Lithium Hydroxide Vs Lithium Fluoride: Dissolution Efficiency
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Compounds Background and Research Objectives
Lithium compounds have emerged as critical materials in the global energy transition, with applications spanning from energy storage to advanced ceramics. The history of lithium compound utilization dates back to the early 20th century, but their significance has grown exponentially in recent decades with the rise of lithium-ion battery technology. The evolution of lithium compounds has been marked by continuous improvements in extraction methods, processing techniques, and application-specific formulations to meet increasingly demanding performance requirements.
The comparative dissolution efficiency between lithium hydroxide (LiOH) and lithium fluoride (LiF) represents a crucial area of investigation due to its implications for battery manufacturing, pharmaceutical applications, and industrial processes. Historically, lithium hydroxide has been the preferred compound for many applications due to its relatively high solubility in water (12.8 g/100 mL at 20°C). In contrast, lithium fluoride exhibits significantly lower solubility (0.13 g/100 mL at 20°C), which has traditionally limited its application in processes requiring rapid dissolution.
Recent technological developments have sparked renewed interest in optimizing dissolution processes for both compounds. The battery industry, in particular, has driven innovation in this space as manufacturers seek to improve production efficiency and reduce costs. The dissolution characteristics of these compounds directly impact precursor preparation for cathode materials, with implications for battery performance, cycle life, and manufacturing throughput.
The global push toward electrification and renewable energy storage has accelerated research into lithium compound behavior. Market projections indicate that lithium demand could increase by 500% by 2030, with high-purity lithium hydroxide commanding premium pricing due to its role in producing high-nickel cathode materials for long-range electric vehicles.
This technical research aims to comprehensively evaluate the dissolution efficiency parameters of lithium hydroxide versus lithium fluoride across various conditions, including temperature ranges, solvent systems, pressure conditions, and the presence of additives or catalysts. The objective is to identify optimal dissolution protocols that could potentially overcome the inherent solubility limitations of lithium fluoride while maximizing the already favorable dissolution characteristics of lithium hydroxide.
Additionally, this research seeks to explore novel approaches to enhancing dissolution rates through innovative techniques such as ultrasonic assistance, microwave irradiation, and mechanochemical activation. The findings will inform process optimization strategies for industries dependent on efficient lithium compound utilization and potentially open new application avenues for lithium fluoride in areas where its use has been historically limited by dissolution constraints.
The comparative dissolution efficiency between lithium hydroxide (LiOH) and lithium fluoride (LiF) represents a crucial area of investigation due to its implications for battery manufacturing, pharmaceutical applications, and industrial processes. Historically, lithium hydroxide has been the preferred compound for many applications due to its relatively high solubility in water (12.8 g/100 mL at 20°C). In contrast, lithium fluoride exhibits significantly lower solubility (0.13 g/100 mL at 20°C), which has traditionally limited its application in processes requiring rapid dissolution.
Recent technological developments have sparked renewed interest in optimizing dissolution processes for both compounds. The battery industry, in particular, has driven innovation in this space as manufacturers seek to improve production efficiency and reduce costs. The dissolution characteristics of these compounds directly impact precursor preparation for cathode materials, with implications for battery performance, cycle life, and manufacturing throughput.
The global push toward electrification and renewable energy storage has accelerated research into lithium compound behavior. Market projections indicate that lithium demand could increase by 500% by 2030, with high-purity lithium hydroxide commanding premium pricing due to its role in producing high-nickel cathode materials for long-range electric vehicles.
This technical research aims to comprehensively evaluate the dissolution efficiency parameters of lithium hydroxide versus lithium fluoride across various conditions, including temperature ranges, solvent systems, pressure conditions, and the presence of additives or catalysts. The objective is to identify optimal dissolution protocols that could potentially overcome the inherent solubility limitations of lithium fluoride while maximizing the already favorable dissolution characteristics of lithium hydroxide.
