How To Maximize Lithium Hydroxide's Use In CO2 Scrubbing
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Scrubbing Technology Evolution and Objectives
Carbon dioxide (CO2) scrubbing technology has evolved significantly over the past century, transitioning from basic chemical absorption methods to sophisticated integrated systems. The earliest industrial CO2 capture techniques emerged in the 1930s, primarily using monoethanolamine (MEA) solutions. These systems, while effective, were energy-intensive and faced challenges with solvent degradation and equipment corrosion.
The 1970s marked a turning point with growing environmental awareness, leading to increased research in flue gas treatment technologies. By the 1990s, CO2 capture became a focal point in climate change mitigation efforts, accelerating technological development. Traditional amine-based systems were gradually improved with more efficient solvents and process optimizations.
Recent years have witnessed a paradigm shift toward more sustainable and cost-effective solutions. Lithium hydroxide (LiOH) has emerged as a promising alternative to conventional scrubbing agents due to its high CO2 absorption capacity and favorable reaction kinetics. Unlike traditional amines, LiOH forms stable lithium carbonate (Li2CO3) when reacting with CO2, offering potential for both capture and utilization pathways.
The primary objective in maximizing lithium hydroxide's use in CO2 scrubbing is to develop scalable, energy-efficient systems that can be deployed across various industrial sectors. This includes power generation, cement production, and other carbon-intensive industries where point-source emissions can be effectively captured. A key goal is to reduce the energy penalty associated with traditional capture methods, which typically consume 20-30% of a power plant's output.
Another critical objective is to address the full lifecycle of lithium resources, from extraction to regeneration. Developing closed-loop systems where spent lithium carbonate can be efficiently converted back to lithium hydroxide is essential for economic viability and sustainability. This regeneration process represents a significant technical challenge that must be overcome for widespread adoption.
The technology evolution is now trending toward hybrid systems that combine lithium hydroxide with other sorbents or membranes to enhance performance across varying operating conditions. Research is also focusing on novel reactor designs that maximize contact efficiency between CO2 and lithium hydroxide while minimizing pressure drops and energy requirements.
Looking forward, the integration of lithium-based CO2 capture with utilization pathways represents a promising frontier. Converting captured CO2 into valuable products using lithium-mediated processes could transform carbon capture from a cost center to a potential revenue stream, fundamentally altering the economics of emissions reduction.
The 1970s marked a turning point with growing environmental awareness, leading to increased research in flue gas treatment technologies. By the 1990s, CO2 capture became a focal point in climate change mitigation efforts, accelerating technological development. Traditional amine-based systems were gradually improved with more efficient solvents and process optimizations.
Recent years have witnessed a paradigm shift toward more sustainable and cost-effective solutions. Lithium hydroxide (LiOH) has emerged as a promising alternative to conventional scrubbing agents due to its high CO2 absorption capacity and favorable reaction kinetics. Unlike traditional amines, LiOH forms stable lithium carbonate (Li2CO3) when reacting with CO2, offering potential for both capture and utilization pathways.
The primary objective in maximizing lithium hydroxide's use in CO2 scrubbing is to develop scalable, energy-efficient systems that can be deployed across various industrial sectors. This includes power generation, cement production, and other carbon-intensive industries where point-source emissions can be effectively captured. A key goal is to reduce the energy penalty associated with traditional capture methods, which typically consume 20-30% of a power plant's output.
Another critical objective is to address the full lifecycle of lithium resources, from extraction to regeneration. Developing closed-loop systems where spent lithium carbonate can be efficiently converted back to lithium hydroxide is essential for economic viability and sustainability. This regeneration process represents a significant technical challenge that must be overcome for widespread adoption.
The technology evolution is now trending toward hybrid systems that combine lithium hydroxide with other sorbents or membranes to enhance performance across varying operating conditions. Research is also focusing on novel reactor designs that maximize contact efficiency between CO2 and lithium hydroxide while minimizing pressure drops and energy requirements.
