Lithium Hydroxide's Role In Chemical Reaction Kinetics
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Reaction Kinetics Background and Objectives
Lithium hydroxide (LiOH) has emerged as a significant compound in the field of chemical reaction kinetics, with its historical development tracing back to the early 20th century. Initially recognized primarily for its applications in battery technology, the understanding of LiOH's catalytic properties and reaction mechanisms has evolved substantially over the decades. The compound's unique properties, including its strong basicity, small ionic radius of Li+, and high solubility in polar solvents, have positioned it as a versatile reagent in various chemical processes.
The evolution of research on lithium hydroxide's kinetic properties has followed a trajectory from basic characterization studies to sophisticated mechanistic investigations. Early research in the 1950s and 1960s focused on fundamental properties, while the 1970s and 1980s saw increased interest in its role in organic synthesis reactions. The past three decades have witnessed exponential growth in publications related to LiOH's kinetic properties, particularly in catalysis, energy storage, and environmental applications.
Current technological trends indicate a growing interest in lithium hydroxide's application in green chemistry, where its ability to facilitate reactions under milder conditions offers significant advantages. The compound's role in carbon dioxide capture and conversion processes represents an emerging area with substantial environmental implications. Additionally, its application in advanced materials synthesis, particularly for nanomaterials with controlled morphology, demonstrates the expanding scope of LiOH in modern chemical engineering.
The primary technical objectives for research in lithium hydroxide reaction kinetics encompass several dimensions. First, there is a need to develop comprehensive mechanistic models that accurately predict LiOH's behavior across diverse reaction environments. Second, researchers aim to optimize reaction conditions to enhance selectivity and yield in LiOH-mediated transformations. Third, there is significant interest in exploring novel applications in emerging fields such as sustainable chemistry and materials science.
Understanding the fundamental aspects of how lithium hydroxide influences reaction rates, transition states, and activation energies remains a central goal. This includes elucidating the specific role of lithium cations in stabilizing reaction intermediates and the contribution of hydroxide anions to nucleophilic processes. Advanced computational methods, in-situ spectroscopic techniques, and high-throughput experimentation are increasingly being employed to address these questions.
The strategic importance of lithium hydroxide reaction kinetics extends beyond academic interest, with direct implications for industrial processes, energy storage technologies, and environmental remediation strategies. As global demand for lithium compounds continues to rise, driven largely by the electric vehicle revolution, understanding the fundamental kinetic properties of LiOH becomes increasingly critical for developing more efficient and sustainable technologies.
The evolution of research on lithium hydroxide's kinetic properties has followed a trajectory from basic characterization studies to sophisticated mechanistic investigations. Early research in the 1950s and 1960s focused on fundamental properties, while the 1970s and 1980s saw increased interest in its role in organic synthesis reactions. The past three decades have witnessed exponential growth in publications related to LiOH's kinetic properties, particularly in catalysis, energy storage, and environmental applications.
Current technological trends indicate a growing interest in lithium hydroxide's application in green chemistry, where its ability to facilitate reactions under milder conditions offers significant advantages. The compound's role in carbon dioxide capture and conversion processes represents an emerging area with substantial environmental implications. Additionally, its application in advanced materials synthesis, particularly for nanomaterials with controlled morphology, demonstrates the expanding scope of LiOH in modern chemical engineering.
The primary technical objectives for research in lithium hydroxide reaction kinetics encompass several dimensions. First, there is a need to develop comprehensive mechanistic models that accurately predict LiOH's behavior across diverse reaction environments. Second, researchers aim to optimize reaction conditions to enhance selectivity and yield in LiOH-mediated transformations. Third, there is significant interest in exploring novel applications in emerging fields such as sustainable chemistry and materials science.