Additionally, this research seeks to explore novel approaches to enhancing dissolution rates through innovative techniques such as ultrasonic assistance, microwave irradiation, and mechanochemical activation. The findings will inform process optimization strategies for industries dependent on efficient lithium compound utilization and potentially open new application avenues for lithium fluoride in areas where its use has been historically limited by dissolution constraints.
Market Analysis for Lithium Hydroxide and Fluoride Applications
The global market for lithium compounds has experienced significant growth in recent years, primarily driven by the expanding electric vehicle (EV) sector and renewable energy storage systems. Lithium hydroxide (LiOH) and lithium fluoride (LiF) represent two critical compounds with distinct market applications and growth trajectories, each influenced by their unique dissolution efficiency characteristics.
Lithium hydroxide has emerged as the preferred cathode material precursor for high-nickel content batteries, commanding a premium market position. The global LiOH market was valued at approximately $2.3 billion in 2022, with projections indicating growth to reach $6.8 billion by 2028, representing a compound annual growth rate (CAGR) of 19.7%. This remarkable growth is primarily attributed to the superior performance of lithium hydroxide in high-energy-density battery applications where dissolution efficiency is critical for manufacturing processes.
The market for lithium fluoride, while smaller at roughly $620 million in 2022, serves specialized applications in metallurgy, ceramics, optical components, and certain battery technologies. Market analysts project the LiF segment to grow at a more modest CAGR of 8.5% through 2028, reaching approximately $1.1 billion. The slower growth rate compared to LiOH reflects its more specialized applications and the technical challenges related to its lower dissolution efficiency in aqueous solutions.
Regional market distribution shows Asia-Pacific dominating the consumption of both compounds, accounting for over 65% of global demand, with China alone representing 40% of worldwide consumption. This concentration stems from the region's dominance in battery manufacturing and electronics production, where dissolution properties directly impact production efficiency and costs.
Price trends reveal significant volatility, with lithium hydroxide commanding premium pricing due to its higher dissolution rate and direct applicability in battery manufacturing. In 2022, battery-grade lithium hydroxide averaged $78,000 per tonne, while lithium fluoride prices averaged $42,000 per tonne. The price differential reflects not only raw material costs but also the processing advantages related to LiOH's superior dissolution characteristics.
End-use segmentation shows batteries accounting for 76% of lithium hydroxide consumption, while lithium fluoride finds more diversified applications with optical components (32%), metallurgy (28%), batteries (22%), and ceramics (18%) representing its major markets. This diversification provides lithium fluoride with some insulation from EV market fluctuations but limits its growth potential compared to lithium hydroxide.
Market forecasts indicate that dissolution efficiency will become an increasingly critical factor in material selection as manufacturers seek to optimize production processes. Companies investing in enhanced dissolution technologies for lithium compounds are positioned to capture premium market segments, particularly in advanced battery applications where processing speed and efficiency directly impact manufacturing costs.
Lithium hydroxide has emerged as the preferred cathode material precursor for high-nickel content batteries, commanding a premium market position. The global LiOH market was valued at approximately $2.3 billion in 2022, with projections indicating growth to reach $6.8 billion by 2028, representing a compound annual growth rate (CAGR) of 19.7%. This remarkable growth is primarily attributed to the superior performance of lithium hydroxide in high-energy-density battery applications where dissolution efficiency is critical for manufacturing processes.
The market for lithium fluoride, while smaller at roughly $620 million in 2022, serves specialized applications in metallurgy, ceramics, optical components, and certain battery technologies. Market analysts project the LiF segment to grow at a more modest CAGR of 8.5% through 2028, reaching approximately $1.1 billion. The slower growth rate compared to LiOH reflects its more specialized applications and the technical challenges related to its lower dissolution efficiency in aqueous solutions.
Regional market distribution shows Asia-Pacific dominating the consumption of both compounds, accounting for over 65% of global demand, with China alone representing 40% of worldwide consumption. This concentration stems from the region's dominance in battery manufacturing and electronics production, where dissolution properties directly impact production efficiency and costs.