Looking forward, the integration of lithium-based CO2 capture with utilization pathways represents a promising frontier. Converting captured CO2 into valuable products using lithium-mediated processes could transform carbon capture from a cost center to a potential revenue stream, fundamentally altering the economics of emissions reduction.
Market Analysis for Lithium Hydroxide-Based Carbon Capture
The global market for lithium hydroxide in carbon capture applications is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. Current market size estimates place the carbon capture, utilization, and storage (CCUS) sector at approximately $2.5 billion, with lithium hydroxide-based solutions representing an emerging segment within this broader market. Growth projections suggest a compound annual growth rate of 15-20% over the next five years for specialized chemical sorbents like lithium hydroxide.
Key market drivers include the implementation of carbon pricing mechanisms in over 40 countries, creating economic incentives for CO2 reduction technologies. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are accelerating adoption of efficient carbon capture solutions. Additionally, major industrial players have committed to net-zero emissions targets, creating substantial demand for cost-effective carbon capture technologies.
Market segmentation reveals several promising application areas for lithium hydroxide-based carbon capture. The power generation sector, particularly natural gas and coal-fired plants, represents the largest potential market due to concentrated emission sources. Industrial applications in cement, steel, and chemical manufacturing constitute the second-largest segment, where high-temperature processes benefit from lithium hydroxide's thermal stability properties.
Direct air capture (DAC) applications represent a smaller but rapidly growing segment, with venture capital investments in DAC technologies exceeding $1 billion in recent years. Submarine and spacecraft life support systems form a niche but premium market segment where lithium hydroxide has established applications that could be optimized further.
Regional market analysis indicates North America and Europe currently lead in carbon capture technology adoption, with combined market share exceeding 60%. However, Asia-Pacific regions, particularly China and India, are projected to see the fastest growth rates due to their expanding industrial bases and increasing environmental regulations.
Competitive landscape assessment reveals that traditional carbon capture is dominated by amine-based solutions from companies like Fluor Corporation and Mitsubishi Heavy Industries. However, specialized chemical companies including Albemarle, SQM, and Ganfeng Lithium are positioned to capitalize on lithium hydroxide applications in carbon capture. Several startups focused on novel sorbent technologies have also secured significant funding to develop enhanced lithium-based carbon capture solutions.
Market barriers include high implementation costs, with current carbon capture technologies adding 25-40% to operational expenses in many industrial applications. Supply chain constraints in lithium production and processing capacity may also impact scaling potential, particularly as demand from electric vehicle battery production continues to grow.
Key market drivers include the implementation of carbon pricing mechanisms in over 40 countries, creating economic incentives for CO2 reduction technologies. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are accelerating adoption of efficient carbon capture solutions. Additionally, major industrial players have committed to net-zero emissions targets, creating substantial demand for cost-effective carbon capture technologies.
Market segmentation reveals several promising application areas for lithium hydroxide-based carbon capture. The power generation sector, particularly natural gas and coal-fired plants, represents the largest potential market due to concentrated emission sources. Industrial applications in cement, steel, and chemical manufacturing constitute the second-largest segment, where high-temperature processes benefit from lithium hydroxide's thermal stability properties.
Direct air capture (DAC) applications represent a smaller but rapidly growing segment, with venture capital investments in DAC technologies exceeding $1 billion in recent years. Submarine and spacecraft life support systems form a niche but premium market segment where lithium hydroxide has established applications that could be optimized further.
Regional market analysis indicates North America and Europe currently lead in carbon capture technology adoption, with combined market share exceeding 60%. However, Asia-Pacific regions, particularly China and India, are projected to see the fastest growth rates due to their expanding industrial bases and increasing environmental regulations.
Competitive landscape assessment reveals that traditional carbon capture is dominated by amine-based solutions from companies like Fluor Corporation and Mitsubishi Heavy Industries. However, specialized chemical companies including Albemarle, SQM, and Ganfeng Lithium are positioned to capitalize on lithium hydroxide applications in carbon capture. Several startups focused on novel sorbent technologies have also secured significant funding to develop enhanced lithium-based carbon capture solutions.
