Comparing Lithium Hydroxide's Effect On Battery Cycle Life
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Battery Technology Background and Objectives
Lithium-ion batteries have revolutionized portable electronics and electric vehicles since their commercial introduction in the early 1990s. The evolution of these energy storage systems has been marked by continuous improvements in energy density, cycle life, and safety characteristics. Within this technological landscape, lithium hydroxide (LiOH) has emerged as a critical component that significantly influences battery performance, particularly regarding cycle life and longevity.
The historical development of lithium battery technology began with primary lithium cells in the 1970s, followed by rechargeable lithium-ion configurations in subsequent decades. Throughout this evolution, researchers have increasingly focused on understanding how various lithium compounds affect electrochemical processes within battery cells. Lithium hydroxide's role has gained particular attention due to its potential to mitigate degradation mechanisms and enhance overall battery durability.
Current technological objectives in this field center on quantifying and optimizing lithium hydroxide's impact on battery cycle life across different cell chemistries and operating conditions. Researchers aim to establish definitive correlations between LiOH concentration, distribution, and the resulting electrochemical stability of battery systems. This includes investigating how lithium hydroxide influences solid-electrolyte interphase (SEI) formation, lithium plating behavior, and structural stability of cathode materials during repeated charge-discharge cycles.
The technological trajectory suggests increasing importance of precise lithium hydroxide management in next-generation battery systems. As energy density requirements continue to rise for applications ranging from consumer electronics to grid-scale storage, understanding how to leverage LiOH's properties becomes essential for extending operational lifetimes. This represents a critical factor in improving the economic viability and sustainability of battery technologies.
Recent research indicates that lithium hydroxide's effects vary significantly across different cathode chemistries, with particularly notable impacts on high-nickel NMC (nickel-manganese-cobalt) and NCA (nickel-cobalt-aluminum) formulations. The technological goal is to develop comprehensive models that predict optimal LiOH concentrations for specific applications and usage profiles, enabling tailored battery designs with enhanced longevity.
The broader objective of this technological investigation extends beyond performance metrics to address sustainability concerns. By extending battery cycle life through optimized lithium hydroxide utilization, manufacturers can reduce resource consumption and minimize environmental impact associated with battery production and disposal. This aligns with global initiatives to develop more sustainable energy storage solutions for the transition to renewable energy systems.
The historical development of lithium battery technology began with primary lithium cells in the 1970s, followed by rechargeable lithium-ion configurations in subsequent decades. Throughout this evolution, researchers have increasingly focused on understanding how various lithium compounds affect electrochemical processes within battery cells. Lithium hydroxide's role has gained particular attention due to its potential to mitigate degradation mechanisms and enhance overall battery durability.
Current technological objectives in this field center on quantifying and optimizing lithium hydroxide's impact on battery cycle life across different cell chemistries and operating conditions. Researchers aim to establish definitive correlations between LiOH concentration, distribution, and the resulting electrochemical stability of battery systems. This includes investigating how lithium hydroxide influences solid-electrolyte interphase (SEI) formation, lithium plating behavior, and structural stability of cathode materials during repeated charge-discharge cycles.
The technological trajectory suggests increasing importance of precise lithium hydroxide management in next-generation battery systems. As energy density requirements continue to rise for applications ranging from consumer electronics to grid-scale storage, understanding how to leverage LiOH's properties becomes essential for extending operational lifetimes. This represents a critical factor in improving the economic viability and sustainability of battery technologies.
Recent research indicates that lithium hydroxide's effects vary significantly across different cathode chemistries, with particularly notable impacts on high-nickel NMC (nickel-manganese-cobalt) and NCA (nickel-cobalt-aluminum) formulations. The technological goal is to develop comprehensive models that predict optimal LiOH concentrations for specific applications and usage profiles, enabling tailored battery designs with enhanced longevity.
The broader objective of this technological investigation extends beyond performance metrics to address sustainability concerns. By extending battery cycle life through optimized lithium hydroxide utilization, manufacturers can reduce resource consumption and minimize environmental impact associated with battery production and disposal. This aligns with global initiatives to develop more sustainable energy storage solutions for the transition to renewable energy systems.