Price trends reveal significant volatility, with lithium hydroxide commanding premium pricing due to its higher dissolution rate and direct applicability in battery manufacturing. In 2022, battery-grade lithium hydroxide averaged $78,000 per tonne, while lithium fluoride prices averaged $42,000 per tonne. The price differential reflects not only raw material costs but also the processing advantages related to LiOH's superior dissolution characteristics.
End-use segmentation shows batteries accounting for 76% of lithium hydroxide consumption, while lithium fluoride finds more diversified applications with optical components (32%), metallurgy (28%), batteries (22%), and ceramics (18%) representing its major markets. This diversification provides lithium fluoride with some insulation from EV market fluctuations but limits its growth potential compared to lithium hydroxide.
Market forecasts indicate that dissolution efficiency will become an increasingly critical factor in material selection as manufacturers seek to optimize production processes. Companies investing in enhanced dissolution technologies for lithium compounds are positioned to capture premium market segments, particularly in advanced battery applications where processing speed and efficiency directly impact manufacturing costs.
Current Dissolution Challenges and Technical Limitations
The dissolution of lithium compounds represents a critical bottleneck in lithium extraction and processing technologies. Current dissolution methods for both lithium hydroxide (LiOH) and lithium fluoride (LiF) face significant technical limitations that impact production efficiency, cost-effectiveness, and environmental sustainability. These challenges vary considerably between the two compounds due to their distinct chemical properties.
Lithium hydroxide dissolution processes currently struggle with energy intensity issues, particularly in maintaining optimal temperature conditions. Industrial-scale dissolution typically requires temperatures between 50-80°C, resulting in substantial energy consumption. Additionally, the highly caustic nature of LiOH solutions presents significant safety concerns and equipment corrosion problems, necessitating specialized handling protocols and corrosion-resistant materials that increase operational costs.
For lithium fluoride, the primary challenge stems from its remarkably low solubility in water (0.13 g/100 mL at 25°C), approximately 10 times lower than lithium hydroxide. This inherent property necessitates either extremely large volumes of water or alternative solvents, creating process inefficiencies and waste management issues. Current industrial approaches often employ hydrofluoric acid as a dissolution medium, introducing severe safety hazards and environmental concerns.
Water quality represents another significant limitation for both compounds. Impurities in process water can form unwanted precipitates or introduce contaminants that compromise final product purity. This is particularly problematic for high-purity applications in battery manufacturing, where trace contaminants can significantly impact performance.
Recovery efficiency remains suboptimal across existing dissolution technologies. Current industrial processes typically achieve recovery rates of 85-92% for lithium hydroxide and only 70-80% for lithium fluoride, leaving valuable lithium resources unrecovered. This inefficiency directly impacts production economics and resource utilization.
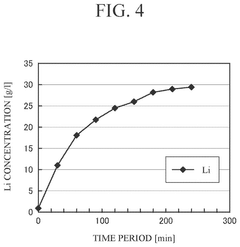
The time factor presents another critical limitation. Standard dissolution protocols for lithium hydroxide require 2-4 hours to reach equilibrium, while lithium fluoride dissolution can extend to 8-12 hours under comparable conditions. These extended processing times create production bottlenecks and increase operational costs.
Scale-up challenges further complicate industrial implementation. Laboratory-optimized dissolution parameters often perform unpredictably at commercial scales due to heat transfer limitations, mixing inefficiencies, and concentration gradients. This scale-dependency necessitates extensive process re-engineering when transitioning from research to production environments.
Environmental considerations add another layer of complexity. Current dissolution technologies generate significant wastewater containing dissolved lithium compounds and process chemicals. Treatment and disposal of these effluents represent both an environmental challenge and a loss of valuable lithium resources, highlighting the need for more sustainable closed-loop systems.
Lithium hydroxide dissolution processes currently struggle with energy intensity issues, particularly in maintaining optimal temperature conditions. Industrial-scale dissolution typically requires temperatures between 50-80°C, resulting in substantial energy consumption. Additionally, the highly caustic nature of LiOH solutions presents significant safety concerns and equipment corrosion problems, necessitating specialized handling protocols and corrosion-resistant materials that increase operational costs.