Understanding the fundamental aspects of how lithium hydroxide influences reaction rates, transition states, and activation energies remains a central goal. This includes elucidating the specific role of lithium cations in stabilizing reaction intermediates and the contribution of hydroxide anions to nucleophilic processes. Advanced computational methods, in-situ spectroscopic techniques, and high-throughput experimentation are increasingly being employed to address these questions.
The strategic importance of lithium hydroxide reaction kinetics extends beyond academic interest, with direct implications for industrial processes, energy storage technologies, and environmental remediation strategies. As global demand for lithium compounds continues to rise, driven largely by the electric vehicle revolution, understanding the fundamental kinetic properties of LiOH becomes increasingly critical for developing more efficient and sustainable technologies.
Market Applications and Demand Analysis for Lithium Hydroxide
The lithium hydroxide market has experienced significant growth in recent years, primarily driven by its expanding applications in various industries. The global lithium hydroxide market was valued at approximately 10.6 billion USD in 2022 and is projected to grow at a compound annual growth rate of 16.2% through 2030. This remarkable growth trajectory is largely attributed to the burgeoning electric vehicle (EV) industry, where lithium hydroxide serves as a critical component in high-performance lithium-ion batteries.
In the battery sector, lithium hydroxide is increasingly preferred over lithium carbonate for cathode production, particularly for nickel-rich chemistries such as NMC (Nickel Manganese Cobalt) and NCA (Nickel Cobalt Aluminum) batteries. These advanced battery formulations require lithium hydroxide due to its superior reaction kinetics at lower temperatures, which results in more efficient manufacturing processes and enhanced battery performance characteristics including higher energy density and longer cycle life.
Beyond the dominant EV market, lithium hydroxide finds substantial application in industrial processes where its role in reaction kinetics is particularly valuable. In the chemical industry, it serves as a catalyst and pH regulator, accelerating reaction rates while maintaining optimal alkaline conditions. The pharmaceutical sector utilizes lithium hydroxide in synthesis processes for various medications, leveraging its ability to facilitate specific chemical transformations with high selectivity.
The ceramics and glass industries represent another significant market segment, where lithium hydroxide is employed to lower melting temperatures and improve product durability. Its ability to modify reaction kinetics during the vitrification process results in energy savings and enhanced material properties. Similarly, in lubricant manufacturing, lithium hydroxide-based greases demonstrate superior thermal stability and water resistance due to the unique chemical interactions facilitated by lithium hydroxide.
Market demand analysis reveals regional variations in lithium hydroxide consumption patterns. Asia-Pacific, particularly China, dominates global demand due to its extensive battery manufacturing capacity. North America and Europe are experiencing accelerated growth rates as they establish domestic battery supply chains to support their automotive industries' transition toward electrification.
Supply constraints represent a significant market challenge, with lithium hydroxide production capacity struggling to keep pace with rapidly increasing demand. This supply-demand imbalance has contributed to price volatility, with lithium hydroxide prices reaching historic highs in recent years. Industry forecasts suggest continued tight market conditions through mid-decade until new production capacity comes online.
The market is also witnessing a shift toward higher purity requirements, with battery-grade lithium hydroxide (99.5%+ purity) commanding premium prices due to its critical role in ensuring optimal reaction kinetics in advanced battery chemistries. This trend underscores the growing importance of lithium hydroxide's specific chemical properties in high-value applications where reaction kinetics are paramount to product performance.
In the battery sector, lithium hydroxide is increasingly preferred over lithium carbonate for cathode production, particularly for nickel-rich chemistries such as NMC (Nickel Manganese Cobalt) and NCA (Nickel Cobalt Aluminum) batteries. These advanced battery formulations require lithium hydroxide due to its superior reaction kinetics at lower temperatures, which results in more efficient manufacturing processes and enhanced battery performance characteristics including higher energy density and longer cycle life.
