competitive advantages. Market research shows consumers are willing to pay premium prices for EVs offering 20% faster charging times and demonstrably longer battery lifespans, directly correlating to electrolyte performance characteristics.
Industry segmentation analysis reveals automotive applications currently represent the largest market share at 65%, followed by consumer electronics at 18% and grid storage solutions at 12%. The remaining market share is distributed across various industrial applications. The grid storage segment is demonstrating the fastest growth rate, expanding at over 22% annually as renewable energy integration drives demand for efficient energy storage solutions.
Market barriers include supply chain constraints for key electrolyte additives, regulatory hurdles related to transportation of certain chemical components, and price sensitivity in emerging markets. These challenges create strategic opportunities for companies that can develop innovative formulations using more readily available materials or that can demonstrate substantial performance improvements justifying premium pricing.
Forecasting models predict particularly strong growth in electrolyte solutions optimized for extreme temperature operation, silicon-anode compatibility, and reduced flammability - all areas where lithium hydroxide electrolyte optimization shows significant promise for market differentiation.
Current Challenges in Lithium Hydroxide Electrolyte Technology
Despite significant advancements in lithium-ion battery technology, lithium hydroxide electrolyte formulations face several persistent challenges that impede optimal performance and widespread adoption. The primary obstacle remains the inherent reactivity of lithium hydroxide with conventional carbonate-based solvents, leading to parasitic reactions that compromise long-term stability and cycling efficiency. This reactivity creates a fundamental trade-off between high ionic conductivity and chemical stability that researchers continue to struggle with.
Concentration optimization presents another significant challenge, as lithium hydroxide exhibits limited solubility in many organic solvents. At high concentrations necessary for practical energy density, precipitation and crystallization issues frequently occur, particularly at lower operating temperatures. This concentration limitation directly impacts power density and rate capability of resulting battery systems.
Interface stability between the electrolyte and electrode materials represents a critical concern. The highly alkaline nature of lithium hydroxide electrolytes accelerates degradation of common cathode materials, particularly at elevated voltages above 4.2V. This interfacial instability leads to capacity fade and reduced cycle life, necessitating complex surface modification strategies or protective coatings that add cost and manufacturing complexity.
Temperature sensitivity further complicates lithium hydroxide electrolyte optimization. Performance metrics show dramatic variation across operating temperature ranges, with significant conductivity drops below 10°C and accelerated degradation above 45°C. This narrow effective temperature window limits application in extreme environments and requires sophisticated thermal management systems.
Safety concerns persist due to the corrosive nature of concentrated hydroxide solutions and their potential reactivity with cell components under abuse conditions. Thermal runaway risks remain higher compared to state-of-the-art LiPF6-based systems, creating regulatory and consumer acceptance barriers.
Manufacturing scalability presents additional challenges, as precise control of water content and atmospheric exposure during electrolyte preparation and cell assembly is essential but difficult to maintain in high-volume production environments. Even trace moisture contamination can dramatically alter electrolyte properties and cell performance.
Analytical characterization of complex hydroxide-containing electrolyte systems remains inadequate, with limited in-situ and operando techniques capable of monitoring speciation changes and interfacial phenomena during cycling. This knowledge gap hinders systematic optimization approaches and often forces researchers into empirical trial-and-error methodologies.
Computational modeling capabilities for hydroxide-based systems lag behind those for conventional electrolytes, with current models struggling to accurately predict transport properties, solvation structures, and interfacial reactions in these complex, highly-correlated ionic environments.
Concentration optimization presents another significant challenge, as lithium hydroxide exhibits limited solubility in many organic solvents. At high concentrations necessary for practical energy density, precipitation and crystallization issues frequently occur, particularly at lower operating temperatures. This concentration limitation directly impacts power density and rate capability of resulting battery systems.
Interface stability between the electrolyte and electrode materials represents a critical concern. The highly alkaline nature of lithium hydroxide electrolytes accelerates degradation of common cathode materials, particularly at elevated voltages above 4.2V. This interfacial instability leads to capacity fade and reduced cycle life, necessitating complex surface modification strategies or protective coatings that add cost and manufacturing complexity.
