How To Benchmark Lithium Hydroxide Solubility In Water
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Solubility Background and Objectives
Lithium hydroxide (LiOH) has emerged as a critical compound in the global transition toward sustainable energy systems, primarily due to its essential role in lithium-ion battery production. The historical development of lithium hydroxide technology can be traced back to the early 20th century, but its significance has grown exponentially in recent decades with the rise of electric vehicles and renewable energy storage solutions.
The evolution of lithium hydroxide technology has been characterized by continuous improvements in production methods, from traditional processes involving lithium carbonate conversion to more advanced direct extraction techniques from brines and hard rock sources. This technological progression has been driven by increasing demand for higher purity lithium hydroxide, particularly battery-grade material that meets stringent specifications for modern high-nickel cathode materials.
Current market dynamics indicate a substantial growth trajectory, with global lithium hydroxide demand projected to increase at a CAGR of approximately 25-30% through 2030. This growth is primarily fueled by the electric vehicle revolution and grid-scale energy storage deployments, creating urgent need for reliable benchmarking methodologies to assess lithium hydroxide quality and performance characteristics.
Solubility represents one of the fundamental physicochemical properties of lithium hydroxide that significantly impacts its processing, purification, and application performance. Despite its importance, standardized benchmarking protocols for lithium hydroxide solubility in water remain inconsistently applied across the industry, creating challenges for quality control, process optimization, and material comparability.
The primary technical objective of this investigation is to establish robust, reproducible methodologies for benchmarking lithium hydroxide solubility in water across varying temperature ranges, concentrations, and in the presence of common impurities. This includes developing standardized experimental protocols, analytical techniques, and data reporting frameworks that can be universally adopted by manufacturers, researchers, and end-users.
Secondary objectives include quantifying the impact of common impurities on solubility behavior, establishing temperature-dependent solubility curves with high precision, and creating reference materials that can serve as calibration standards for the industry. Additionally, this research aims to correlate solubility characteristics with downstream performance in battery applications, particularly regarding electrolyte preparation and cathode synthesis processes.
The anticipated outcomes of this technical investigation will provide valuable tools for quality assessment, process optimization, and material selection across the lithium battery value chain, ultimately contributing to improved battery performance, manufacturing efficiency, and resource utilization in this rapidly evolving technological landscape.
The evolution of lithium hydroxide technology has been characterized by continuous improvements in production methods, from traditional processes involving lithium carbonate conversion to more advanced direct extraction techniques from brines and hard rock sources. This technological progression has been driven by increasing demand for higher purity lithium hydroxide, particularly battery-grade material that meets stringent specifications for modern high-nickel cathode materials.
Current market dynamics indicate a substantial growth trajectory, with global lithium hydroxide demand projected to increase at a CAGR of approximately 25-30% through 2030. This growth is primarily fueled by the electric vehicle revolution and grid-scale energy storage deployments, creating urgent need for reliable benchmarking methodologies to assess lithium hydroxide quality and performance characteristics.
Solubility represents one of the fundamental physicochemical properties of lithium hydroxide that significantly impacts its processing, purification, and application performance. Despite its importance, standardized benchmarking protocols for lithium hydroxide solubility in water remain inconsistently applied across the industry, creating challenges for quality control, process optimization, and material comparability.
The primary technical objective of this investigation is to establish robust, reproducible methodologies for benchmarking lithium hydroxide solubility in water across varying temperature ranges, concentrations, and in the presence of common impurities. This includes developing standardized experimental protocols, analytical techniques, and data reporting frameworks that can be universally adopted by manufacturers, researchers, and end-users.
Secondary objectives include quantifying the impact of common impurities on solubility behavior, establishing temperature-dependent solubility curves with high precision, and creating reference materials that can serve as calibration standards for the industry. Additionally, this research aims to correlate solubility characteristics with downstream performance in battery applications, particularly regarding electrolyte preparation and cathode synthesis processes.
The anticipated outcomes of this technical investigation will provide valuable tools for quality assessment, process optimization, and material selection across the lithium battery value chain, ultimately contributing to improved battery performance, manufacturing efficiency, and resource utilization in this rapidly evolving technological landscape.