Market Analysis of Lithium Hydroxide in Battery Applications
The global lithium hydroxide market has experienced significant growth in recent years, primarily driven by its critical role in high-performance lithium-ion battery production. As of 2023, the market size has reached approximately $2.3 billion, with projections indicating a compound annual growth rate (CAGR) of 9.2% through 2030. This growth trajectory is directly linked to the expanding electric vehicle (EV) sector, which has seen annual sales increases exceeding 40% in major markets including China, Europe, and North America.
Lithium hydroxide's superior performance in nickel-rich cathode materials, particularly NCM (Nickel-Cobalt-Manganese) and NCA (Nickel-Cobalt-Aluminum) chemistries, has positioned it as the preferred lithium compound for high-energy density batteries. These advanced cathode formulations require lithium hydroxide rather than lithium carbonate to achieve optimal cycle life performance, especially in applications demanding extended range and durability.
Market segmentation reveals that approximately 65% of lithium hydroxide production is now directed toward battery applications, with the remaining 35% distributed across traditional industrial uses including lubricants, ceramics, and chemical processing. Within the battery segment, automotive applications account for 78% of demand, followed by energy storage systems at 14% and consumer electronics at 8%.
Regional analysis shows China dominating both production and consumption, controlling approximately 51% of global lithium hydroxide manufacturing capacity. Australia has emerged as the primary source of raw materials, with its spodumene concentrate feeding conversion facilities predominantly located in China. North American and European markets are rapidly expanding their domestic production capabilities to reduce supply chain vulnerabilities, with several major projects under development that could add 100,000+ tons of annual capacity by 2026.
Price volatility has been a significant market characteristic, with lithium hydroxide prices fluctuating between $15,000 and $85,000 per ton over the past three years. This volatility reflects both supply constraints and accelerating demand from battery manufacturers. Industry analysts project more stable pricing as new production capacity comes online, though premiums for battery-grade material with proven cycle life benefits will likely persist.
The competitive landscape features established mining companies like Albemarle, SQM, and Ganfeng Lithium alongside newer specialized producers. Battery manufacturers and automotive OEMs are increasingly securing supply through direct investments and long-term agreements, reflecting lithium hydroxide's strategic importance in achieving battery performance targets, particularly extended cycle life in premium EV applications.
Lithium hydroxide's superior performance in nickel-rich cathode materials, particularly NCM (Nickel-Cobalt-Manganese) and NCA (Nickel-Cobalt-Aluminum) chemistries, has positioned it as the preferred lithium compound for high-energy density batteries. These advanced cathode formulations require lithium hydroxide rather than lithium carbonate to achieve optimal cycle life performance, especially in applications demanding extended range and durability.
Market segmentation reveals that approximately 65% of lithium hydroxide production is now directed toward battery applications, with the remaining 35% distributed across traditional industrial uses including lubricants, ceramics, and chemical processing. Within the battery segment, automotive applications account for 78% of demand, followed by energy storage systems at 14% and consumer electronics at 8%.
Regional analysis shows China dominating both production and consumption, controlling approximately 51% of global lithium hydroxide manufacturing capacity. Australia has emerged as the primary source of raw materials, with its spodumene concentrate feeding conversion facilities predominantly located in China. North American and European markets are rapidly expanding their domestic production capabilities to reduce supply chain vulnerabilities, with several major projects under development that could add 100,000+ tons of annual capacity by 2026.
Price volatility has been a significant market characteristic, with lithium hydroxide prices fluctuating between $15,000 and $85,000 per ton over the past three years. This volatility reflects both supply constraints and accelerating demand from battery manufacturers. Industry analysts project more stable pricing as new production capacity comes online, though premiums for battery-grade material with proven cycle life benefits will likely persist.
The competitive landscape features established mining companies like Albemarle, SQM, and Ganfeng Lithium alongside newer specialized producers. Battery manufacturers and automotive OEMs are increasingly securing supply through direct investments and long-term agreements, reflecting lithium hydroxide's strategic importance in achieving battery performance targets, particularly extended cycle life in premium EV applications.