Temperature sensitivity further complicates lithium hydroxide electrolyte optimization. Performance metrics show dramatic variation across operating temperature ranges, with significant conductivity drops below 10°C and accelerated degradation above 45°C. This narrow effective temperature window limits application in extreme environments and requires sophisticated thermal management systems.
Safety concerns persist due to the corrosive nature of concentrated hydroxide solutions and their potential reactivity with cell components under abuse conditions. Thermal runaway risks remain higher compared to state-of-the-art LiPF6-based systems, creating regulatory and consumer acceptance barriers.
Manufacturing scalability presents additional challenges, as precise control of water content and atmospheric exposure during electrolyte preparation and cell assembly is essential but difficult to maintain in high-volume production environments. Even trace moisture contamination can dramatically alter electrolyte properties and cell performance.
Analytical characterization of complex hydroxide-containing electrolyte systems remains inadequate, with limited in-situ and operando techniques capable of monitoring speciation changes and interfacial phenomena during cycling. This knowledge gap hinders systematic optimization approaches and often forces researchers into empirical trial-and-error methodologies.
Computational modeling capabilities for hydroxide-based systems lag behind those for conventional electrolytes, with current models struggling to accurately predict transport properties, solvation structures, and interfacial reactions in these complex, highly-correlated ionic environments.
Mainstream Lithium Hydroxide Electrolyte Optimization Approaches
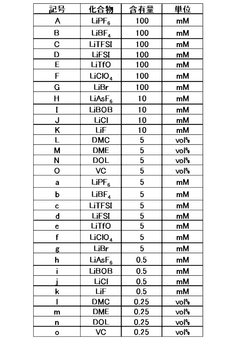
01 Lithium hydroxide concentration optimization in electrolytes
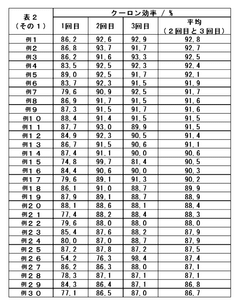
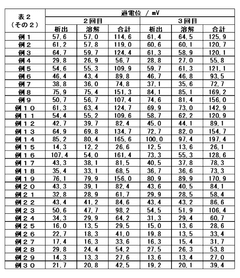
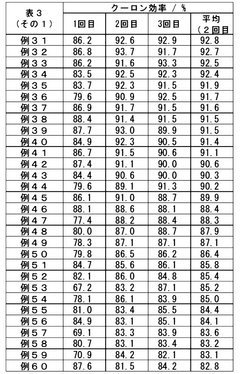
Optimizing the concentration of lithium hydroxide in electrolyte solutions is crucial for battery performance. Research shows that carefully controlled lithium hydroxide concentrations can enhance ionic conductivity, improve charge-discharge efficiency, and extend battery life. The optimal concentration depends on the specific battery chemistry and operating conditions, with studies indicating that balanced formulations prevent unwanted side reactions while maintaining high energy density.- Lithium hydroxide concentration optimization in electrolytes: Optimizing the concentration of lithium hydroxide in electrolytes can significantly improve battery performance. The proper concentration balance enhances ionic conductivity while minimizing unwanted side reactions. Research shows that controlled lithium hydroxide levels can extend battery cycle life and improve capacity retention. This optimization approach involves precise formulation to maintain pH stability and prevent precipitation of lithium compounds during battery operation.
- Additives for lithium hydroxide electrolyte stabilization: Various additives can be incorporated into lithium hydroxide electrolytes to enhance stability and performance. These additives include film-forming compounds, pH buffers, and chelating agents that prevent unwanted side reactions. The strategic use of additives can mitigate lithium dendrite formation, reduce electrolyte decomposition, and improve the solid-electrolyte interphase quality. Properly selected additives work synergistically with lithium hydroxide to optimize overall battery efficiency and longevity.