Market Applications and Demand Analysis
The lithium hydroxide market has experienced significant growth in recent years, primarily driven by the expanding electric vehicle (EV) industry. Lithium hydroxide is a critical component in the production of high-performance lithium-ion batteries, particularly those utilizing nickel-rich cathode materials that deliver higher energy density and longer driving ranges. Understanding the solubility benchmarking of lithium hydroxide in water is essential for optimizing production processes and ensuring product quality across various applications.
The global lithium hydroxide market was valued at approximately $1.2 billion in 2020 and is projected to reach $3.5 billion by 2027, growing at a CAGR of 16.5% during the forecast period. This growth is predominantly fueled by the automotive sector's transition toward electrification, with major manufacturers committing to electric vehicle production targets.
Battery manufacturing represents the largest application segment, accounting for over 65% of lithium hydroxide consumption. The increasing preference for high-nickel cathodes (NCM 811 and NCA) in EV batteries has specifically boosted demand for battery-grade lithium hydroxide, as it enables better performance at high temperatures compared to lithium carbonate alternatives.
Beyond the EV sector, lithium hydroxide finds applications in various industrial processes. The lubricant industry utilizes lithium hydroxide for producing lithium-based greases, which offer excellent water resistance and high-temperature stability. This segment constitutes approximately 12% of the global market demand.
The glass and ceramics industry represents another significant application area, where lithium hydroxide serves as a flux agent that lowers melting temperatures and improves product durability. This sector accounts for roughly 8% of global consumption, with steady growth projected as construction activities increase worldwide.
Air treatment systems also utilize lithium hydroxide as a carbon dioxide absorbent in closed environments such as spacecraft, submarines, and mining operations. Though smaller in volume, this application commands premium pricing due to its critical nature in life-support systems.
Regional demand analysis shows Asia-Pacific dominating the market with over 70% share, led by China's massive battery manufacturing capacity. North America and Europe are experiencing the fastest growth rates as they establish domestic battery supply chains to support their automotive industries.
The accurate benchmarking of lithium hydroxide solubility in water directly impacts production efficiency, product purity, and ultimately manufacturing costs across these applications. As manufacturers seek to optimize processes and reduce water consumption, precise solubility data becomes increasingly valuable for sustainable production methods and quality control protocols.
The global lithium hydroxide market was valued at approximately $1.2 billion in 2020 and is projected to reach $3.5 billion by 2027, growing at a CAGR of 16.5% during the forecast period. This growth is predominantly fueled by the automotive sector's transition toward electrification, with major manufacturers committing to electric vehicle production targets.
Battery manufacturing represents the largest application segment, accounting for over 65% of lithium hydroxide consumption. The increasing preference for high-nickel cathodes (NCM 811 and NCA) in EV batteries has specifically boosted demand for battery-grade lithium hydroxide, as it enables better performance at high temperatures compared to lithium carbonate alternatives.
Beyond the EV sector, lithium hydroxide finds applications in various industrial processes. The lubricant industry utilizes lithium hydroxide for producing lithium-based greases, which offer excellent water resistance and high-temperature stability. This segment constitutes approximately 12% of the global market demand.
The glass and ceramics industry represents another significant application area, where lithium hydroxide serves as a flux agent that lowers melting temperatures and improves product durability. This sector accounts for roughly 8% of global consumption, with steady growth projected as construction activities increase worldwide.
Air treatment systems also utilize lithium hydroxide as a carbon dioxide absorbent in closed environments such as spacecraft, submarines, and mining operations. Though smaller in volume, this application commands premium pricing due to its critical nature in life-support systems.
Regional demand analysis shows Asia-Pacific dominating the market with over 70% share, led by China's massive battery manufacturing capacity. North America and Europe are experiencing the fastest growth rates as they establish domestic battery supply chains to support their automotive industries.
The accurate benchmarking of lithium hydroxide solubility in water directly impacts production efficiency, product purity, and ultimately manufacturing costs across these applications. As manufacturers seek to optimize processes and reduce water consumption, precise solubility data becomes increasingly valuable for sustainable production methods and quality control protocols.