Current Challenges in Lithium Hydroxide Battery Technology
Despite significant advancements in lithium-ion battery technology, several critical challenges persist in the application of lithium hydroxide (LiOH) for high-performance batteries. The primary obstacle remains the precise control of LiOH concentration during battery manufacturing processes. Excess LiOH can lead to unwanted side reactions with electrolytes, while insufficient amounts fail to adequately compensate for lithium loss during cycling, resulting in capacity degradation.
Temperature sensitivity presents another significant challenge, as LiOH reactivity varies considerably across different thermal environments. This creates complications for batteries operating in extreme conditions, where performance consistency becomes difficult to maintain. The manufacturing process itself introduces variability, with batch-to-batch inconsistencies in LiOH purity and particle size distribution directly impacting cycle life performance.
The interaction between LiOH and various cathode materials remains incompletely understood. While beneficial effects have been documented with nickel-rich cathodes (NMC811, NCA), the mechanisms differ substantially with other compositions such as LFP or LNMO cathodes. This knowledge gap hampers optimization efforts across diverse battery chemistries and applications.
Long-term stability issues also plague current LiOH implementations. The compound's hygroscopic nature leads to moisture absorption during manufacturing and storage, potentially introducing water into the battery system. This can trigger parasitic reactions that accelerate capacity fade and reduce overall cycle life, particularly in humid environments.
Cost considerations further complicate widespread adoption, as high-purity LiOH suitable for battery applications commands premium pricing in the market. The economic viability of LiOH-enhanced batteries depends heavily on achieving the right balance between performance improvements and additional material costs.
Analytical challenges persist in accurately measuring and monitoring LiOH distribution within electrode materials. Current characterization techniques provide limited spatial resolution, making it difficult to optimize LiOH incorporation strategies. This technical limitation slows the development of more effective formulations and processing methods.
Safety concerns also merit attention, as LiOH can potentially contribute to thermal runaway under certain failure conditions. Understanding these safety implications across different battery designs and operating conditions remains an active research area requiring further investigation.
Addressing these interconnected challenges requires coordinated efforts across materials science, electrochemistry, and manufacturing engineering disciplines to fully realize the potential benefits of lithium hydroxide in extending battery cycle life.
Temperature sensitivity presents another significant challenge, as LiOH reactivity varies considerably across different thermal environments. This creates complications for batteries operating in extreme conditions, where performance consistency becomes difficult to maintain. The manufacturing process itself introduces variability, with batch-to-batch inconsistencies in LiOH purity and particle size distribution directly impacting cycle life performance.
The interaction between LiOH and various cathode materials remains incompletely understood. While beneficial effects have been documented with nickel-rich cathodes (NMC811, NCA), the mechanisms differ substantially with other compositions such as LFP or LNMO cathodes. This knowledge gap hampers optimization efforts across diverse battery chemistries and applications.
Long-term stability issues also plague current LiOH implementations. The compound's hygroscopic nature leads to moisture absorption during manufacturing and storage, potentially introducing water into the battery system. This can trigger parasitic reactions that accelerate capacity fade and reduce overall cycle life, particularly in humid environments.
Cost considerations further complicate widespread adoption, as high-purity LiOH suitable for battery applications commands premium pricing in the market. The economic viability of LiOH-enhanced batteries depends heavily on achieving the right balance between performance improvements and additional material costs.
Analytical challenges persist in accurately measuring and monitoring LiOH distribution within electrode materials. Current characterization techniques provide limited spatial resolution, making it difficult to optimize LiOH incorporation strategies. This technical limitation slows the development of more effective formulations and processing methods.
Safety concerns also merit attention, as LiOH can potentially contribute to thermal runaway under certain failure conditions. Understanding these safety implications across different battery designs and operating conditions remains an active research area requiring further investigation.
Addressing these interconnected challenges requires coordinated efforts across materials science, electrochemistry, and manufacturing engineering disciplines to fully realize the potential benefits of lithium hydroxide in extending battery cycle life.