- Temperature effects on lithium hydroxide electrolyte performance: The performance of lithium hydroxide electrolytes is significantly influenced by operating temperature. Research demonstrates that temperature optimization can enhance ionic mobility, reduce internal resistance, and improve overall battery efficiency. Studies have identified specific temperature ranges where lithium hydroxide electrolytes exhibit optimal conductivity while minimizing degradation mechanisms. Temperature management strategies include controlled heating/cooling systems and thermal insulation techniques to maintain ideal operating conditions.
- Novel electrolyte compositions incorporating lithium hydroxide: Innovative electrolyte formulations that incorporate lithium hydroxide with other components show promising results for next-generation batteries. These novel compositions include hybrid organic-inorganic electrolytes, polymer-based systems, and ionic liquid mixtures. The strategic combination of lithium hydroxide with these materials can yield electrolytes with enhanced safety, wider electrochemical stability windows, and improved compatibility with high-voltage cathodes. These advanced formulations represent a significant advancement in battery technology.
- Manufacturing processes for optimized lithium hydroxide electrolytes: Specialized manufacturing techniques are crucial for producing high-quality lithium hydroxide electrolytes. These processes include precise purification methods, controlled mixing protocols, and advanced quality control measures. Innovations in manufacturing technology enable the production of electrolytes with consistent composition, minimal impurities, and optimal performance characteristics. Continuous flow synthesis, ultrasonic mixing, and other advanced processing methods have been developed to enhance the quality and reproducibility of lithium hydroxide electrolytes.
02 Additives for lithium hydroxide electrolyte stability
Various additives can be incorporated into lithium hydroxide electrolytes to enhance stability and performance. These additives include film-forming compounds, ionic conductivity enhancers, and stabilizing agents that prevent degradation during cycling. The strategic combination of additives with lithium hydroxide creates synergistic effects that improve the electrochemical window, reduce unwanted reactions at electrode interfaces, and enhance overall battery safety and longevity.Expand Specific Solutions03 Temperature effects on lithium hydroxide electrolyte performance
Temperature significantly impacts the performance of lithium hydroxide electrolytes. Research demonstrates that optimizing electrolyte formulations for specific temperature ranges can improve battery operation in extreme conditions. Advanced thermal management techniques and temperature-responsive electrolyte compositions help maintain optimal ionic conductivity and prevent precipitation or degradation issues. These temperature-optimized formulations are particularly important for applications requiring operation in variable environmental conditions.Expand Specific Solutions04 Novel solvent systems for lithium hydroxide electrolytes
Innovative solvent systems are being developed to enhance the performance of lithium hydroxide electrolytes. These include mixed organic solvent systems, ionic liquids, and hybrid electrolyte formulations that improve lithium ion transport while reducing flammability. The careful selection of solvent components affects critical properties such as viscosity, dielectric constant, and electrode wetting characteristics. Advanced solvent systems also enable higher voltage operation and improved low-temperature performance in lithium-based batteries.Expand Specific Solutions05 Interface engineering for lithium hydroxide electrolytes
Interface engineering techniques optimize the interaction between lithium hydroxide electrolytes and electrode surfaces. These approaches include surface coatings, functional electrolyte additives, and specialized electrode treatments that promote stable solid-electrolyte interphase formation. By controlling interfacial reactions, these methods minimize impedance growth, prevent lithium dendrite formation, and enhance cycling stability. Advanced interface engineering strategies also address challenges related to volume changes during cycling and improve the compatibility between high-voltage cathodes and lithium hydroxide-containing electrolytes.Expand Specific Solutions
Leading Companies in Battery Electrolyte Research
The lithium hydroxide electrolyte formulation optimization market is currently in a growth phase, with an estimated global market size exceeding $5 billion and projected to expand at a CAGR of 8-10% through 2030. The competitive landscape features established players like Samsung SDI, Tesla, and BASF alongside emerging specialists such as Wildcat Discovery Technologies and Sila Nanotechnologies. Technical maturity varies significantly across applications, with automotive-grade formulations more advanced than those for grid storage. Leading companies including Ningde Amperex Technology (CATL) and Samsung are focusing on silicon-anode compatible electrolytes, while research institutions like Beijing Institute of Technology and National Institute for Materials Science are pioneering novel additives to enhance thermal stability and cycle life. The industry is transitioning from proprietary formulations toward standardized solutions with customizable performance characteristics.
