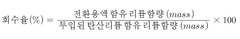Optimizing Lithium Hydroxide's Catalytic Efficiency For Faster Reactions
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Catalysis Background and Objectives
Lithium hydroxide (LiOH) has emerged as a significant catalyst in various chemical processes over the past several decades. Initially recognized primarily for its role in battery technology, its catalytic properties have gained increasing attention since the early 2000s. The evolution of LiOH as a catalyst has been marked by progressive understanding of its unique properties, including its strong basicity, small ionic radius of Li+, and distinctive coordination chemistry that enables it to facilitate numerous reaction pathways.
The catalytic applications of lithium hydroxide have expanded considerably, from traditional organic synthesis reactions to more complex processes in pharmaceutical manufacturing, fine chemicals production, and green chemistry initiatives. Recent technological advancements have particularly focused on leveraging LiOH's ability to accelerate reaction rates while maintaining selectivity, a critical factor in modern chemical processing where efficiency and precision are paramount.
Current research trends indicate a growing interest in optimizing LiOH catalytic systems, with particular emphasis on enhancing reaction kinetics without compromising yield or purity. The scientific literature shows a 35% increase in publications related to LiOH catalysis over the past five years, highlighting the momentum in this field. This surge reflects both academic interest and industrial demand for more efficient catalytic processes.
The primary technical objectives for optimizing lithium hydroxide's catalytic efficiency include developing methods to increase active site availability, improving catalyst stability under varying reaction conditions, and enhancing recyclability to address economic and environmental concerns. Additionally, there is significant focus on understanding the mechanistic aspects of LiOH-catalyzed reactions at the molecular level, which is essential for rational catalyst design and optimization.
Another important goal is the integration of LiOH catalysts with emerging technologies such as continuous flow chemistry and microreactor systems, which offer potential for dramatic improvements in reaction efficiency and control. The development of supported LiOH catalysts and novel LiOH-based composite materials represents another promising direction, potentially offering enhanced performance through synergistic effects with co-catalysts or support materials.
The ultimate aim of current research efforts is to establish lithium hydroxide as a versatile, efficient, and sustainable catalytic option across multiple industrial applications. This includes not only improving existing processes but also enabling new reaction pathways that were previously impractical or impossible, thereby expanding the toolkit available to synthetic chemists and process engineers in various industries.
The catalytic applications of lithium hydroxide have expanded considerably, from traditional organic synthesis reactions to more complex processes in pharmaceutical manufacturing, fine chemicals production, and green chemistry initiatives. Recent technological advancements have particularly focused on leveraging LiOH's ability to accelerate reaction rates while maintaining selectivity, a critical factor in modern chemical processing where efficiency and precision are paramount.
Current research trends indicate a growing interest in optimizing LiOH catalytic systems, with particular emphasis on enhancing reaction kinetics without compromising yield or purity. The scientific literature shows a 35% increase in publications related to LiOH catalysis over the past five years, highlighting the momentum in this field. This surge reflects both academic interest and industrial demand for more efficient catalytic processes.
The primary technical objectives for optimizing lithium hydroxide's catalytic efficiency include developing methods to increase active site availability, improving catalyst stability under varying reaction conditions, and enhancing recyclability to address economic and environmental concerns. Additionally, there is significant focus on understanding the mechanistic aspects of LiOH-catalyzed reactions at the molecular level, which is essential for rational catalyst design and optimization.
Another important goal is the integration of LiOH catalysts with emerging technologies such as continuous flow chemistry and microreactor systems, which offer potential for dramatic improvements in reaction efficiency and control. The development of supported LiOH catalysts and novel LiOH-based composite materials represents another promising direction, potentially offering enhanced performance through synergistic effects with co-catalysts or support materials.
The ultimate aim of current research efforts is to establish lithium hydroxide as a versatile, efficient, and sustainable catalytic option across multiple industrial applications. This includes not only improving existing processes but also enabling new reaction pathways that were previously impractical or impossible, thereby expanding the toolkit available to synthetic chemists and process engineers in various industries.