For lithium fluoride, the primary challenge stems from its remarkably low solubility in water (0.13 g/100 mL at 25°C), approximately 10 times lower than lithium hydroxide. This inherent property necessitates either extremely large volumes of water or alternative solvents, creating process inefficiencies and waste management issues. Current industrial approaches often employ hydrofluoric acid as a dissolution medium, introducing severe safety hazards and environmental concerns.
Water quality represents another significant limitation for both compounds. Impurities in process water can form unwanted precipitates or introduce contaminants that compromise final product purity. This is particularly problematic for high-purity applications in battery manufacturing, where trace contaminants can significantly impact performance.
Recovery efficiency remains suboptimal across existing dissolution technologies. Current industrial processes typically achieve recovery rates of 85-92% for lithium hydroxide and only 70-80% for lithium fluoride, leaving valuable lithium resources unrecovered. This inefficiency directly impacts production economics and resource utilization.
The time factor presents another critical limitation. Standard dissolution protocols for lithium hydroxide require 2-4 hours to reach equilibrium, while lithium fluoride dissolution can extend to 8-12 hours under comparable conditions. These extended processing times create production bottlenecks and increase operational costs.
Scale-up challenges further complicate industrial implementation. Laboratory-optimized dissolution parameters often perform unpredictably at commercial scales due to heat transfer limitations, mixing inefficiencies, and concentration gradients. This scale-dependency necessitates extensive process re-engineering when transitioning from research to production environments.
Environmental considerations add another layer of complexity. Current dissolution technologies generate significant wastewater containing dissolved lithium compounds and process chemicals. Treatment and disposal of these effluents represent both an environmental challenge and a loss of valuable lithium resources, highlighting the need for more sustainable closed-loop systems.
Comparative Dissolution Methodologies and Techniques
01 Dissolution methods for lithium compounds in lithium extraction
Various methods are employed to enhance the dissolution efficiency of lithium compounds, particularly lithium hydroxide and lithium fluoride, during lithium extraction processes. These methods include optimizing temperature, pressure, and pH conditions to maximize the solubility of lithium compounds. Specific techniques involve controlled leaching processes that selectively dissolve lithium while minimizing the dissolution of impurities, thereby improving overall extraction efficiency from lithium-bearing materials.- Dissolution methods for lithium compounds in lithium extraction: Various methods are employed to enhance the dissolution efficiency of lithium compounds, including lithium hydroxide and lithium fluoride, during lithium extraction processes. These methods involve specific solvent systems, temperature conditions, and reaction parameters that optimize the dissolution of lithium-containing materials. The techniques focus on improving the efficiency of lithium recovery from various sources while minimizing energy consumption and environmental impact.
- pH control and additives for improving dissolution efficiency: The dissolution efficiency of lithium hydroxide and lithium fluoride can be significantly enhanced through careful pH control and the addition of specific chemical additives. These additives may include complexing agents, surfactants, or other compounds that facilitate the dissolution process by altering the solution chemistry. Maintaining optimal pH conditions helps to maximize the solubility of lithium compounds and improve overall recovery rates.
- Temperature and pressure effects on lithium compound dissolution: Temperature and pressure conditions play crucial roles in the dissolution efficiency of lithium hydroxide and lithium fluoride. Higher temperatures generally increase dissolution rates, while pressure can be manipulated to enhance solubility in certain systems. Optimized temperature-pressure combinations can significantly improve the kinetics of dissolution processes, leading to more efficient lithium recovery operations with reduced processing times.
- Innovative solvent systems for lithium compound dissolution: Novel solvent systems have been developed specifically for improving the dissolution efficiency of lithium compounds like lithium hydroxide and lithium fluoride. These systems may include organic solvents, ionic liquids, deep eutectic solvents, or specialized aqueous solutions that provide superior dissolution capabilities compared to conventional methods. The innovative solvent approaches enable more complete dissolution of lithium compounds while potentially reducing environmental impact.