BASF Corp.
Technical Solution: BASF has pioneered a systematic approach to lithium hydroxide electrolyte optimization through their "Electrolyte Designer" platform. Their technology focuses on molecular engineering of electrolyte components, particularly solvents and additives that work synergistically with controlled amounts of lithium hydroxide. BASF's formulations utilize proprietary lithium salt blends where LiOH is precisely incorporated (typically 5-100 ppm) to neutralize acidic impurities and modify the solid electrolyte interphase chemistry. Their research has demonstrated that controlled LiOH addition can significantly improve the thermal stability of conventional LiPF6-based electrolytes by up to 20°C[3]. BASF employs high-throughput screening methods to evaluate thousands of potential electrolyte combinations, optimizing for specific performance metrics like conductivity, voltage stability, and temperature performance. Their latest generation electrolytes incorporate specially designed boron-based additives that work synergistically with lithium hydroxide to form more stable interfaces with both cathode and anode materials[4].
Strengths: Comprehensive chemical expertise across the entire battery value chain; sophisticated modeling and simulation capabilities for electrolyte design; global R&D network enabling rapid innovation. Weaknesses: Less direct battery manufacturing experience compared to cell manufacturers; some advanced formulations face challenges in cost-effective scaling.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology Ltd.) has developed advanced lithium hydroxide electrolyte formulations incorporating fluorinated solvents and lithium salts to enhance battery performance. Their proprietary electrolyte system utilizes optimized LiOH concentrations balanced with other lithium salts (LiPF6, LiFSI) to improve ionic conductivity while maintaining stability. CATL's formulations incorporate film-forming additives like vinylene carbonate and fluoroethylene carbonate at precisely controlled ratios (0.5-2%) to create stable solid electrolyte interphase layers. Their research has demonstrated that controlling lithium hydroxide content within 10-50 ppm in electrolytes can significantly reduce gas generation during cycling[1]. CATL's electrolytes feature multi-functional additives that simultaneously address multiple performance parameters, resulting in batteries with enhanced cycle life (>2000 cycles at 80% capacity retention) and improved fast-charging capabilities[2].
Strengths: Industry-leading manufacturing scale enables rapid commercialization of new formulations; extensive real-world validation data across various applications; proprietary additive packages that address multiple performance parameters simultaneously. Weaknesses: Higher production costs compared to standard electrolytes; some formulations may require specialized handling due to sensitivity to moisture and air.
Key Patents and Innovations in Electrolyte Chemistry
Electrolyte formulations for optimal performance in Si-containing lithium ion batteries
PatentActiveUS11990585B2
Innovation
- The development of electrolyte compositions comprising multiple components like solvents, co-solvents, salts, and additives, which improve thermal stability and interfacial compatibility, and the use of silicon-dominant anodes with conductive additives and carbonized polymers to enhance electrical conductivity and mechanical robustness.
Optimization system, optimization method, program, electrolytic solution for lithium secondary battery, and lithium secondary battery
PatentWO2022102188A1
Innovation
- An optimization system that combines local search methods with Bayesian optimization to efficiently search for combinations of additives, updates the additive list by removing frequently used additives, and iteratively generates and evaluates new electrolyte formulations until predetermined termination conditions are met.
Environmental Impact and Sustainability Considerations
The optimization of lithium hydroxide electrolyte formulations must be evaluated not only for performance but also for their environmental footprint throughout the entire lifecycle. Current lithium extraction processes are resource-intensive, consuming approximately 500,000 gallons of water per ton of lithium produced in traditional evaporation pond methods. This significant water usage occurs predominantly in water-stressed regions, creating ecological imbalances and potential conflicts with local communities.