Current Benchmarking Methodologies and Challenges
The benchmarking of lithium hydroxide solubility in water currently employs several established methodologies, each with specific advantages and limitations. The most common approach involves gravimetric analysis, where precise amounts of lithium hydroxide are added to water at controlled temperatures until saturation occurs. The solution is then filtered, and the dissolved content is determined by evaporation and weighing. This method, while reliable, is time-consuming and requires meticulous laboratory technique to ensure accuracy.
Titration methods represent another widely used approach, particularly acid-base titrations with standardized acids to determine hydroxide concentration. Potentiometric titration offers enhanced precision by monitoring pH changes during the neutralization process, allowing for more accurate endpoint detection. However, these methods can be affected by the presence of impurities or other dissolved species that may interfere with the titration process.
Spectroscopic techniques have gained prominence in recent years, including atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS) for lithium ion concentration determination. These methods offer high sensitivity and can detect lithium at parts-per-billion levels, though they require sophisticated instrumentation and skilled operators. The challenge lies in correlating the measured lithium concentration with actual hydroxide solubility, as other lithium species may be present.
Conductometric methods measure the electrical conductivity of lithium hydroxide solutions, which increases with concentration until saturation. While this approach allows for continuous monitoring, it requires careful calibration and can be influenced by temperature fluctuations and the presence of other electrolytes.
A significant challenge across all methodologies is temperature control, as lithium hydroxide solubility exhibits strong temperature dependence. Even minor temperature variations can lead to substantial measurement discrepancies, necessitating precise temperature regulation systems. Most laboratories maintain temperature control within ±0.1°C, though achieving this precision consistently remains challenging.
Standardization presents another obstacle, as different laboratories often employ varying protocols, equipment, and analytical techniques. This lack of standardization complicates direct comparison of results across different research groups and industrial settings. The absence of universally accepted reference materials specifically for lithium hydroxide solubility further compounds this issue.
Emerging challenges include the need for real-time monitoring systems capable of tracking solubility under dynamic conditions, particularly important for industrial applications where temperature and pressure may fluctuate. Additionally, the increasing demand for high-purity lithium compounds in battery manufacturing has raised the bar for analytical precision, requiring more sensitive and accurate benchmarking methodologies that can detect even minute variations in solubility.
Titration methods represent another widely used approach, particularly acid-base titrations with standardized acids to determine hydroxide concentration. Potentiometric titration offers enhanced precision by monitoring pH changes during the neutralization process, allowing for more accurate endpoint detection. However, these methods can be affected by the presence of impurities or other dissolved species that may interfere with the titration process.
Spectroscopic techniques have gained prominence in recent years, including atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS) for lithium ion concentration determination. These methods offer high sensitivity and can detect lithium at parts-per-billion levels, though they require sophisticated instrumentation and skilled operators. The challenge lies in correlating the measured lithium concentration with actual hydroxide solubility, as other lithium species may be present.
Conductometric methods measure the electrical conductivity of lithium hydroxide solutions, which increases with concentration until saturation. While this approach allows for continuous monitoring, it requires careful calibration and can be influenced by temperature fluctuations and the presence of other electrolytes.
A significant challenge across all methodologies is temperature control, as lithium hydroxide solubility exhibits strong temperature dependence. Even minor temperature variations can lead to substantial measurement discrepancies, necessitating precise temperature regulation systems. Most laboratories maintain temperature control within ±0.1°C, though achieving this precision consistently remains challenging.
Standardization presents another obstacle, as different laboratories often employ varying protocols, equipment, and analytical techniques. This lack of standardization complicates direct comparison of results across different research groups and industrial settings. The absence of universally accepted reference materials specifically for lithium hydroxide solubility further compounds this issue.
Emerging challenges include the need for real-time monitoring systems capable of tracking solubility under dynamic conditions, particularly important for industrial applications where temperature and pressure may fluctuate. Additionally, the increasing demand for high-purity lithium compounds in battery manufacturing has raised the bar for analytical precision, requiring more sensitive and accurate benchmarking methodologies that can detect even minute variations in solubility.