Beyond the dominant EV market, lithium hydroxide finds substantial application in industrial processes where its role in reaction kinetics is particularly valuable. In the chemical industry, it serves as a catalyst and pH regulator, accelerating reaction rates while maintaining optimal alkaline conditions. The pharmaceutical sector utilizes lithium hydroxide in synthesis processes for various medications, leveraging its ability to facilitate specific chemical transformations with high selectivity.
The ceramics and glass industries represent another significant market segment, where lithium hydroxide is employed to lower melting temperatures and improve product durability. Its ability to modify reaction kinetics during the vitrification process results in energy savings and enhanced material properties. Similarly, in lubricant manufacturing, lithium hydroxide-based greases demonstrate superior thermal stability and water resistance due to the unique chemical interactions facilitated by lithium hydroxide.
Market demand analysis reveals regional variations in lithium hydroxide consumption patterns. Asia-Pacific, particularly China, dominates global demand due to its extensive battery manufacturing capacity. North America and Europe are experiencing accelerated growth rates as they establish domestic battery supply chains to support their automotive industries' transition toward electrification.
Supply constraints represent a significant market challenge, with lithium hydroxide production capacity struggling to keep pace with rapidly increasing demand. This supply-demand imbalance has contributed to price volatility, with lithium hydroxide prices reaching historic highs in recent years. Industry forecasts suggest continued tight market conditions through mid-decade until new production capacity comes online.
The market is also witnessing a shift toward higher purity requirements, with battery-grade lithium hydroxide (99.5%+ purity) commanding premium prices due to its critical role in ensuring optimal reaction kinetics in advanced battery chemistries. This trend underscores the growing importance of lithium hydroxide's specific chemical properties in high-value applications where reaction kinetics are paramount to product performance.
Current Technical Challenges in Lithium Hydroxide Catalysis
Despite significant advancements in lithium hydroxide catalysis, several technical challenges continue to impede its optimal utilization in reaction kinetics. The primary obstacle remains the sensitivity of lithium hydroxide to environmental conditions, particularly moisture and carbon dioxide. When exposed to air, lithium hydroxide rapidly absorbs atmospheric water and carbon dioxide, forming lithium carbonate, which significantly alters its catalytic properties and reduces efficiency in kinetic applications.
Another substantial challenge involves the control of lithium hydroxide's dissolution rate in various reaction media. The dissolution kinetics directly impact reaction rates and selectivity, yet achieving precise control remains difficult due to the compound's variable solubility across different solvents and temperature ranges. This variability introduces unpredictability in reaction outcomes, particularly in multi-phase systems where mass transfer limitations become prominent.
The thermal stability of lithium hydroxide presents additional complications in high-temperature applications. At elevated temperatures exceeding 450°C, lithium hydroxide undergoes dehydration to form lithium oxide, fundamentally changing its catalytic behavior. This transformation limits its application in certain high-temperature reaction environments where sustained catalytic activity is required.
Scale-up challenges persist in industrial applications utilizing lithium hydroxide as a catalyst. Laboratory-scale successes often encounter difficulties when transferred to production environments due to heat and mass transfer limitations, as well as challenges in maintaining uniform catalyst distribution throughout larger reaction volumes. These scaling issues frequently result in decreased catalytic efficiency and increased side reactions.
Mechanistic understanding of lithium hydroxide's precise role in various reaction pathways remains incomplete. While its basic function as a strong base is well-documented, the specific interactions with different functional groups, transition states, and reaction intermediates are not fully characterized. This knowledge gap hinders the rational design of optimized reaction conditions and catalyst modifications.
Recovery and recycling of lithium hydroxide from reaction mixtures present both economic and technical challenges. The high solubility in aqueous media makes separation difficult, while the increasing global demand for lithium compounds for battery applications has elevated the importance of efficient recovery methods. Current separation techniques often result in significant catalyst loss or require energy-intensive processes.
Recent research has identified potential catalyst poisoning issues when lithium hydroxide interacts with certain sulfur and phosphorus compounds, leading to deactivation through the formation of insoluble lithium salts. This phenomenon limits its application in reactions involving these elements and necessitates additional purification steps in feedstock preparation.