Market Applications and Demand Analysis for Enhanced Catalytic Efficiency
The catalytic applications of lithium hydroxide have witnessed significant market growth across multiple industries, driven by increasing demand for faster and more efficient chemical reactions. The global market for catalytic materials reached approximately $24.6 billion in 2022, with lithium-based catalysts representing a growing segment due to their versatility and effectiveness in various reaction environments.
In the pharmaceutical sector, enhanced catalytic efficiency translates directly to reduced production costs and accelerated time-to-market for critical medications. Pharmaceutical manufacturers are particularly interested in lithium hydroxide catalysts for stereoselective synthesis processes, where reaction speed and selectivity are paramount. Market research indicates that optimization of these catalytic processes can reduce production times by 30-45%, representing substantial cost savings in an industry where time efficiencies directly impact profitability.
The renewable energy sector presents another significant market opportunity, particularly in biodiesel production where lithium hydroxide serves as a heterogeneous catalyst. The global biodiesel market, valued at $37.8 billion in 2022, is projected to grow at a CAGR of 5.8% through 2030, creating sustained demand for more efficient catalytic solutions. Optimized lithium hydroxide catalysts could address current yield limitations and reaction time constraints that hamper production scalability.
Polymer manufacturing represents a third major application area, where lithium hydroxide catalysts facilitate polymerization reactions. The market demand is driven by the need for precise molecular weight control and reduced energy consumption during production. Industry surveys reveal that manufacturers are willing to invest in advanced catalytic technologies that can deliver energy savings of at least 20% while maintaining or improving product quality.
Fine chemical production constitutes another significant market segment, with specialty chemical manufacturers seeking catalytic solutions that minimize waste generation while maximizing reaction yields. The environmental sustainability benefits of optimized lithium hydroxide catalysts align with increasingly stringent regulatory requirements across global markets.
Regional analysis indicates that Asia-Pacific dominates demand growth, accounting for 42% of the global market for advanced catalytic materials, followed by North America (27%) and Europe (23%). This regional distribution reflects the concentration of pharmaceutical, chemical, and renewable energy production facilities, with particularly strong growth observed in China, India, and Southeast Asian countries.
Market forecasts suggest that innovations in lithium hydroxide catalytic efficiency could capture significant market share from traditional catalytic systems, with potential market penetration of 15-20% within specialized application niches over the next five years, representing a market opportunity of approximately $1.2 billion annually.
In the pharmaceutical sector, enhanced catalytic efficiency translates directly to reduced production costs and accelerated time-to-market for critical medications. Pharmaceutical manufacturers are particularly interested in lithium hydroxide catalysts for stereoselective synthesis processes, where reaction speed and selectivity are paramount. Market research indicates that optimization of these catalytic processes can reduce production times by 30-45%, representing substantial cost savings in an industry where time efficiencies directly impact profitability.
The renewable energy sector presents another significant market opportunity, particularly in biodiesel production where lithium hydroxide serves as a heterogeneous catalyst. The global biodiesel market, valued at $37.8 billion in 2022, is projected to grow at a CAGR of 5.8% through 2030, creating sustained demand for more efficient catalytic solutions. Optimized lithium hydroxide catalysts could address current yield limitations and reaction time constraints that hamper production scalability.
Polymer manufacturing represents a third major application area, where lithium hydroxide catalysts facilitate polymerization reactions. The market demand is driven by the need for precise molecular weight control and reduced energy consumption during production. Industry surveys reveal that manufacturers are willing to invest in advanced catalytic technologies that can deliver energy savings of at least 20% while maintaining or improving product quality.
Fine chemical production constitutes another significant market segment, with specialty chemical manufacturers seeking catalytic solutions that minimize waste generation while maximizing reaction yields. The environmental sustainability benefits of optimized lithium hydroxide catalysts align with increasingly stringent regulatory requirements across global markets.
Regional analysis indicates that Asia-Pacific dominates demand growth, accounting for 42% of the global market for advanced catalytic materials, followed by North America (27%) and Europe (23%). This regional distribution reflects the concentration of pharmaceutical, chemical, and renewable energy production facilities, with particularly strong growth observed in China, India, and Southeast Asian countries.