Market barriers include high implementation costs, with current carbon capture technologies adding 25-40% to operational expenses in many industrial applications. Supply chain constraints in lithium production and processing capacity may also impact scaling potential, particularly as demand from electric vehicle battery production continues to grow.
Current Limitations and Challenges in CO2 Scrubbing Technologies
Current carbon dioxide scrubbing technologies face significant limitations that hinder their widespread adoption and efficiency. Traditional amine-based scrubbing systems, while effective at capturing CO2, suffer from high energy requirements during the regeneration phase, with thermal energy demands often exceeding 3.5 GJ/ton of CO2 captured. This energy penalty substantially reduces the net environmental benefit and economic viability of carbon capture operations.
Material degradation presents another critical challenge, as conventional sorbents like monoethanolamine (MEA) experience oxidative and thermal degradation, requiring frequent replacement and generating hazardous waste streams. The degradation rate accelerates in the presence of oxygen and other flue gas contaminants, limiting operational lifespans to approximately 1-2 years in industrial settings.
Scaling issues further complicate implementation, as current technologies struggle with the transition from laboratory demonstrations to industrial-scale operations. The equipment footprint for large-scale carbon capture can occupy substantial space, making retrofitting existing facilities particularly challenging. For instance, a typical 500 MW coal-fired power plant would require a CO2 capture system occupying approximately 4-5 acres of land.
Economic barriers remain formidable, with current cost estimates for CO2 capture ranging from $40-100 per ton, significantly above carbon pricing in most markets. This cost gap creates a substantial barrier to commercial deployment without regulatory mandates or additional incentives.
Water consumption represents another environmental concern, as conventional wet scrubbing processes require 1-2 tons of water per ton of CO2 captured. This water intensity creates sustainability challenges, particularly in water-stressed regions where competing demands for water resources already exist.
Regarding lithium hydroxide specifically, its application in CO2 scrubbing faces unique challenges. Despite its high theoretical CO2 absorption capacity (1 mole of LiOH can capture 0.5 moles of CO2), practical implementation is constrained by several factors. The relatively high cost of lithium compounds compared to traditional sorbents like calcium hydroxide creates economic barriers to widespread adoption.
Additionally, lithium hydroxide's reaction kinetics with CO2 are suboptimal at ambient conditions, requiring either elevated temperatures or engineered particle structures to achieve commercially viable capture rates. The regeneration of lithium carbonate back to lithium hydroxide also demands significant energy input, potentially offsetting some environmental benefits of the capture process.
Competing demands for lithium resources from the battery industry further complicate supply chains, with electric vehicle production consuming an increasing share of global lithium production. This competition drives price volatility and raises questions about long-term supply security for large-scale carbon capture applications.
Material degradation presents another critical challenge, as conventional sorbents like monoethanolamine (MEA) experience oxidative and thermal degradation, requiring frequent replacement and generating hazardous waste streams. The degradation rate accelerates in the presence of oxygen and other flue gas contaminants, limiting operational lifespans to approximately 1-2 years in industrial settings.
Scaling issues further complicate implementation, as current technologies struggle with the transition from laboratory demonstrations to industrial-scale operations. The equipment footprint for large-scale carbon capture can occupy substantial space, making retrofitting existing facilities particularly challenging. For instance, a typical 500 MW coal-fired power plant would require a CO2 capture system occupying approximately 4-5 acres of land.
Economic barriers remain formidable, with current cost estimates for CO2 capture ranging from $40-100 per ton, significantly above carbon pricing in most markets. This cost gap creates a substantial barrier to commercial deployment without regulatory mandates or additional incentives.
Water consumption represents another environmental concern, as conventional wet scrubbing processes require 1-2 tons of water per ton of CO2 captured. This water intensity creates sustainability challenges, particularly in water-stressed regions where competing demands for water resources already exist.