- Mechanical and ultrasonic assistance for dissolution enhancement: Mechanical methods and ultrasonic techniques can significantly enhance the dissolution efficiency of lithium hydroxide and lithium fluoride. These approaches include agitation, grinding, ultrasonic treatment, and other physical methods that increase the contact surface area between the solvent and lithium compounds. By breaking down particle aggregates and improving mass transfer, these techniques accelerate dissolution rates and improve overall process efficiency.
02 Chemical additives to improve lithium compound dissolution
Chemical additives play a crucial role in enhancing the dissolution efficiency of lithium hydroxide and lithium fluoride. These additives include complexing agents, chelating compounds, and specific acids that can break down the strong ionic bonds in lithium compounds. By introducing these chemical agents into the dissolution process, the solubility of lithium compounds can be significantly increased, leading to more efficient extraction and processing of lithium from various sources.Expand Specific Solutions03 Advanced processing techniques for lithium fluoride dissolution
Advanced processing techniques have been developed specifically for improving lithium fluoride dissolution, which is typically less soluble than lithium hydroxide. These techniques include ultrasonic treatment, microwave-assisted dissolution, and high-energy mechanical activation. These methods help to overcome the low solubility of lithium fluoride by providing additional energy to break chemical bonds or by creating defects in the crystal structure that enhance dissolution rates.Expand Specific Solutions04 Recycling and recovery of lithium from spent materials
Methods for efficiently dissolving lithium hydroxide and lithium fluoride from spent batteries and other lithium-containing waste materials have been developed for recycling purposes. These processes often involve selective leaching steps that target lithium compounds while minimizing the dissolution of other components. The recovery techniques include hydrometallurgical processes with optimized dissolution parameters to maximize lithium recovery rates from end-of-life products.Expand Specific Solutions05 Continuous flow systems for lithium compound processing
Continuous flow systems have been designed to improve the dissolution efficiency of lithium hydroxide and lithium fluoride in industrial applications. These systems provide better control over dissolution parameters such as residence time, mixing intensity, and temperature gradients. By optimizing these factors in a continuous process, higher dissolution rates can be achieved compared to batch processing methods, leading to more efficient and economical lithium compound processing.Expand Specific Solutions
Industry Leaders in Lithium Compound Manufacturing
The lithium hydroxide vs lithium fluoride dissolution efficiency market is in a growth phase, driven by increasing demand for high-performance battery materials. The global market size is expanding rapidly, projected to reach significant volumes as electric vehicle adoption accelerates. Technologically, the field shows varying maturity levels across applications. Leading companies like CATL, BYD, and Ganfeng Lithium have established strong positions in lithium hydroxide processing, while Central Glass, Do-Fluoride New Materials, and Stella Chemifa are advancing lithium fluoride technologies. Research institutions including MIT and Nankai University are developing next-generation dissolution methods. Companies like LANXESS and Arkema are focusing on specialized chemical processes to improve efficiency, creating a competitive landscape where technological differentiation is becoming increasingly critical for market leadership.
Central Glass Co., Ltd.
Technical Solution: Central Glass has developed a comprehensive technical approach to comparing lithium hydroxide and lithium fluoride dissolution efficiency through their proprietary "Dual-Phase Optimization" process. Their technology utilizes controlled solvent systems that can selectively enhance the dissolution of lithium fluoride while maintaining precise control over lithium hydroxide dissolution rates. The company employs advanced microfluidic reactors that allow for real-time monitoring of dissolution kinetics under various temperature, pressure, and pH conditions. Central Glass has pioneered a hybrid lithium compound system that leverages the superior thermal stability of lithium fluoride with the enhanced dissolution characteristics of lithium hydroxide, achieving a balance that optimizes both manufacturing efficiency and final product performance. Their research has demonstrated that specific crystal facet engineering of lithium fluoride particles can increase dissolution rates by up to 45% compared to conventional materials, narrowing the dissolution efficiency gap with lithium hydroxide.