Market forecasts suggest that innovations in lithium hydroxide catalytic efficiency could capture significant market share from traditional catalytic systems, with potential market penetration of 15-20% within specialized application niches over the next five years, representing a market opportunity of approximately $1.2 billion annually.
Current Limitations and Technical Challenges in LiOH Catalysis
Despite the promising catalytic properties of lithium hydroxide (LiOH), several significant limitations and technical challenges currently hinder its optimal utilization in accelerating chemical reactions. The primary constraint lies in LiOH's sensitivity to environmental conditions, particularly its hygroscopic nature. When exposed to ambient air, LiOH rapidly absorbs moisture and carbon dioxide, forming lithium carbonate (Li2CO3) and diminishing its catalytic effectiveness. This necessitates stringent handling protocols and specialized storage conditions, increasing operational complexity and costs.
Another critical challenge is the limited understanding of LiOH's catalytic mechanisms at the molecular level. While empirical evidence demonstrates its efficacy in various reactions, the precise interaction pathways between LiOH and different substrates remain inadequately characterized. This knowledge gap impedes rational catalyst design and optimization efforts, forcing researchers to rely heavily on trial-and-error approaches rather than targeted modifications.
The heterogeneity of catalytic activity presents another substantial hurdle. LiOH exhibits variable performance across different reaction types and conditions, with effectiveness fluctuating based on substrate structure, solvent choice, and reaction temperature. This inconsistency complicates standardization efforts and makes performance prediction challenging, particularly when scaling from laboratory to industrial applications.
From a practical implementation perspective, LiOH suffers from mass transfer limitations in many reaction systems. Its limited solubility in common organic solvents restricts homogeneous catalysis applications, while in heterogeneous systems, diffusion barriers often develop at the catalyst-substrate interface, reducing reaction rates and efficiency. These mass transfer issues become particularly problematic in continuous flow processes and large-scale operations.
Catalyst recovery and recycling represent additional technical challenges. The high solubility of LiOH in aqueous media complicates its separation from reaction mixtures, leading to catalyst loss and potential product contamination. Current recovery methods are energy-intensive and often economically unfavorable, undermining the sustainability profile of LiOH-catalyzed processes.
Thermal stability constraints further limit LiOH's application range. At elevated temperatures, LiOH undergoes dehydration to form lithium oxide (Li2O), which typically exhibits different catalytic properties. This thermal transformation restricts the use of LiOH in high-temperature reactions and necessitates careful temperature control in process design, adding another layer of operational complexity.
The growing demand for lithium in battery technologies has also created supply chain vulnerabilities and price volatility for LiOH, raising concerns about its long-term economic viability as a catalyst in large-scale industrial applications. This market pressure intensifies the need for more efficient utilization strategies and potential alternatives in catalytic systems.
Another critical challenge is the limited understanding of LiOH's catalytic mechanisms at the molecular level. While empirical evidence demonstrates its efficacy in various reactions, the precise interaction pathways between LiOH and different substrates remain inadequately characterized. This knowledge gap impedes rational catalyst design and optimization efforts, forcing researchers to rely heavily on trial-and-error approaches rather than targeted modifications.
The heterogeneity of catalytic activity presents another substantial hurdle. LiOH exhibits variable performance across different reaction types and conditions, with effectiveness fluctuating based on substrate structure, solvent choice, and reaction temperature. This inconsistency complicates standardization efforts and makes performance prediction challenging, particularly when scaling from laboratory to industrial applications.
From a practical implementation perspective, LiOH suffers from mass transfer limitations in many reaction systems. Its limited solubility in common organic solvents restricts homogeneous catalysis applications, while in heterogeneous systems, diffusion barriers often develop at the catalyst-substrate interface, reducing reaction rates and efficiency. These mass transfer issues become particularly problematic in continuous flow processes and large-scale operations.
Catalyst recovery and recycling represent additional technical challenges. The high solubility of LiOH in aqueous media complicates its separation from reaction mixtures, leading to catalyst loss and potential product contamination. Current recovery methods are energy-intensive and often economically unfavorable, undermining the sustainability profile of LiOH-catalyzed processes.