Regarding lithium hydroxide specifically, its application in CO2 scrubbing faces unique challenges. Despite its high theoretical CO2 absorption capacity (1 mole of LiOH can capture 0.5 moles of CO2), practical implementation is constrained by several factors. The relatively high cost of lithium compounds compared to traditional sorbents like calcium hydroxide creates economic barriers to widespread adoption.
Additionally, lithium hydroxide's reaction kinetics with CO2 are suboptimal at ambient conditions, requiring either elevated temperatures or engineered particle structures to achieve commercially viable capture rates. The regeneration of lithium carbonate back to lithium hydroxide also demands significant energy input, potentially offsetting some environmental benefits of the capture process.
Competing demands for lithium resources from the battery industry further complicate supply chains, with electric vehicle production consuming an increasing share of global lithium production. This competition drives price volatility and raises questions about long-term supply security for large-scale carbon capture applications.
Existing Lithium Hydroxide Implementation Methods for CO2 Removal
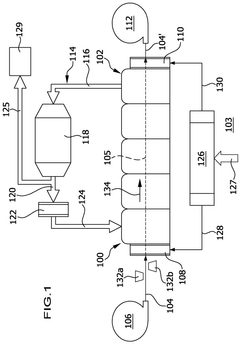
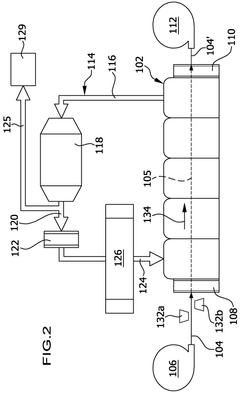
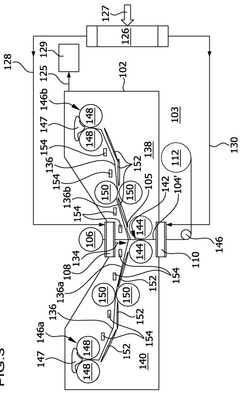
01 Lithium hydroxide-based CO2 capture systems
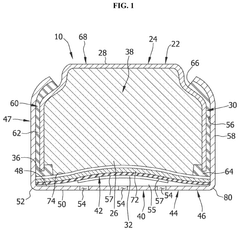
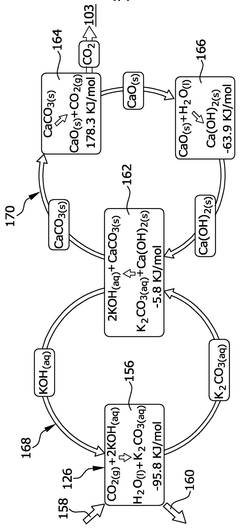
Lithium hydroxide (LiOH) is used as an effective CO2 scrubbing agent in various applications due to its high absorption capacity. The reaction between lithium hydroxide and carbon dioxide produces lithium carbonate (Li2CO3). These systems are particularly valuable in enclosed environments where efficient CO2 removal is critical. The high efficiency of lithium hydroxide in CO2 scrubbing makes it suitable for applications requiring compact and lightweight solutions.- Lithium hydroxide-based CO2 capture systems: Lithium hydroxide (LiOH) is used as an effective CO2 scrubbing agent in various applications. When CO2 comes into contact with LiOH, it forms lithium carbonate (Li2CO3) through a chemical reaction. This process is particularly valuable in closed environments where efficient CO2 removal is critical. The high absorption capacity of lithium hydroxide makes it suitable for applications requiring compact scrubbing systems with minimal weight and volume requirements.
- Regeneration methods for lithium-based CO2 sorbents: Various techniques have been developed to regenerate lithium-based CO2 sorbents after they have been converted to lithium carbonate. These methods include thermal regeneration, electrochemical processes, and chemical treatments that convert lithium carbonate back to lithium hydroxide. Regeneration improves the economic viability of lithium-based CO2 capture systems by allowing the sorbent to be reused multiple times, reducing operational costs and environmental impact while maintaining scrubbing efficiency.