The manufacturing of electrolytes involves volatile organic compounds (VOCs) and fluorinated substances that pose substantial environmental risks. These compounds contribute to air pollution, water contamination, and potential long-term ecological damage. Recent studies indicate that fluorinated electrolyte components can persist in the environment for decades, with bioaccumulation potential in various organisms.
Carbon emissions associated with electrolyte production represent another critical concern. The energy-intensive processes required for synthesizing high-purity electrolyte components generate approximately 15-20 kg CO2 equivalent per kilogram of electrolyte produced. This carbon footprint can be significantly reduced through renewable energy integration and process optimization.
Sustainable alternatives are emerging in response to these challenges. Bio-derived solvents and water-based processing techniques show promise in reducing environmental impact while maintaining electrochemical performance. Research indicates that certain plant-derived solvents can reduce toxic waste generation by up to 60% compared to conventional methods, while achieving comparable ionic conductivity.
Circular economy approaches present additional sustainability opportunities. Closed-loop systems for electrolyte recovery and recycling can reclaim up to 80% of valuable components, substantially reducing virgin material requirements. Advanced separation technologies utilizing supercritical CO2 extraction demonstrate particular promise for selective recovery of electrolyte components without generating secondary waste streams.
Regulatory frameworks worldwide are increasingly emphasizing environmental considerations in battery technology development. The European Union's Battery Directive and similar regulations in North America and Asia are establishing stringent requirements for lifecycle assessment and environmental impact disclosure. These regulations will likely accelerate the transition toward greener electrolyte formulations with reduced ecological footprints.
Industry leaders are responding by implementing sustainability metrics in their R&D processes, with environmental impact becoming a key performance indicator alongside traditional electrochemical parameters. This holistic approach ensures that future electrolyte formulations will balance performance requirements with ecological responsibility.
The manufacturing of electrolytes involves volatile organic compounds (VOCs) and fluorinated substances that pose substantial environmental risks. These compounds contribute to air pollution, water contamination, and potential long-term ecological damage. Recent studies indicate that fluorinated electrolyte components can persist in the environment for decades, with bioaccumulation potential in various organisms.
Carbon emissions associated with electrolyte production represent another critical concern. The energy-intensive processes required for synthesizing high-purity electrolyte components generate approximately 15-20 kg CO2 equivalent per kilogram of electrolyte produced. This carbon footprint can be significantly reduced through renewable energy integration and process optimization.
Sustainable alternatives are emerging in response to these challenges. Bio-derived solvents and water-based processing techniques show promise in reducing environmental impact while maintaining electrochemical performance. Research indicates that certain plant-derived solvents can reduce toxic waste generation by up to 60% compared to conventional methods, while achieving comparable ionic conductivity.
Circular economy approaches present additional sustainability opportunities. Closed-loop systems for electrolyte recovery and recycling can reclaim up to 80% of valuable components, substantially reducing virgin material requirements. Advanced separation technologies utilizing supercritical CO2 extraction demonstrate particular promise for selective recovery of electrolyte components without generating secondary waste streams.
Regulatory frameworks worldwide are increasingly emphasizing environmental considerations in battery technology development. The European Union's Battery Directive and similar regulations in North America and Asia are establishing stringent requirements for lifecycle assessment and environmental impact disclosure. These regulations will likely accelerate the transition toward greener electrolyte formulations with reduced ecological footprints.
Industry leaders are responding by implementing sustainability metrics in their R&D processes, with environmental impact becoming a key performance indicator alongside traditional electrochemical parameters. This holistic approach ensures that future electrolyte formulations will balance performance requirements with ecological responsibility.