Another substantial challenge involves the control of lithium hydroxide's dissolution rate in various reaction media. The dissolution kinetics directly impact reaction rates and selectivity, yet achieving precise control remains difficult due to the compound's variable solubility across different solvents and temperature ranges. This variability introduces unpredictability in reaction outcomes, particularly in multi-phase systems where mass transfer limitations become prominent.
The thermal stability of lithium hydroxide presents additional complications in high-temperature applications. At elevated temperatures exceeding 450°C, lithium hydroxide undergoes dehydration to form lithium oxide, fundamentally changing its catalytic behavior. This transformation limits its application in certain high-temperature reaction environments where sustained catalytic activity is required.
Scale-up challenges persist in industrial applications utilizing lithium hydroxide as a catalyst. Laboratory-scale successes often encounter difficulties when transferred to production environments due to heat and mass transfer limitations, as well as challenges in maintaining uniform catalyst distribution throughout larger reaction volumes. These scaling issues frequently result in decreased catalytic efficiency and increased side reactions.
Mechanistic understanding of lithium hydroxide's precise role in various reaction pathways remains incomplete. While its basic function as a strong base is well-documented, the specific interactions with different functional groups, transition states, and reaction intermediates are not fully characterized. This knowledge gap hinders the rational design of optimized reaction conditions and catalyst modifications.
Recovery and recycling of lithium hydroxide from reaction mixtures present both economic and technical challenges. The high solubility in aqueous media makes separation difficult, while the increasing global demand for lithium compounds for battery applications has elevated the importance of efficient recovery methods. Current separation techniques often result in significant catalyst loss or require energy-intensive processes.
Recent research has identified potential catalyst poisoning issues when lithium hydroxide interacts with certain sulfur and phosphorus compounds, leading to deactivation through the formation of insoluble lithium salts. This phenomenon limits its application in reactions involving these elements and necessitates additional purification steps in feedstock preparation.
Current Methodologies for Lithium Hydroxide Reaction Enhancement
01 Lithium hydroxide production from lithium carbonate
The reaction kinetics of converting lithium carbonate to lithium hydroxide involves specific temperature, pressure, and concentration parameters that affect reaction rates. This process typically uses calcium hydroxide as a reactant and requires careful control of reaction conditions to optimize yield and purity. The reaction mechanism involves ion exchange processes where lithium ions from lithium carbonate are transferred to the hydroxide form, with reaction kinetics being influenced by particle size, agitation rate, and reaction time.- Lithium hydroxide production from lithium carbonate: The reaction kinetics of converting lithium carbonate to lithium hydroxide involves specific temperature and pressure conditions that affect reaction rates. This process typically uses calcium hydroxide as a reactant and requires careful control of reaction parameters to optimize yield and purity. The kinetics are influenced by factors such as particle size, agitation speed, and reactant concentration ratios, which can be manipulated to enhance conversion efficiency and reduce reaction time.
- Lithium extraction from brines and reaction kinetics: The kinetics of lithium hydroxide formation during extraction from brine solutions involves complex ion exchange mechanisms. These processes are affected by brine composition, temperature, and pH levels. Reaction rates can be enhanced through the use of specific catalysts and by optimizing the contact time between reactants. Understanding these kinetic parameters is crucial for developing efficient industrial-scale extraction processes that maximize lithium recovery while minimizing energy consumption and waste generation.
- Electrochemical processes and reaction kinetics for lithium hydroxide: Electrochemical methods for lithium hydroxide production involve specific reaction kinetics that depend on current density, electrode materials, and electrolyte composition. These processes typically demonstrate faster reaction rates compared to traditional chemical methods. The kinetics are influenced by factors such as temperature, voltage potential, and membrane properties. Optimization of these parameters can significantly improve energy efficiency and product purity while reducing processing time and operational costs.