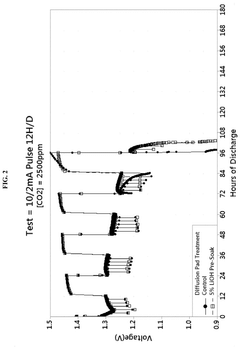
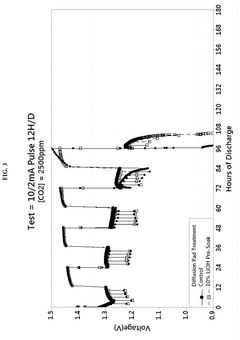
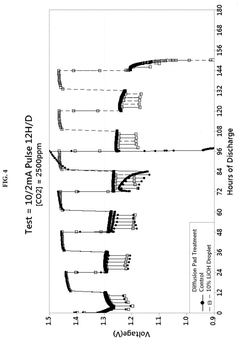
- Enhanced CO2 scrubbing efficiency through material modifications: The efficiency of lithium hydroxide for CO2 scrubbing can be enhanced through various material modifications. These include increasing the surface area of the sorbent, incorporating supportive materials, developing composite structures, and adding catalysts. Modified lithium hydroxide materials demonstrate improved reaction kinetics, higher CO2 absorption capacity, and better stability during multiple absorption-regeneration cycles, resulting in more efficient CO2 capture systems.
- Integration of lithium hydroxide scrubbers in environmental control systems: Lithium hydroxide CO2 scrubbers are integrated into various environmental control systems, particularly in spacecraft, submarines, and other enclosed environments. These integrated systems often combine lithium hydroxide with other components such as moisture control mechanisms, air circulation devices, and monitoring sensors. The integration allows for optimized performance, reduced power consumption, and enhanced reliability of the overall life support system while maintaining effective CO2 removal.
- Comparative analysis of lithium hydroxide against other CO2 scrubbing technologies: Studies comparing lithium hydroxide with alternative CO2 scrubbing technologies evaluate factors such as absorption capacity, reaction kinetics, energy requirements, and operational constraints. While lithium hydroxide offers advantages in terms of high absorption capacity and low energy requirements for CO2 capture, it faces challenges related to regeneration complexity and resource availability. These comparative analyses help in selecting the most appropriate CO2 capture technology for specific applications based on efficiency, cost, and environmental considerations.
02 Regeneration methods for lithium-based CO2 sorbents
Various techniques have been developed to regenerate lithium-based CO2 sorbents after saturation, improving their lifecycle efficiency and economic viability. These methods include thermal regeneration, electrochemical processes, and chemical treatments that convert lithium carbonate back to lithium hydroxide. Regeneration processes typically involve heating the spent sorbent under controlled conditions or using other energy inputs to release the captured CO2 and restore the original absorption capacity of the material.Expand Specific Solutions03 Enhanced formulations with additives and support materials
The efficiency of lithium hydroxide for CO2 scrubbing can be significantly improved by incorporating various additives and support materials. These formulations may include porous substrates, catalysts, or other alkaline compounds that work synergistically with lithium hydroxide. The enhanced formulations provide benefits such as increased surface area, improved reaction kinetics, reduced pressure drop, and better moisture handling capabilities, all contributing to higher overall CO2 removal efficiency.Expand Specific Solutions04 Integration of lithium hydroxide scrubbers in air purification systems
Lithium hydroxide CO2 scrubbers are integrated into comprehensive air purification systems for various applications including spacecraft, submarines, and other enclosed environments. These integrated systems often combine CO2 removal with humidity control, temperature regulation, and removal of other contaminants. The design considerations include optimizing airflow patterns, minimizing energy consumption, and ensuring reliable operation under varying conditions while maintaining high CO2 scrubbing efficiency.Expand Specific Solutions05 Monitoring and control systems for optimizing scrubbing efficiency
Advanced monitoring and control systems have been developed to optimize the performance of lithium hydroxide CO2 scrubbing processes. These systems employ sensors to track parameters such as CO2 concentration, temperature, humidity, and sorbent saturation levels. Real-time data analysis enables dynamic adjustment of operating conditions to maintain peak efficiency. Some systems incorporate predictive algorithms to anticipate maintenance needs and prevent performance degradation, ensuring consistent CO2 removal efficiency throughout the operational lifecycle.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Scrubbing
The CO2 scrubbing market using lithium hydroxide is in an early growth phase, with increasing demand driven by carbon capture initiatives across industrial sectors. The market size is expanding rapidly as environmental regulations tighten globally, though still relatively modest compared to lithium's battery applications. Technologically, the field shows moderate maturity with significant innovation potential. Leading players include Air Liquide Deutschland and Messer Group with established gas processing expertise, while specialized companies like Clean Energy Systems and Nanoscale Components are advancing novel carbon capture solutions. Chinese institutions (Institute of Process Engineering CAS) and lithium producers (EcoPro Innovation, Qinghai Dongtai Jinel) are increasingly active, leveraging their materials expertise to develop more efficient lithium hydroxide-based CO2 absorption systems with improved regeneration capabilities.