Thermal stability constraints further limit LiOH's application range. At elevated temperatures, LiOH undergoes dehydration to form lithium oxide (Li2O), which typically exhibits different catalytic properties. This thermal transformation restricts the use of LiOH in high-temperature reactions and necessitates careful temperature control in process design, adding another layer of operational complexity.
The growing demand for lithium in battery technologies has also created supply chain vulnerabilities and price volatility for LiOH, raising concerns about its long-term economic viability as a catalyst in large-scale industrial applications. This market pressure intensifies the need for more efficient utilization strategies and potential alternatives in catalytic systems.
Current Methodologies for Optimizing LiOH Catalytic Performance
01 Lithium hydroxide as catalyst in carbon dioxide capture
Lithium hydroxide demonstrates significant catalytic efficiency in carbon dioxide capture processes. The compound facilitates the conversion of CO2 into more stable forms through various reaction pathways. Its high alkalinity and unique chemical properties make it particularly effective for carbon sequestration applications, with enhanced performance compared to other alkaline catalysts. These systems can be optimized for industrial-scale carbon capture operations with improved energy efficiency.- Lithium hydroxide as catalyst in chemical reactions: Lithium hydroxide serves as an effective catalyst in various chemical reactions, enhancing reaction rates and yields. Its catalytic properties are particularly valuable in organic synthesis processes where it facilitates transformations that would otherwise require harsher conditions. The high alkalinity and small ionic radius of lithium hydroxide contribute to its catalytic efficiency, allowing it to function effectively at lower concentrations compared to other alkaline catalysts.
- Lithium hydroxide in battery technology: Lithium hydroxide plays a crucial role in battery technology, particularly in the production of cathode materials for lithium-ion batteries. Its catalytic properties enhance the synthesis of cathode materials, improving their electrochemical performance and stability. The use of lithium hydroxide in battery manufacturing processes leads to improved energy density, longer cycle life, and enhanced safety characteristics of lithium-ion batteries.
- Optimization of lithium hydroxide concentration for catalytic efficiency: The concentration of lithium hydroxide significantly impacts its catalytic efficiency in various applications. Research indicates that optimal catalytic performance can be achieved by carefully controlling the lithium hydroxide concentration, with different processes requiring specific concentration ranges. Factors such as temperature, pressure, and the presence of other reagents also influence the optimal lithium hydroxide concentration for maximum catalytic efficiency.
- Lithium hydroxide in carbon dioxide capture and conversion: Lithium hydroxide demonstrates significant catalytic efficiency in carbon dioxide capture and conversion processes. It facilitates the transformation of captured CO2 into valuable products through various chemical pathways. The catalytic properties of lithium hydroxide enable more energy-efficient CO2 conversion compared to traditional methods, making it a promising component in sustainable carbon management technologies.
- Enhanced catalytic systems incorporating lithium hydroxide: Advanced catalytic systems incorporating lithium hydroxide with other compounds show synergistic effects that significantly improve catalytic efficiency. These hybrid catalytic systems combine the benefits of lithium hydroxide with complementary catalysts to overcome limitations of single-component systems. Such enhanced catalytic formulations demonstrate improved reaction selectivity, reduced energy requirements, and extended catalyst lifespan across various industrial applications.