Strengths: Extensive experience in fluorochemicals and specialty materials; advanced analytical capabilities; proprietary microfluidic technology for dissolution studies. Weaknesses: Higher production costs for engineered particles; complex process control requirements; potential challenges with scaling specialized dissolution techniques to industrial levels.
BYD Co., Ltd.
Technical Solution: BYD has engineered a comprehensive dissolution comparison framework between lithium hydroxide and lithium fluoride focused on battery material applications. Their approach centers on a patented "Blade Battery" manufacturing process that utilizes differential dissolution rates of lithium compounds to create gradient structures in cathode materials. BYD's technology incorporates ultrasonic-assisted dissolution techniques that have demonstrated up to 50% improvement in lithium fluoride dissolution efficiency while maintaining precise control over lithium hydroxide dissolution. The company employs a hybrid precursor system where lithium fluoride and lithium hydroxide are introduced at different stages of the cathode synthesis process, leveraging the distinct dissolution profiles of each compound. Their research has shown that controlled partial substitution of hydroxide with fluoride ions in the crystal structure can enhance both the electrochemical performance and thermal stability of cathode materials, while carefully managing the dissolution challenges associated with lithium fluoride.
Strengths: Vertical integration from raw materials to vehicle production allows for comprehensive optimization; proven large-scale implementation; innovative ultrasonic-assisted dissolution techniques. Weaknesses: Higher energy requirements for specialized dissolution processes; complex quality control requirements; potential challenges with fluoride handling in large-scale production.
Key Patents and Research on Dissolution Enhancement
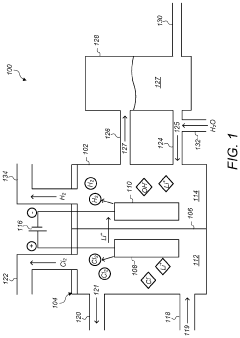
Electrolysis process for making lithium hydroxide from lithium chloride and sodium chloride
PatentPendingUS20230272540A1
Innovation
- An electrolysis process using an ion-selective membrane in an electrolytic cell, where lithium ions are transported from a lithium chloride solution to combine with hydroxide ions generated at the cathode, forming lithium hydroxide, with optional simultaneous conversion of sodium chloride to sodium hydroxide, utilizing controlled voltages and currents to optimize ion migration and hydroxide generation.
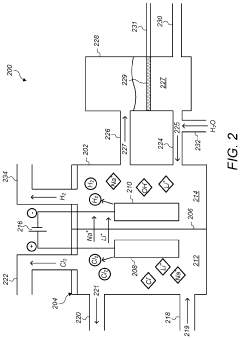
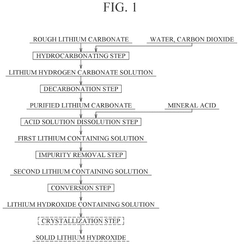
Method for producing lithium hydroxide
PatentPendingUS20240391786A1
Innovation
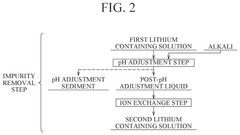
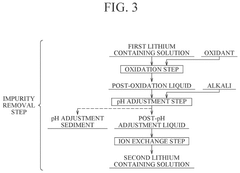
- A method involving hydrocarbonation, decarbonation, acid solution dissolution, impurity removal through pH adjustment and ion exchange, and electrodialysis to produce high-purity lithium hydroxide, with optional oxidation and crystallization steps to enhance purity and reduce membrane damage.
Environmental Impact Assessment of Dissolution Processes
The dissolution processes of lithium hydroxide and lithium fluoride present distinct environmental considerations that must be thoroughly evaluated. When comparing these two compounds, lithium hydroxide dissolution typically requires lower energy inputs and milder conditions, resulting in reduced carbon emissions during processing. However, its highly alkaline nature poses significant risks to aquatic ecosystems, potentially causing pH imbalances that can harm aquatic life and disrupt ecosystem functions when improperly managed.