Air Liquide Deutschland GmbH
Technical Solution: Air Liquide has pioneered an advanced lithium hydroxide-based CO2 capture system called "LiOH-CarbonCapture" that maximizes efficiency through a multi-stage absorption process. Their technology utilizes a specially formulated lithium hydroxide slurry with proprietary additives that enhance CO2 absorption kinetics while preventing precipitation issues. The system achieves absorption rates up to 40% faster than conventional lithium-based systems through optimized reactor design and controlled reaction conditions. Air Liquide's process incorporates a novel thermal swing regeneration method that operates at lower temperatures (around 550°C versus 700°C for traditional methods), reducing energy requirements by approximately 25%. The company has implemented this technology in industrial settings, demonstrating CO2 removal efficiencies of 98% with significantly reduced equipment footprint compared to traditional scrubbing technologies.
Strengths: Exceptional CO2 removal efficiency; reduced energy consumption for regeneration; compact equipment design; long-term stability of the lithium hydroxide solution. Weaknesses: Requires precise temperature and flow control; higher complexity in system operation; potential for lithium losses during extended operation cycles.
Messer Group GmbH
Technical Solution: Messer Group has developed the "LiOH-Enhanced Membrane Contactor" technology that maximizes lithium hydroxide utilization in CO2 scrubbing through an innovative membrane-based approach. Their system uses hollow fiber membrane contactors where a specially formulated lithium hydroxide solution flows on one side while CO2-containing gas passes on the other, allowing efficient gas-liquid contact without mixing. This approach prevents common issues like foaming and entrainment while achieving 2-3 times higher mass transfer rates compared to conventional packed column absorbers. Messer's technology incorporates a proprietary lithium hydroxide solution with stabilizing additives that prevent precipitation and extend solution lifetime. The system features an integrated electrochemical regeneration unit that converts lithium carbonate back to lithium hydroxide using renewable electricity, eliminating the high thermal energy requirements of conventional regeneration methods and reducing overall energy consumption by up to 40%.
Strengths: Superior mass transfer efficiency; lower pressure drop across the system; reduced energy requirements through electrochemical regeneration; compact design suitable for retrofitting existing facilities. Weaknesses: Higher membrane replacement costs; potential for membrane fouling over time; requires careful control of solution chemistry to prevent membrane damage.
Key Patents and Research on Lithium Hydroxide CO2 Absorption
Zinc-air electrochemical cells with carbon dioxide scavengers
PatentActiveUS12107280B2
Innovation
- Incorporating a carbon dioxide scrubbing agent, such as lithium hydroxide or soda lime, within the battery or its packaging to react with CO2, maintaining electrolyte conductivity and cathode porosity by combining it with hygroscopic materials like polyvinyl alcohol, which increases its accessibility to water.