Safety Standards and Testing Protocols for Electrolyte Formulations
The development of safety standards for lithium hydroxide electrolyte formulations has evolved significantly over the past decade, driven by increasing concerns about battery thermal runaway incidents and the growing adoption of lithium-ion technologies across multiple industries. Current safety protocols encompass both international standards such as IEC 62133, UL 1642, and UN 38.3, as well as regional requirements including China's GB/T 31485 and Europe's EN 62619.
Thermal stability testing represents a cornerstone of electrolyte safety assessment, typically involving differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) to evaluate exothermic reactions under various temperature conditions. These tests determine the onset temperature for thermal decomposition and quantify heat generation rates, critical parameters for predicting cell behavior under abuse conditions.
Flammability testing protocols have become increasingly sophisticated, moving beyond simple flash point determination to include self-extinguishing time measurements and heat release rate quantification. The standardized Cone Calorimeter Test provides valuable data on smoke production, heat release patterns, and combustion characteristics of electrolyte formulations under controlled ignition scenarios.
Chemical compatibility testing examines the long-term interaction between electrolyte components and cell materials, with particular emphasis on electrode degradation mechanisms and gas generation. Protocols typically mandate storage at elevated temperatures (45-85°C) for extended periods (500-1000 hours) to accelerate aging effects, followed by comprehensive analysis of decomposition products using gas chromatography-mass spectrometry (GC-MS).
Electrochemical stability window (ESW) determination has been standardized through cyclic voltammetry protocols, establishing minimum requirements for oxidative and reductive stability limits. Current standards require lithium hydroxide electrolytes to maintain stability between 0V and 4.5V vs. Li/Li+ to accommodate next-generation high-voltage cathode materials.
Toxicity assessment frameworks have expanded beyond traditional LD50 measurements to include comprehensive environmental impact evaluations. Modern protocols require quantification of volatile organic compounds (VOCs) emissions during normal operation and abuse scenarios, with particular attention to fluorinated decomposition products that may form during thermal events.
Recent updates to testing standards have incorporated real-world abuse scenarios, including nail penetration tests, crush tests, and external short circuit simulations. These protocols evaluate electrolyte behavior under catastrophic failure conditions, providing critical data for safety system design and risk mitigation strategies in consumer electronics, electric vehicles, and grid storage applications.
Thermal stability testing represents a cornerstone of electrolyte safety assessment, typically involving differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) to evaluate exothermic reactions under various temperature conditions. These tests determine the onset temperature for thermal decomposition and quantify heat generation rates, critical parameters for predicting cell behavior under abuse conditions.
Flammability testing protocols have become increasingly sophisticated, moving beyond simple flash point determination to include self-extinguishing time measurements and heat release rate quantification. The standardized Cone Calorimeter Test provides valuable data on smoke production, heat release patterns, and combustion characteristics of electrolyte formulations under controlled ignition scenarios.
Chemical compatibility testing examines the long-term interaction between electrolyte components and cell materials, with particular emphasis on electrode degradation mechanisms and gas generation. Protocols typically mandate storage at elevated temperatures (45-85°C) for extended periods (500-1000 hours) to accelerate aging effects, followed by comprehensive analysis of decomposition products using gas chromatography-mass spectrometry (GC-MS).
Electrochemical stability window (ESW) determination has been standardized through cyclic voltammetry protocols, establishing minimum requirements for oxidative and reductive stability limits. Current standards require lithium hydroxide electrolytes to maintain stability between 0V and 4.5V vs. Li/Li+ to accommodate next-generation high-voltage cathode materials.
Toxicity assessment frameworks have expanded beyond traditional LD50 measurements to include comprehensive environmental impact evaluations. Modern protocols require quantification of volatile organic compounds (VOCs) emissions during normal operation and abuse scenarios, with particular attention to fluorinated decomposition products that may form during thermal events.
Recent updates to testing standards have incorporated real-world abuse scenarios, including nail penetration tests, crush tests, and external short circuit simulations. These protocols evaluate electrolyte behavior under catastrophic failure conditions, providing critical data for safety system design and risk mitigation strategies in consumer electronics, electric vehicles, and grid storage applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!