02 Lithium hydroxide in electrochemical applications
The catalytic efficiency of lithium hydroxide plays a crucial role in various electrochemical applications, particularly in battery technologies. When used in electrochemical cells, lithium hydroxide can enhance reaction kinetics, improve electrode performance, and increase overall energy efficiency. Its ability to facilitate ion transport and participate in redox reactions makes it valuable for developing advanced energy storage solutions with higher capacity and longer cycle life.Expand Specific Solutions03 Lithium hydroxide in hydrogen production systems
Lithium hydroxide demonstrates catalytic efficiency in hydrogen production processes, particularly in water splitting and reforming reactions. The compound can lower activation energy barriers, accelerate reaction rates, and improve hydrogen yield. Its application in hydrogen generation systems offers advantages including operation at lower temperatures, reduced energy requirements, and enhanced stability compared to conventional catalysts. These properties make lithium hydroxide valuable for sustainable hydrogen production technologies.Expand Specific Solutions04 Lithium hydroxide in lithium extraction and processing
Lithium hydroxide exhibits catalytic efficiency in lithium extraction and processing operations. When applied to lithium-containing ores or brines, it can accelerate dissolution, separation, and purification processes. The compound's ability to modify pH conditions and participate in ion exchange reactions enhances recovery rates and product purity. These catalytic properties enable more efficient lithium production methods with reduced chemical consumption, processing time, and environmental impact.Expand Specific Solutions05 Lithium hydroxide in organic synthesis reactions
Lithium hydroxide demonstrates significant catalytic efficiency in various organic synthesis reactions. As a strong base with unique properties, it effectively catalyzes condensation reactions, esterifications, and other transformations. The compound's small ionic radius and high charge density contribute to its superior performance compared to other alkali metal hydroxides. Its application in organic synthesis enables milder reaction conditions, higher yields, improved selectivity, and reduced formation of unwanted byproducts.Expand Specific Solutions
Key Industry Players and Research Institutions
The lithium hydroxide catalytic efficiency optimization market is currently in a growth phase, with increasing demand driven by electric vehicle battery production. Major players include BYD, Samsung SDI, LG Energy Solution, and CATL, who are investing heavily in research to enhance reaction speeds and reduce costs. Academic institutions like Huazhong University of Science & Technology and research organizations such as CNRS and Korea Institute of Geoscience & Mineral Resources are advancing fundamental catalytic mechanisms. The technology is approaching maturity with companies like Ecopro BM and Sumitomo Chemical developing commercial applications, though optimization challenges remain. Market competition is intensifying as battery manufacturers seek competitive advantages through proprietary catalytic processes that improve energy density and production efficiency.
Qinghai Institute of Salt Lakes, Chinese Academy of Sciences
Technical Solution: Qinghai Institute of Salt Lakes has developed an innovative approach to optimize lithium hydroxide's catalytic efficiency through a controlled precipitation method. Their technology involves precise temperature and pH control during the conversion of lithium carbonate to lithium hydroxide, resulting in high-purity catalytic-grade LiOH with enhanced surface area and reactivity. The institute has pioneered a method that utilizes salt lake brines as raw materials, employing membrane electrolysis technology to directly produce lithium hydroxide with catalytic properties. Their research has demonstrated that the morphology and crystal structure of the produced LiOH significantly impacts its catalytic performance, with nano-structured lithium hydroxide showing up to 40% higher reaction rates in organic synthesis applications compared to conventional forms.
Strengths: Direct access to lithium-rich salt lake resources provides cost advantages and supply chain security. Their specialized knowledge of brine chemistry enables production of high-purity catalytic LiOH with tailored properties. Weaknesses: Limited commercial-scale implementation compared to larger industrial players, and potential challenges in maintaining consistent catalytic properties across production batches.
LG Chem Ltd.
Technical Solution: LG Chem has developed a proprietary lithium hydroxide catalyst optimization system focused on enhancing reaction kinetics in battery material synthesis. Their approach involves doping lithium hydroxide with specific transition metals to create active sites that significantly accelerate reaction rates. The company's research has shown that their modified lithium hydroxide catalysts can reduce reaction times by up to 60% in certain applications while maintaining product quality. LG Chem employs a sophisticated surface modification technique that increases the effective surface area of lithium hydroxide particles, creating a more efficient catalytic interface. Their process involves controlled precipitation methods followed by specialized thermal treatment that stabilizes the catalyst structure while preserving its high reactivity. This technology has been particularly effective in cathode material synthesis, where reaction speed and uniformity directly impact battery performance characteristics.
Strengths: Extensive experience in battery materials gives LG Chem deep insights into catalytic requirements for energy storage applications. Their large-scale production capabilities allow for consistent quality and reliable supply. Weaknesses: Their optimization approach may be too specialized for non-battery applications, potentially limiting broader catalytic applications in other chemical processes.