Standard Protocols for LiOH Solubility Testing
01 Solubility characteristics of lithium hydroxide in various solutions
Lithium hydroxide exhibits specific solubility behaviors in different solutions, which is crucial for various industrial applications. The solubility of lithium hydroxide varies with temperature, pH, and the presence of other ions in solution. Understanding these solubility characteristics is essential for optimizing extraction processes and purification methods in lithium production.- Solubility characteristics of lithium hydroxide in various solutions: Lithium hydroxide exhibits specific solubility characteristics in different solutions, which is important for various industrial applications. The solubility of lithium hydroxide varies with temperature, pH, and the presence of other ions in solution. Understanding these solubility parameters is crucial for optimizing extraction processes and purification methods in lithium production.
- Methods to enhance lithium hydroxide solubility for extraction processes: Various techniques can be employed to enhance the solubility of lithium hydroxide during extraction processes. These methods include temperature modulation, pH adjustment, and the addition of specific reagents that can increase dissolution rates. Enhanced solubility facilitates more efficient extraction of lithium from ores and brines, improving overall process economics in lithium production.
- Solubility control in lithium hydroxide purification processes: Controlling the solubility of lithium hydroxide is essential in purification processes to achieve high-purity products. Techniques such as selective crystallization, fractional precipitation, and solvent extraction leverage the differential solubility of lithium hydroxide and impurities. These methods allow for the separation of lithium hydroxide from contaminants based on their distinct solubility behaviors under controlled conditions.
- Impact of impurities on lithium hydroxide solubility: The presence of impurities significantly affects the solubility of lithium hydroxide in solution. Common impurities in lithium processing, such as sodium, calcium, and magnesium salts, can either increase or decrease lithium hydroxide solubility through common ion effects or by forming complex compounds. Understanding these interactions is crucial for designing effective separation and purification strategies in lithium hydroxide production.
- Temperature-dependent solubility behavior of lithium hydroxide: The solubility of lithium hydroxide demonstrates significant temperature dependence, which is leveraged in industrial processes. Unlike many compounds, lithium hydroxide exhibits inverse solubility behavior in certain temperature ranges, where solubility decreases with increasing temperature. This unique property is utilized in crystallization processes for the production of high-purity lithium hydroxide and in the design of lithium recovery systems from various sources.
02 Methods to enhance lithium hydroxide solubility for extraction processes
Various techniques can be employed to enhance the solubility of lithium hydroxide during extraction processes. These methods include temperature modulation, pH adjustment, and the addition of specific reagents that can increase dissolution rates. Enhanced solubility facilitates more efficient extraction of lithium from brine solutions and mineral sources, improving overall process economics.Expand Specific Solutions03 Precipitation and crystallization of lithium hydroxide from solutions
Controlling the precipitation and crystallization of lithium hydroxide from solutions is critical for producing high-purity lithium compounds. Factors affecting crystallization include solution concentration, cooling rate, seeding techniques, and the presence of impurities. Optimized crystallization processes yield lithium hydroxide with desired particle size distribution and purity levels suitable for battery applications.Expand Specific Solutions04 Impact of impurities on lithium hydroxide solubility
The presence of impurities significantly affects the solubility behavior of lithium hydroxide in solution. Common impurities include sodium, potassium, calcium, and magnesium ions, which can form complex interactions with lithium hydroxide. Understanding these interactions is essential for developing effective purification strategies and producing battery-grade lithium hydroxide with minimal contaminants.Expand Specific Solutions05 Solubility considerations in lithium hydroxide production for battery applications
Battery-grade lithium hydroxide production requires careful control of solubility parameters throughout the manufacturing process. Specific solubility considerations include water content management, temperature control during processing stages, and prevention of unwanted precipitation. These factors directly impact the quality and performance of lithium hydroxide used in cathode materials for lithium-ion batteries.Expand Specific Solutions
Leading Research Institutions and Industry Players
The lithium hydroxide solubility benchmarking market is in a growth phase, with increasing demand driven by the expanding electric vehicle battery sector. Key players include major battery manufacturers like LG Energy Solution, BYD, and Tianjin Lishen, alongside chemical companies such as LG Chem, Solvay, and Hanwha Chemical. The technology maturity varies across regions, with companies like POSCO Holdings and Jiangxi Yunwei New Materials demonstrating advanced capabilities in lithium processing. Research institutions including Korea Atomic Energy Research Institute and Dalian University of Technology are contributing significant advancements to measurement methodologies. The competitive landscape is characterized by increasing collaboration between battery manufacturers and chemical suppliers to optimize lithium hydroxide properties for next-generation batteries.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a specialized benchmarking methodology for lithium hydroxide solubility tailored specifically for battery material qualification. Their approach combines traditional solubility measurements with electrochemical performance testing to establish correlations between solubility characteristics and battery performance metrics. The company employs automated dissolution testing equipment with integrated sampling systems that feed directly into analytical instruments including atomic absorption spectroscopy (AAS) and ion chromatography. LG Energy Solution's protocol includes standardized agitation methods and precise temperature control to ensure reproducible dissolution conditions. Their benchmarking system incorporates a database of historical solubility data linked to battery performance outcomes, enabling predictive quality assessment of lithium hydroxide materials based on their solubility profiles under standardized conditions.