System and method for treating a material to be treated, involving the removal of carbon dioxide
PatentWO2025162541A1
Innovation
- A system and method to minimize CO2 content in treatment areas by using CO2 reduction devices, such as membrane contactors and sorbents, to maintain an atmosphere with low CO2 levels, preferably below 200 ppm, and optionally incorporating air curtains and recirculation circuits to further reduce CO2 exposure.
Environmental Impact Assessment of Lithium Hydroxide CO2 Scrubbing
The environmental implications of lithium hydroxide-based CO2 scrubbing systems must be thoroughly evaluated to ensure sustainable implementation. When compared to traditional amine-based carbon capture technologies, lithium hydroxide offers several environmental advantages, including lower energy requirements for regeneration and reduced volatile organic compound emissions. However, the full lifecycle assessment reveals important considerations that must be addressed.
Lithium mining operations, particularly in water-stressed regions like the Lithium Triangle in South America, pose significant environmental challenges. Extraction processes can consume between 500,000 to 2 million gallons of water per ton of lithium produced, potentially exacerbating water scarcity issues in vulnerable ecosystems. Additionally, the creation of evaporation ponds for lithium extraction has been linked to soil contamination and disruption of local hydrological systems.
The carbon footprint of lithium hydroxide production must be factored into the overall environmental assessment of CO2 scrubbing systems. Current manufacturing processes generate approximately 5-15 tons of CO2 equivalent per ton of lithium hydroxide produced, depending on energy sources and production methods. This upstream carbon debt must be offset by the system's operational carbon capture efficiency to achieve net environmental benefits.
Waste management presents another critical environmental consideration. Spent lithium hydroxide sorbents require proper disposal or recycling protocols to prevent potential contamination of soil and water resources. Recent advancements in lithium recycling technologies have improved recovery rates to 80-95%, significantly reducing the environmental burden of disposal while creating a more circular material economy.
The implementation of lithium hydroxide CO2 scrubbing at scale could potentially impact global lithium markets, redirecting supplies from other applications such as electric vehicle batteries. This market competition necessitates careful resource allocation planning to prevent unintended consequences in other green technology sectors.
Water usage during the operational phase of lithium hydroxide scrubbing systems is substantially lower than conventional amine-based systems, requiring approximately 30-40% less cooling water. This represents a significant environmental advantage in regions where water conservation is prioritized.
Regulatory frameworks governing lithium hydroxide handling, transportation, and disposal vary significantly across jurisdictions, creating compliance challenges for global implementation. Harmonization of environmental standards would facilitate more consistent environmental performance across different geographical deployments of this technology.
Lithium mining operations, particularly in water-stressed regions like the Lithium Triangle in South America, pose significant environmental challenges. Extraction processes can consume between 500,000 to 2 million gallons of water per ton of lithium produced, potentially exacerbating water scarcity issues in vulnerable ecosystems. Additionally, the creation of evaporation ponds for lithium extraction has been linked to soil contamination and disruption of local hydrological systems.
The carbon footprint of lithium hydroxide production must be factored into the overall environmental assessment of CO2 scrubbing systems. Current manufacturing processes generate approximately 5-15 tons of CO2 equivalent per ton of lithium hydroxide produced, depending on energy sources and production methods. This upstream carbon debt must be offset by the system's operational carbon capture efficiency to achieve net environmental benefits.
Waste management presents another critical environmental consideration. Spent lithium hydroxide sorbents require proper disposal or recycling protocols to prevent potential contamination of soil and water resources. Recent advancements in lithium recycling technologies have improved recovery rates to 80-95%, significantly reducing the environmental burden of disposal while creating a more circular material economy.
The implementation of lithium hydroxide CO2 scrubbing at scale could potentially impact global lithium markets, redirecting supplies from other applications such as electric vehicle batteries. This market competition necessitates careful resource allocation planning to prevent unintended consequences in other green technology sectors.
Water usage during the operational phase of lithium hydroxide scrubbing systems is substantially lower than conventional amine-based systems, requiring approximately 30-40% less cooling water. This represents a significant environmental advantage in regions where water conservation is prioritized.