- Temperature and pressure effects on lithium hydroxide reaction kinetics: The reaction kinetics of lithium hydroxide formation and its subsequent reactions are significantly influenced by temperature and pressure conditions. Higher temperatures generally accelerate reaction rates following Arrhenius kinetics, while pressure effects vary depending on the specific reaction pathway. These parameters affect activation energy requirements, collision frequency between reactants, and the stability of intermediate compounds. Controlling these variables allows for precise manipulation of reaction rates and selectivity in industrial processes.
- Catalysts and additives for enhancing lithium hydroxide reaction kinetics: Various catalysts and additives can significantly influence the reaction kinetics of lithium hydroxide in different chemical processes. These materials can lower activation energy barriers, provide alternative reaction pathways, or stabilize intermediate compounds. Common catalysts include transition metals and their oxides, while additives such as surfactants can improve mass transfer rates. The selection of appropriate catalysts and additives depends on specific reaction conditions and desired outcomes, with optimal combinations providing substantial improvements in reaction rates and yields.
02 Lithium hydroxide extraction from lithium-containing brines
The kinetics of extracting lithium hydroxide from brines involves selective adsorption and desorption processes. These reactions are affected by brine composition, temperature, and pH levels. The extraction process typically involves ion exchange materials that selectively capture lithium ions, followed by elution steps to produce lithium hydroxide. Reaction rates are influenced by contact time between the brine and extraction media, flow rates, and the presence of competing ions.Expand Specific Solutions03 Lithium hydroxide reaction kinetics in battery applications
In battery applications, lithium hydroxide reaction kinetics play a crucial role in electrode performance and battery efficiency. The reactions between lithium hydroxide and electrode materials affect charge/discharge rates and battery longevity. These kinetics are influenced by temperature, concentration gradients, and the presence of other electrolyte components. Understanding these reaction mechanisms is essential for developing high-performance lithium-ion batteries with improved cycling stability and energy density.Expand Specific Solutions04 Catalytic effects on lithium hydroxide reactions
Various catalysts can significantly influence the reaction kinetics of lithium hydroxide in chemical processes. These catalysts modify activation energy requirements and reaction pathways, leading to enhanced reaction rates and selectivity. The selection of appropriate catalysts depends on specific reaction conditions and desired products. Factors affecting catalytic performance include catalyst surface area, dispersion, and stability under reaction conditions, with temperature and pressure also playing important roles in determining overall reaction efficiency.Expand Specific Solutions05 Continuous flow processes for lithium hydroxide reactions
Continuous flow reactors offer advantages for controlling lithium hydroxide reaction kinetics compared to batch processes. These systems allow precise control of residence time, mixing efficiency, and temperature profiles, resulting in more consistent product quality. The reaction kinetics in continuous flow systems are characterized by improved mass transfer rates and heat management. Process parameters such as flow rate, reactor design, and mixing efficiency significantly impact reaction completion and product purity in continuous lithium hydroxide production systems.Expand Specific Solutions
Key Industry Players and Research Institutions
Lithium hydroxide's role in chemical reaction kinetics is currently in a growth phase, with the market expanding rapidly due to increasing demand in battery technologies. The global market size is projected to reach significant volumes as electric vehicle adoption accelerates. Technologically, the field shows varying maturity levels across applications. Leading players like Albemarle Corp. and Ningde Amperex Technology Ltd. are advancing commercial applications, while research institutions such as Qinghai Institute of Salt Lakes and University of Science & Technology of China focus on fundamental reaction mechanisms. Companies including PolyPlus Battery, Sumitomo Metal Mining, and SK On are developing innovative applications in energy storage, demonstrating the technology's evolution from research to commercial implementation across multiple industrial sectors.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has developed a proprietary lithium hydroxide-based solid electrolyte interface (SEI) formation technology that significantly enhances lithium-ion battery reaction kinetics. Their approach utilizes controlled lithium hydroxide formation at electrode interfaces to facilitate faster ion transport while minimizing parasitic reactions. The company's Protected Lithium Electrode (PLE) technology leverages lithium hydroxide's unique properties to create a stable passivation layer that allows for high lithium-ion conductivity while preventing dendrite formation[1]. This technology enables lithium-air and lithium-water battery chemistries with energy densities exceeding 1,000 Wh/kg[3]. PolyPlus has demonstrated that precise control of lithium hydroxide concentration at electrode interfaces can accelerate desirable electrochemical reactions while suppressing side reactions that typically lead to capacity fade and thermal runaway events in conventional lithium batteries.