Strengths: Direct correlation between solubility data and battery performance metrics; automated workflow minimizing human error; extensive historical database for comparative analysis. Weaknesses: Specialized focus on battery applications may limit broader applicability; complex integration of multiple analytical techniques; requires significant historical data for optimal predictive performance.
LG Chem Ltd.
Technical Solution: LG Chem has pioneered an integrated benchmarking system for lithium hydroxide solubility specifically designed for battery material quality control. Their methodology employs automated sampling systems coupled with inductively coupled plasma mass spectrometry (ICP-MS) for precise quantification of dissolved lithium ions. The company's approach features temperature-controlled microfluidic devices that enable rapid assessment of solubility kinetics under varying conditions. LG Chem's benchmarking protocol includes standardized reference materials for calibration and validation, ensuring reproducibility across different manufacturing batches. Their system incorporates computational modeling to predict solubility behavior based on empirical data, allowing for optimization of battery material processing parameters and quality control specifications.
Strengths: High-throughput capability ideal for production environments; excellent reproducibility through standardized reference materials; integration with computational modeling for predictive applications. Weaknesses: Requires significant initial investment in specialized equipment; limited flexibility for non-standard testing conditions; higher operational complexity requiring specialized technical expertise.
Critical Parameters Affecting Measurement Accuracy
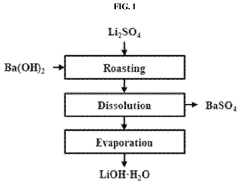
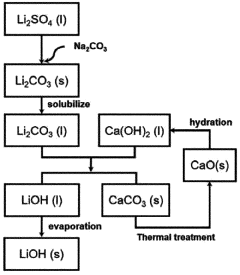
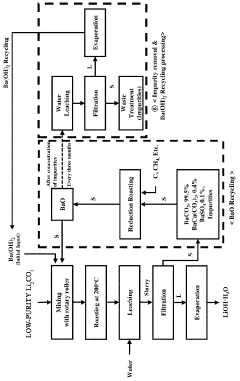
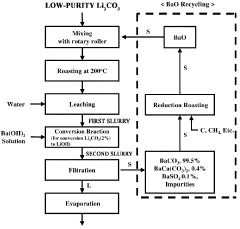
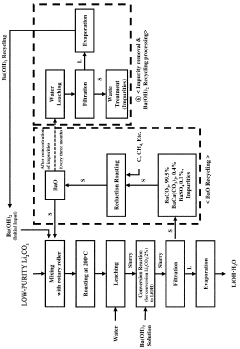
Method for preparing lithium hydroxide using lithium sulfate and barium hydroxide
PatentPendingUS20230373802A1
Innovation
- A method involving the mixing of lithium sulfate and barium hydroxide, followed by roasting to form insoluble barium sulfate and water-soluble lithium hydroxide, allowing for solid-liquid separation and evaporation to obtain high-purity lithium hydroxide with low lithium loss and no waste generation.