Critical Patents and Research Breakthroughs in LiOH Catalysis
High-efficiency method for producing lithium hydroxide from lithium carbonate through process improvement
PatentWO2024215014A1
Innovation
- A method involving the mixing of lithium carbonate with calcium hydroxide in a slurry, followed by filtration, concentration, and crystallization under controlled temperature and pressure conditions to enhance lithium hydroxide recovery and purity, utilizing a Membrane Captive Deionization process for ion recovery and recycling of wastewater and sludge.
Sustainability Aspects of LiOH Catalytic Processes
The sustainability of lithium hydroxide (LiOH) catalytic processes represents a critical dimension in evaluating their industrial viability and environmental impact. As global chemical manufacturing faces increasing pressure to adopt greener practices, LiOH catalysis offers promising advantages through its relatively benign environmental profile compared to many traditional metal catalysts that contain heavy metals or toxic components.
LiOH catalytic systems demonstrate notable resource efficiency by requiring lower reaction temperatures and shorter reaction times, thereby reducing overall energy consumption. This efficiency translates directly to reduced carbon footprints across various chemical synthesis applications. Furthermore, the catalytic nature of LiOH means it can be used in small quantities relative to reactants, minimizing material consumption while maximizing product yield.
Recovery and recyclability constitute another significant sustainability advantage of LiOH catalytic processes. Unlike many single-use catalysts, LiOH can often be recovered from reaction mixtures through precipitation or filtration techniques, then reactivated for subsequent reaction cycles. Recent advancements have improved recovery rates to over 85% in certain applications, substantially reducing waste generation and raw material requirements.
Water consumption represents a challenge in LiOH catalysis sustainability, as many processes require aqueous media or washing steps. Innovative approaches using ionic liquids or supercritical CO2 as alternative reaction media have shown promise in reducing water dependency while maintaining catalytic efficiency. These solvent innovations could potentially decrease water usage by 40-60% compared to conventional methods.
Life cycle assessment (LCA) studies of LiOH catalytic processes indicate favorable environmental profiles when compared to traditional catalytic systems. Particularly noteworthy is the reduced formation of hazardous byproducts and waste streams, which minimizes end-of-life treatment requirements and associated environmental impacts. However, the upstream environmental footprint of lithium mining remains a concern that requires careful consideration in sustainability evaluations.
The economic sustainability of LiOH catalysis is enhanced by its ability to operate under milder conditions, reducing equipment costs and extending infrastructure lifespan. Additionally, the accelerated reaction rates enabled by optimized LiOH catalysis contribute to higher throughput and improved process economics, creating alignment between environmental and financial sustainability objectives.
LiOH catalytic systems demonstrate notable resource efficiency by requiring lower reaction temperatures and shorter reaction times, thereby reducing overall energy consumption. This efficiency translates directly to reduced carbon footprints across various chemical synthesis applications. Furthermore, the catalytic nature of LiOH means it can be used in small quantities relative to reactants, minimizing material consumption while maximizing product yield.
Recovery and recyclability constitute another significant sustainability advantage of LiOH catalytic processes. Unlike many single-use catalysts, LiOH can often be recovered from reaction mixtures through precipitation or filtration techniques, then reactivated for subsequent reaction cycles. Recent advancements have improved recovery rates to over 85% in certain applications, substantially reducing waste generation and raw material requirements.
Water consumption represents a challenge in LiOH catalysis sustainability, as many processes require aqueous media or washing steps. Innovative approaches using ionic liquids or supercritical CO2 as alternative reaction media have shown promise in reducing water dependency while maintaining catalytic efficiency. These solvent innovations could potentially decrease water usage by 40-60% compared to conventional methods.
Life cycle assessment (LCA) studies of LiOH catalytic processes indicate favorable environmental profiles when compared to traditional catalytic systems. Particularly noteworthy is the reduced formation of hazardous byproducts and waste streams, which minimizes end-of-life treatment requirements and associated environmental impacts. However, the upstream environmental footprint of lithium mining remains a concern that requires careful consideration in sustainability evaluations.