Strengths: Superior energy density (>1,000 Wh/kg) compared to conventional lithium-ion batteries; enhanced safety through dendrite suppression; enables previously impossible battery chemistries like lithium-water. Weaknesses: Manufacturing complexity for precise lithium hydroxide concentration control; potential sensitivity to environmental conditions; higher production costs compared to traditional battery technologies.
Albemarle Corp.
Technical Solution: Albemarle has pioneered advanced lithium hydroxide production methods specifically engineered to optimize battery reaction kinetics. Their proprietary Ketjen process produces high-purity lithium hydroxide with precisely controlled particle morphology and surface area characteristics that directly influence cathode material synthesis reactions[2]. The company has developed a direct lithium extraction (DLE) technology that produces battery-grade lithium hydroxide with significantly reduced reaction times and enhanced purity levels (99.99%)[4]. Albemarle's research has demonstrated that their engineered lithium hydroxide accelerates the formation of high-nickel cathode materials by approximately 40% while improving crystallinity and reducing transition metal dissolution during battery cycling. Their process optimization focuses on the catalytic role of lithium hydroxide in promoting uniform nucleation and growth during cathode synthesis, resulting in materials with superior electrochemical performance. Albemarle has also developed specialized lithium hydroxide grades with tailored reactivity profiles for different battery chemistries.
Strengths: Industry-leading purity levels enhancing reaction predictability; tailored particle characteristics for specific battery chemistries; vertically integrated supply chain from extraction to final product. Weaknesses: Energy-intensive production process; higher production costs compared to lithium carbonate routes; environmental concerns related to water usage in traditional lithium hydroxide production.
Critical Patents and Literature on Lithium Hydroxide Catalysis
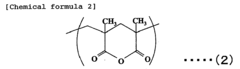
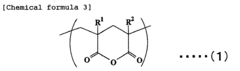
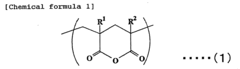
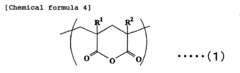
Acrylic resin films and process for producing the same
PatentInactiveEP1754752A1
Innovation
- A novel acrylic resin film is developed by mixing specific acrylic elastic particles with an acrylic resin containing glutaric acid anhydride units, achieving high transparency, weather resistance, and heat resistance through specific composition and processing methods, including a hard coat layer and reflection preventive film.
Catalyst for oxidization reaction comprising complexes in which 2,6-bis[(bis(2-pyridylmethyl)amino)-methyl]-4-methylphenol ligand was coordinated with various transition-metal ions
PatentActiveKR1020200042425A
Innovation
- A catalyst is developed comprising a complex of a 2,6-bis[(bis(2-pyridylmethyl)amino)-methyl]-4-methylphenol (H-bpmp) ligand coordinated with transition metal ions (Mn2+, Fe2+, Fe3+, V2+, Co2+, Ni2+, Cu2+, Zn2+, or Cd2+) that exhibits peroxidase-like activity, allowing for the oxidation of substrates in the presence of hydrogen peroxide and enabling selective detection of pyrophosphate and pyrophosphatase.