Regulatory frameworks governing lithium hydroxide handling, transportation, and disposal vary significantly across jurisdictions, creating compliance challenges for global implementation. Harmonization of environmental standards would facilitate more consistent environmental performance across different geographical deployments of this technology.
Economic Feasibility and Scaling Considerations
The economic viability of lithium hydroxide (LiOH) for CO2 scrubbing depends significantly on several interrelated factors including raw material costs, processing expenses, and operational efficiency. Current market prices for lithium hydroxide range from $15,000 to $20,000 per metric ton, making large-scale implementation potentially cost-prohibitive compared to traditional scrubbing agents like monoethanolamine (MEA) which costs approximately $1,500-2,500 per ton. However, LiOH's superior CO2 absorption capacity and regeneration potential could offset these higher initial costs over extended operational periods.
Capital expenditure for retrofitting existing scrubbing systems or constructing new LiOH-based facilities represents a significant barrier to adoption. Engineering estimates suggest conversion costs between $150-300 per kilowatt for power plants, with new installations potentially reaching $500-700 per kilowatt. These figures necessitate careful financial modeling that accounts for the extended operational lifespan of LiOH systems compared to conventional alternatives.
Scaling considerations present both challenges and opportunities. Laboratory-scale successes with LiOH scrubbing systems have demonstrated theoretical CO2 capture efficiencies of 85-95%, but industrial implementation faces diminishing returns as system size increases. Pilot projects at the 1-5 MW scale have shown efficiency drops to 70-80% due to mass transfer limitations and heat management issues. These challenges require innovative engineering solutions such as advanced contactor designs and optimized regeneration cycles.
Supply chain constraints represent another critical scaling consideration. Global lithium production capacity currently stands at approximately 82,000 metric tons annually, with significant competition from battery manufacturers. Widespread adoption of LiOH for carbon capture could potentially require 15,000-25,000 metric tons annually by 2030, necessitating expanded mining operations or development of lithium recycling technologies to prevent supply bottlenecks.
Economic modeling suggests that LiOH scrubbing systems become cost-competitive with traditional methods when carbon prices exceed $60-75 per ton, assuming current lithium prices. Sensitivity analysis indicates that a 20% reduction in lithium costs would lower this threshold to $45-55 per ton, highlighting the importance of continued innovation in lithium extraction and processing technologies. Government incentives, carbon taxes, and regulatory frameworks will significantly influence adoption timelines across different regions and industries.
Capital expenditure for retrofitting existing scrubbing systems or constructing new LiOH-based facilities represents a significant barrier to adoption. Engineering estimates suggest conversion costs between $150-300 per kilowatt for power plants, with new installations potentially reaching $500-700 per kilowatt. These figures necessitate careful financial modeling that accounts for the extended operational lifespan of LiOH systems compared to conventional alternatives.
Scaling considerations present both challenges and opportunities. Laboratory-scale successes with LiOH scrubbing systems have demonstrated theoretical CO2 capture efficiencies of 85-95%, but industrial implementation faces diminishing returns as system size increases. Pilot projects at the 1-5 MW scale have shown efficiency drops to 70-80% due to mass transfer limitations and heat management issues. These challenges require innovative engineering solutions such as advanced contactor designs and optimized regeneration cycles.
Supply chain constraints represent another critical scaling consideration. Global lithium production capacity currently stands at approximately 82,000 metric tons annually, with significant competition from battery manufacturers. Widespread adoption of LiOH for carbon capture could potentially require 15,000-25,000 metric tons annually by 2030, necessitating expanded mining operations or development of lithium recycling technologies to prevent supply bottlenecks.
Economic modeling suggests that LiOH scrubbing systems become cost-competitive with traditional methods when carbon prices exceed $60-75 per ton, assuming current lithium prices. Sensitivity analysis indicates that a 20% reduction in lithium costs would lower this threshold to $45-55 per ton, highlighting the importance of continued innovation in lithium extraction and processing technologies. Government incentives, carbon taxes, and regulatory frameworks will significantly influence adoption timelines across different regions and industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!