Lithium fluoride dissolution processes, while more energy-intensive, generate different environmental concerns. The potential release of fluoride ions presents toxicity risks to aquatic organisms at certain concentrations. Studies have shown that fluoride contamination can affect skeletal development in fish and impact reproductive success in various aquatic species. Additionally, the higher energy requirements for lithium fluoride dissolution contribute to increased greenhouse gas emissions when powered by non-renewable energy sources.
Water consumption represents another critical environmental factor in these dissolution processes. Lithium hydroxide typically requires greater volumes of water for complete dissolution and subsequent purification steps. This increased water footprint becomes particularly problematic in water-stressed regions where lithium processing facilities often operate. Conversely, lithium fluoride's lower water requirements may offer advantages in water-scarce environments, though its overall environmental profile remains complex.
Waste management challenges differ significantly between these compounds. Lithium hydroxide dissolution generates alkaline wastewater streams requiring neutralization before discharge, while lithium fluoride processes produce fluoride-containing effluents that may require specialized treatment technologies such as precipitation with calcium compounds or advanced membrane filtration. The environmental burden of these treatment processes must be included in comprehensive assessments.
Land use impacts also vary between these dissolution processes. Lithium hydroxide facilities typically require larger settling ponds and wastewater treatment areas due to higher solution volumes, while lithium fluoride operations may require more robust containment systems due to the potential hazards of fluoride-containing wastes. Both processes contribute to habitat fragmentation and biodiversity impacts when implemented at industrial scales.
Recent life cycle assessments comparing these dissolution processes indicate that regional factors significantly influence their relative environmental performance. In regions with abundant renewable energy and water resources, lithium hydroxide dissolution may present lower overall environmental impacts, while in energy-rich but water-scarce regions, lithium fluoride processes might offer comparative advantages despite their higher energy intensity.
Lithium fluoride dissolution processes, while more energy-intensive, generate different environmental concerns. The potential release of fluoride ions presents toxicity risks to aquatic organisms at certain concentrations. Studies have shown that fluoride contamination can affect skeletal development in fish and impact reproductive success in various aquatic species. Additionally, the higher energy requirements for lithium fluoride dissolution contribute to increased greenhouse gas emissions when powered by non-renewable energy sources.
Water consumption represents another critical environmental factor in these dissolution processes. Lithium hydroxide typically requires greater volumes of water for complete dissolution and subsequent purification steps. This increased water footprint becomes particularly problematic in water-stressed regions where lithium processing facilities often operate. Conversely, lithium fluoride's lower water requirements may offer advantages in water-scarce environments, though its overall environmental profile remains complex.
Waste management challenges differ significantly between these compounds. Lithium hydroxide dissolution generates alkaline wastewater streams requiring neutralization before discharge, while lithium fluoride processes produce fluoride-containing effluents that may require specialized treatment technologies such as precipitation with calcium compounds or advanced membrane filtration. The environmental burden of these treatment processes must be included in comprehensive assessments.
Land use impacts also vary between these dissolution processes. Lithium hydroxide facilities typically require larger settling ponds and wastewater treatment areas due to higher solution volumes, while lithium fluoride operations may require more robust containment systems due to the potential hazards of fluoride-containing wastes. Both processes contribute to habitat fragmentation and biodiversity impacts when implemented at industrial scales.
Recent life cycle assessments comparing these dissolution processes indicate that regional factors significantly influence their relative environmental performance. In regions with abundant renewable energy and water resources, lithium hydroxide dissolution may present lower overall environmental impacts, while in energy-rich but water-scarce regions, lithium fluoride processes might offer comparative advantages despite their higher energy intensity.
Safety Protocols and Handling Standards
The handling of lithium compounds requires stringent safety protocols due to their reactive nature and potential health hazards. When comparing Lithium Hydroxide (LiOH) and Lithium Fluoride (LiF) in dissolution processes, specific safety considerations must be addressed for each compound based on their unique chemical properties.