Method for preparing lithium hydroxide by using lithium carbonate and barium compound
PatentPendingAU2022380507A1
Innovation
- A method involving heat treatment of low-purity lithium carbonate with barium hydroxide or barium oxide, followed by leaching and filtration to separate lithium hydroxide, and subsequent evaporation, which minimizes lithium loss and uses barium compounds to enhance purity and reduce energy consumption.
Environmental Factors Influencing Solubility Data
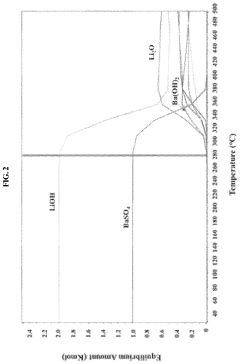
The solubility of lithium hydroxide in water is significantly influenced by various environmental factors that must be carefully controlled and documented during benchmarking procedures. Temperature stands as the most critical parameter affecting solubility, with lithium hydroxide demonstrating a positive correlation between temperature and dissolution rate. Research indicates that solubility increases from approximately 12.8 g/100g water at 20°C to 17.5 g/100g water at 100°C, necessitating precise temperature control during benchmark testing.
Pressure conditions, while less impactful than temperature for this particular compound, can still affect solubility measurements, especially in closed systems or when working near water's boiling point. Standardized pressure conditions should be maintained and recorded to ensure reproducibility of benchmark data.
The presence of other ions in solution creates complex interaction effects that can dramatically alter lithium hydroxide solubility. Common ion effect occurs when additional lithium-containing compounds are present, while diverse electrolytes may either enhance or suppress solubility through salting-in or salting-out phenomena. These ionic interactions must be quantified through controlled experiments to establish reliable benchmarks.
Solution pH represents another critical factor, as lithium hydroxide itself significantly increases solution alkalinity. The self-buffering effect of lithium hydroxide solutions complicates solubility measurements, requiring careful monitoring of pH throughout dissolution processes to accurately interpret results.
Atmospheric conditions, particularly carbon dioxide exposure, introduce additional complexity. CO₂ readily reacts with lithium hydroxide solutions to form lithium carbonate, which has substantially different solubility characteristics. This reaction can lead to misleading solubility data if not properly controlled, necessitating either inert atmosphere conditions or standardized CO₂ exposure protocols.
Agitation methods and rates during dissolution testing significantly impact measured solubility values, especially for determining kinetic solubility parameters. Standardized stirring protocols must be established to ensure comparable results across different testing environments.
Water quality parameters, including initial pH, mineral content, and dissolved gas concentrations, must be rigorously controlled. Research-grade deionized water with documented specifications should be used as the standard dissolution medium to minimize variability from water source differences.
Particle size and morphology of lithium hydroxide samples introduce additional variables affecting dissolution rates and apparent solubility. Standardized sample preparation protocols, including sieving or other particle classification methods, should be implemented to ensure consistent physical properties across benchmark tests.
Pressure conditions, while less impactful than temperature for this particular compound, can still affect solubility measurements, especially in closed systems or when working near water's boiling point. Standardized pressure conditions should be maintained and recorded to ensure reproducibility of benchmark data.
The presence of other ions in solution creates complex interaction effects that can dramatically alter lithium hydroxide solubility. Common ion effect occurs when additional lithium-containing compounds are present, while diverse electrolytes may either enhance or suppress solubility through salting-in or salting-out phenomena. These ionic interactions must be quantified through controlled experiments to establish reliable benchmarks.
Solution pH represents another critical factor, as lithium hydroxide itself significantly increases solution alkalinity. The self-buffering effect of lithium hydroxide solutions complicates solubility measurements, requiring careful monitoring of pH throughout dissolution processes to accurately interpret results.
Atmospheric conditions, particularly carbon dioxide exposure, introduce additional complexity. CO₂ readily reacts with lithium hydroxide solutions to form lithium carbonate, which has substantially different solubility characteristics. This reaction can lead to misleading solubility data if not properly controlled, necessitating either inert atmosphere conditions or standardized CO₂ exposure protocols.
Agitation methods and rates during dissolution testing significantly impact measured solubility values, especially for determining kinetic solubility parameters. Standardized stirring protocols must be established to ensure comparable results across different testing environments.