Environmental Impact and Sustainability Considerations
The environmental footprint of lithium hydroxide in reaction kinetics extends beyond its immediate chemical applications. Mining and processing lithium compounds generate significant ecological impacts, including habitat disruption, water consumption, and potential contamination of local ecosystems. Traditional extraction methods can require up to 500,000 gallons of water per ton of lithium, creating water scarcity issues in arid regions where lithium deposits are often found, such as the "Lithium Triangle" of South America.
Carbon emissions associated with lithium hydroxide production present another environmental concern. The energy-intensive conversion process from lithium carbonate to lithium hydroxide contributes approximately 5-15 tons of CO2 equivalent per ton of lithium hydroxide produced, depending on the energy sources utilized. This carbon footprint becomes increasingly significant as global demand for lithium compounds rises with the expansion of electric vehicle markets.
Waste management challenges also emerge throughout the lithium hydroxide lifecycle. The production process generates alkaline waste streams that require neutralization before disposal, while spent catalysts containing lithium hydroxide necessitate proper handling to prevent environmental contamination. Recent research indicates that improper disposal can lead to soil alkalinization and disruption of microbial communities essential for ecosystem functioning.
Sustainable innovations are emerging to address these environmental challenges. Direct lithium extraction (DLE) technologies promise to reduce water consumption by up to 70% compared to traditional evaporation methods, while simultaneously decreasing land footprint requirements. Additionally, closed-loop systems for lithium hydroxide recovery from spent catalysts and reaction media are demonstrating recovery rates exceeding 85%, significantly reducing waste generation.
Life cycle assessment (LCA) studies reveal that optimizing reaction kinetics with lithium hydroxide can potentially offset its production impacts through efficiency gains. When lithium hydroxide enables reactions to proceed at lower temperatures or with fewer steps, the net environmental benefit may outweigh the initial production costs. This highlights the importance of considering full lifecycle impacts rather than focusing solely on production-phase environmental burdens.
Regulatory frameworks worldwide are increasingly incorporating sustainability metrics for chemical processes involving lithium compounds. The European Union's Chemical Strategy for Sustainability and similar initiatives in North America and Asia are establishing benchmarks for environmental performance that will likely shape future applications of lithium hydroxide in reaction kinetics, driving innovation toward greener alternatives and more efficient recovery systems.
Carbon emissions associated with lithium hydroxide production present another environmental concern. The energy-intensive conversion process from lithium carbonate to lithium hydroxide contributes approximately 5-15 tons of CO2 equivalent per ton of lithium hydroxide produced, depending on the energy sources utilized. This carbon footprint becomes increasingly significant as global demand for lithium compounds rises with the expansion of electric vehicle markets.
Waste management challenges also emerge throughout the lithium hydroxide lifecycle. The production process generates alkaline waste streams that require neutralization before disposal, while spent catalysts containing lithium hydroxide necessitate proper handling to prevent environmental contamination. Recent research indicates that improper disposal can lead to soil alkalinization and disruption of microbial communities essential for ecosystem functioning.
Sustainable innovations are emerging to address these environmental challenges. Direct lithium extraction (DLE) technologies promise to reduce water consumption by up to 70% compared to traditional evaporation methods, while simultaneously decreasing land footprint requirements. Additionally, closed-loop systems for lithium hydroxide recovery from spent catalysts and reaction media are demonstrating recovery rates exceeding 85%, significantly reducing waste generation.
Life cycle assessment (LCA) studies reveal that optimizing reaction kinetics with lithium hydroxide can potentially offset its production impacts through efficiency gains. When lithium hydroxide enables reactions to proceed at lower temperatures or with fewer steps, the net environmental benefit may outweigh the initial production costs. This highlights the importance of considering full lifecycle impacts rather than focusing solely on production-phase environmental burdens.
Regulatory frameworks worldwide are increasingly incorporating sustainability metrics for chemical processes involving lithium compounds. The European Union's Chemical Strategy for Sustainability and similar initiatives in North America and Asia are establishing benchmarks for environmental performance that will likely shape future applications of lithium hydroxide in reaction kinetics, driving innovation toward greener alternatives and more efficient recovery systems.