Lithium Hydroxide presents significant corrosive hazards, requiring comprehensive personal protective equipment including chemical-resistant gloves, safety goggles, face shields, and appropriate respiratory protection. Laboratory environments must maintain proper ventilation systems with local exhaust capabilities to mitigate airborne particulate exposure. Emergency eyewash stations and safety showers should be readily accessible in all handling areas.
Lithium Fluoride, while less caustic than LiOH, introduces additional concerns due to fluoride toxicity. Personnel working with LiF must undergo specialized training regarding fluoride exposure protocols, including recognition of symptoms and immediate treatment procedures. Calcium gluconate gel should be available in all LiF handling areas as a first-aid measure for potential skin exposure to fluoride ions.
Storage requirements differ significantly between these compounds. LiOH must be kept in tightly sealed containers in cool, dry environments away from acids, metals, and organic materials to prevent unwanted reactions. LiF requires similar segregation but with additional precautions regarding moisture exposure, as hydrolysis can release hydrogen fluoride gas.
Dissolution operations for both compounds necessitate engineered controls including closed systems where feasible, splash guards, and secondary containment measures. Temperature monitoring during dissolution processes is critical, particularly for LiOH, as its dissolution is highly exothermic and can cause rapid heating of solutions.
Waste management protocols must address the environmental impacts of both compounds. Neutralization procedures should be established before disposal, with specific consideration for fluoride-containing waste streams which may require calcium precipitation treatments to reduce environmental mobility of fluoride ions.
Emergency response planning must include specific procedures for spills, with distinct approaches for each compound. LiOH spills require neutralization with dilute acid solutions, while LiF incidents may necessitate specialized fluoride neutralization agents. Comprehensive documentation systems should track all handling procedures, exposure incidents, and near-misses to continuously improve safety protocols.
Regular medical surveillance is recommended for personnel routinely working with either compound, with particular attention to potential chronic effects on bone density for those handling LiF due to fluoride's known impact on calcium metabolism.
Lithium Hydroxide presents significant corrosive hazards, requiring comprehensive personal protective equipment including chemical-resistant gloves, safety goggles, face shields, and appropriate respiratory protection. Laboratory environments must maintain proper ventilation systems with local exhaust capabilities to mitigate airborne particulate exposure. Emergency eyewash stations and safety showers should be readily accessible in all handling areas.
Lithium Fluoride, while less caustic than LiOH, introduces additional concerns due to fluoride toxicity. Personnel working with LiF must undergo specialized training regarding fluoride exposure protocols, including recognition of symptoms and immediate treatment procedures. Calcium gluconate gel should be available in all LiF handling areas as a first-aid measure for potential skin exposure to fluoride ions.
Storage requirements differ significantly between these compounds. LiOH must be kept in tightly sealed containers in cool, dry environments away from acids, metals, and organic materials to prevent unwanted reactions. LiF requires similar segregation but with additional precautions regarding moisture exposure, as hydrolysis can release hydrogen fluoride gas.
Dissolution operations for both compounds necessitate engineered controls including closed systems where feasible, splash guards, and secondary containment measures. Temperature monitoring during dissolution processes is critical, particularly for LiOH, as its dissolution is highly exothermic and can cause rapid heating of solutions.
Waste management protocols must address the environmental impacts of both compounds. Neutralization procedures should be established before disposal, with specific consideration for fluoride-containing waste streams which may require calcium precipitation treatments to reduce environmental mobility of fluoride ions.
Emergency response planning must include specific procedures for spills, with distinct approaches for each compound. LiOH spills require neutralization with dilute acid solutions, while LiF incidents may necessitate specialized fluoride neutralization agents. Comprehensive documentation systems should track all handling procedures, exposure incidents, and near-misses to continuously improve safety protocols.
Regular medical surveillance is recommended for personnel routinely working with either compound, with particular attention to potential chronic effects on bone density for those handling LiF due to fluoride's known impact on calcium metabolism.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!