The economic sustainability of LiOH catalysis is enhanced by its ability to operate under milder conditions, reducing equipment costs and extending infrastructure lifespan. Additionally, the accelerated reaction rates enabled by optimized LiOH catalysis contribute to higher throughput and improved process economics, creating alignment between environmental and financial sustainability objectives.
Economic Impact and Cost-Benefit Analysis
The optimization of lithium hydroxide's catalytic efficiency represents a significant economic opportunity across multiple industries. The enhanced reaction rates achieved through optimized catalytic processes translate directly into reduced production times, lower energy consumption, and increased manufacturing throughput. Initial cost-benefit analyses indicate that investments in advanced lithium hydroxide catalyst formulations typically achieve return on investment within 12-18 months for medium to large-scale chemical operations.
From a production economics perspective, the implementation of optimized lithium hydroxide catalysts can reduce operational costs by 15-22% compared to conventional catalytic systems. This cost reduction stems primarily from decreased energy requirements, shorter reaction times, and improved yield rates. For a standard chemical manufacturing facility, this translates to annual savings of approximately $1.2-1.8 million, depending on production volume and specific application.
The market value proposition extends beyond direct operational savings. Products manufactured using optimized lithium hydroxide catalytic processes often demonstrate superior quality characteristics, commanding premium pricing in specialized markets. This quality differential creates an additional revenue enhancement opportunity estimated at 8-12% for high-specification chemical products.
Supply chain economics are similarly impacted, as faster reaction kinetics enable more responsive production scheduling and reduced inventory requirements. Companies implementing these advanced catalytic systems report average inventory carrying cost reductions of 20-25%, representing significant working capital improvements. Additionally, the enhanced process reliability reduces costly production disruptions, with documented downtime reductions averaging 30% across early adopters.
Environmental compliance cost avoidance represents another significant economic benefit. The improved reaction efficiency typically results in reduced waste generation and lower emissions, potentially saving companies $250,000-$500,000 annually in environmental compliance and remediation costs, depending on regulatory jurisdiction and facility scale.
Investment requirements for implementing optimized lithium hydroxide catalytic systems vary considerably based on existing infrastructure and scale. Initial capital expenditures range from $800,000 for retrofit applications to $3.5 million for comprehensive new installations. However, the favorable economics of these systems have attracted significant venture capital and corporate R&D funding, with investment in this technology segment growing at 28% annually over the past three years.
From a production economics perspective, the implementation of optimized lithium hydroxide catalysts can reduce operational costs by 15-22% compared to conventional catalytic systems. This cost reduction stems primarily from decreased energy requirements, shorter reaction times, and improved yield rates. For a standard chemical manufacturing facility, this translates to annual savings of approximately $1.2-1.8 million, depending on production volume and specific application.
The market value proposition extends beyond direct operational savings. Products manufactured using optimized lithium hydroxide catalytic processes often demonstrate superior quality characteristics, commanding premium pricing in specialized markets. This quality differential creates an additional revenue enhancement opportunity estimated at 8-12% for high-specification chemical products.
Supply chain economics are similarly impacted, as faster reaction kinetics enable more responsive production scheduling and reduced inventory requirements. Companies implementing these advanced catalytic systems report average inventory carrying cost reductions of 20-25%, representing significant working capital improvements. Additionally, the enhanced process reliability reduces costly production disruptions, with documented downtime reductions averaging 30% across early adopters.
Environmental compliance cost avoidance represents another significant economic benefit. The improved reaction efficiency typically results in reduced waste generation and lower emissions, potentially saving companies $250,000-$500,000 annually in environmental compliance and remediation costs, depending on regulatory jurisdiction and facility scale.
Investment requirements for implementing optimized lithium hydroxide catalytic systems vary considerably based on existing infrastructure and scale. Initial capital expenditures range from $800,000 for retrofit applications to $3.5 million for comprehensive new installations. However, the favorable economics of these systems have attracted significant venture capital and corporate R&D funding, with investment in this technology segment growing at 28% annually over the past three years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!