Water quality parameters, including initial pH, mineral content, and dissolved gas concentrations, must be rigorously controlled. Research-grade deionized water with documented specifications should be used as the standard dissolution medium to minimize variability from water source differences.
Particle size and morphology of lithium hydroxide samples introduce additional variables affecting dissolution rates and apparent solubility. Standardized sample preparation protocols, including sieving or other particle classification methods, should be implemented to ensure consistent physical properties across benchmark tests.
Data Validation and Reproducibility Standards
Establishing robust data validation and reproducibility standards is critical for accurate benchmarking of lithium hydroxide solubility in water. The scientific community requires standardized protocols to ensure that experimental results can be verified and trusted across different research settings. Primary validation methods should include statistical analysis of multiple measurement series, with clear reporting of standard deviations, confidence intervals, and potential sources of experimental error. Researchers must document all measurement uncertainties, including those related to temperature control, pH monitoring, and analytical instrument calibration.
Reproducibility standards for lithium hydroxide solubility studies should mandate detailed documentation of experimental conditions. This includes precise temperature control (±0.1°C), water purity specifications (conductivity <0.1 μS/cm), vessel material composition, stirring methods and rates, equilibration times, and sampling techniques. The analytical methods employed for concentration determination—whether ICP-MS, atomic absorption spectroscopy, or titration—must be thoroughly described with calibration procedures and detection limits clearly stated.
Cross-validation between different analytical techniques represents another essential component of robust benchmarking. When possible, researchers should employ at least two independent measurement methods to confirm solubility values. Interlaboratory comparison studies provide additional validation by identifying systematic errors that may be specific to particular laboratory environments or methodologies. These collaborative efforts help establish consensus values that can serve as reference points for the scientific community.
Data reporting standards must include complete raw data availability, either within publications or in accessible repositories. This transparency enables independent verification and meta-analysis. Researchers should adopt standardized formats for reporting solubility data, including temperature-dependent equations with clearly defined validity ranges and thermodynamic parameters. The IUPAC-recommended format for reporting solubility data should be followed, with appropriate citation of primary literature sources.
Quality assurance protocols should incorporate regular calibration checks using certified reference materials. For lithium hydroxide specifically, researchers should periodically verify their measurement systems using standardized lithium solutions of known concentration. Blind testing procedures, where analysts measure samples of unknown concentration prepared by independent parties, provide additional validation of measurement accuracy and precision. These rigorous validation and reproducibility standards ensure that benchmarking efforts for lithium hydroxide solubility yield reliable data that can confidently inform industrial applications and academic research.
Reproducibility standards for lithium hydroxide solubility studies should mandate detailed documentation of experimental conditions. This includes precise temperature control (±0.1°C), water purity specifications (conductivity <0.1 μS/cm), vessel material composition, stirring methods and rates, equilibration times, and sampling techniques. The analytical methods employed for concentration determination—whether ICP-MS, atomic absorption spectroscopy, or titration—must be thoroughly described with calibration procedures and detection limits clearly stated.
Cross-validation between different analytical techniques represents another essential component of robust benchmarking. When possible, researchers should employ at least two independent measurement methods to confirm solubility values. Interlaboratory comparison studies provide additional validation by identifying systematic errors that may be specific to particular laboratory environments or methodologies. These collaborative efforts help establish consensus values that can serve as reference points for the scientific community.
Data reporting standards must include complete raw data availability, either within publications or in accessible repositories. This transparency enables independent verification and meta-analysis. Researchers should adopt standardized formats for reporting solubility data, including temperature-dependent equations with clearly defined validity ranges and thermodynamic parameters. The IUPAC-recommended format for reporting solubility data should be followed, with appropriate citation of primary literature sources.
Quality assurance protocols should incorporate regular calibration checks using certified reference materials. For lithium hydroxide specifically, researchers should periodically verify their measurement systems using standardized lithium solutions of known concentration. Blind testing procedures, where analysts measure samples of unknown concentration prepared by independent parties, provide additional validation of measurement accuracy and precision. These rigorous validation and reproducibility standards ensure that benchmarking efforts for lithium hydroxide solubility yield reliable data that can confidently inform industrial applications and academic research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!