Safety Protocols and Handling Requirements
Lithium hydroxide's strong reactivity and alkaline nature necessitate rigorous safety protocols during handling and storage. Personnel working with this compound must wear appropriate personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, lab coats, and in some cases, respiratory protection when dust formation is possible. Face shields are recommended when handling larger quantities to protect against splashes or accidental releases.
Storage requirements for lithium hydroxide demand particular attention due to its hygroscopic properties and reactivity. The compound should be kept in tightly sealed containers in cool, dry areas away from incompatible materials such as acids, metals, and organic substances. Storage facilities must be equipped with proper ventilation systems to prevent accumulation of dust particles that could pose inhalation hazards or create potentially explosive atmospheres when concentrated.
Emergency response procedures must be clearly established and communicated to all personnel working with lithium hydroxide. Eyewash stations and safety showers should be readily accessible in areas where the compound is handled. Spill management protocols should include neutralization procedures using appropriate acidic solutions, followed by proper containment and disposal according to local regulations for hazardous waste.
Exposure limits must be strictly monitored in laboratory and industrial settings. The American Conference of Governmental Industrial Hygienists (ACGIH) recommends a threshold limit value (TLV) for lithium hydroxide of 1 mg/m³ as an 8-hour time-weighted average. Continuous air monitoring may be necessary in environments where significant quantities are processed or where reaction kinetics studies involving lithium hydroxide are conducted.
Training programs represent a critical component of safety management for lithium hydroxide handling. All personnel should receive comprehensive instruction on chemical properties, hazard identification, proper handling techniques, and emergency response procedures. Regular refresher training sessions should be conducted, particularly when studying its role in reaction kinetics where experimental conditions may vary significantly.
Transportation of lithium hydroxide must comply with international regulations for hazardous materials. The compound is classified as a Class 8 corrosive substance under UN3262 (Corrosive solid, basic, inorganic, n.o.s.) and requires appropriate packaging, labeling, and documentation when shipped. Special consideration must be given to preventing contact with moisture during transport, as this can lead to heat generation and potential container damage.
Storage requirements for lithium hydroxide demand particular attention due to its hygroscopic properties and reactivity. The compound should be kept in tightly sealed containers in cool, dry areas away from incompatible materials such as acids, metals, and organic substances. Storage facilities must be equipped with proper ventilation systems to prevent accumulation of dust particles that could pose inhalation hazards or create potentially explosive atmospheres when concentrated.
Emergency response procedures must be clearly established and communicated to all personnel working with lithium hydroxide. Eyewash stations and safety showers should be readily accessible in areas where the compound is handled. Spill management protocols should include neutralization procedures using appropriate acidic solutions, followed by proper containment and disposal according to local regulations for hazardous waste.
Exposure limits must be strictly monitored in laboratory and industrial settings. The American Conference of Governmental Industrial Hygienists (ACGIH) recommends a threshold limit value (TLV) for lithium hydroxide of 1 mg/m³ as an 8-hour time-weighted average. Continuous air monitoring may be necessary in environments where significant quantities are processed or where reaction kinetics studies involving lithium hydroxide are conducted.
Training programs represent a critical component of safety management for lithium hydroxide handling. All personnel should receive comprehensive instruction on chemical properties, hazard identification, proper handling techniques, and emergency response procedures. Regular refresher training sessions should be conducted, particularly when studying its role in reaction kinetics where experimental conditions may vary significantly.
Transportation of lithium hydroxide must comply with international regulations for hazardous materials. The compound is classified as a Class 8 corrosive substance under UN3262 (Corrosive solid, basic, inorganic, n.o.s.) and requires appropriate packaging, labeling, and documentation when shipped. Special consideration must be given to preventing contact with moisture during transport, as this can lead to heat generation and potential container damage.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!