Technical Solutions for Enhancing Battery Cycle Life with Lithium Hydroxide
01 Lithium hydroxide in battery electrolytes for improved cycle life
Lithium hydroxide can be incorporated into battery electrolytes to neutralize acidic impurities and maintain pH balance, which helps prevent electrode degradation during cycling. This addition stabilizes the solid electrolyte interphase (SEI) layer, reducing capacity fade and extending the overall cycle life of lithium-ion batteries. The controlled concentration of lithium hydroxide in electrolyte formulations can significantly improve battery performance under various operating conditions.- Lithium hydroxide in battery electrolyte formulations: Lithium hydroxide can be incorporated into battery electrolyte formulations to improve cycle life. It acts as a pH regulator and helps neutralize acidic components that form during cycling, which can otherwise degrade electrode materials. The addition of controlled amounts of lithium hydroxide to electrolytes can reduce side reactions at electrode surfaces, leading to improved capacity retention and extended cycle life of lithium-ion batteries.
- Surface treatment of cathode materials with lithium hydroxide: Treating cathode active materials with lithium hydroxide creates a protective surface layer that enhances cycle life. This surface modification helps prevent direct contact between the cathode material and the electrolyte, reducing unwanted side reactions. The lithium hydroxide treatment can also repair surface defects and stabilize the crystal structure of cathode materials during repeated charge-discharge cycles, resulting in improved electrochemical performance and longer battery life.
- Lithium hydroxide as precursor for high-performance cathode materials: Using high-purity lithium hydroxide as a precursor in the synthesis of cathode materials can significantly impact cycle life. The quality and characteristics of lithium hydroxide, including particle size, purity, and morphology, directly influence the structural stability and electrochemical properties of the resulting cathode materials. Optimized synthesis processes using carefully selected lithium hydroxide precursors can produce cathode materials with enhanced structural integrity during cycling, leading to improved capacity retention and longer battery lifespan.
- Lithium hydroxide in solid electrolyte interfaces: Lithium hydroxide plays a crucial role in the formation and stability of solid electrolyte interfaces (SEI) on electrode surfaces. When incorporated into SEI layers, lithium hydroxide can help create more stable and ion-conductive interfaces between the electrode and electrolyte. This improved interface reduces impedance growth during cycling and prevents continuous electrolyte decomposition, resulting in better cycling stability and extended battery life. The controlled presence of lithium hydroxide in these interfaces can significantly enhance the long-term performance of lithium-ion batteries.
- Recycling and regeneration of lithium hydroxide for sustainable battery production: Recycling processes that recover and regenerate lithium hydroxide from spent batteries can contribute to improved cycle life in new batteries. These processes focus on extracting high-purity lithium hydroxide that can be reused in the production of new cathode materials. The quality of recycled lithium hydroxide, when properly processed, can match or even exceed that of virgin materials, leading to cathode materials with excellent cycling performance. This approach not only addresses sustainability concerns but also can result in batteries with enhanced cycle life due to the controlled quality of the recycled lithium hydroxide.
02 Lithium hydroxide as cathode material precursor
Using lithium hydroxide as a precursor in cathode material synthesis leads to improved crystallinity and structural stability of the resulting active materials. The controlled reaction of lithium hydroxide with metal compounds produces cathode materials with optimized particle morphology and uniform composition, contributing to enhanced electrochemical performance and extended cycle life. This synthesis approach enables the production of high-capacity cathode materials that maintain structural integrity during repeated charge-discharge cycles.Expand Specific Solutions03 Surface treatment of electrode materials with lithium hydroxide
Surface modification of electrode materials using lithium hydroxide creates a protective layer that prevents direct contact between active materials and the electrolyte. This treatment reduces unwanted side reactions, suppresses transition metal dissolution, and stabilizes the electrode-electrolyte interface. The lithium hydroxide coating also facilitates lithium-ion transport while minimizing structural changes during cycling, resulting in improved capacity retention and extended battery lifespan.Expand Specific Solutions04 Recycling processes for lithium hydroxide recovery
Advanced recycling processes have been developed to recover lithium hydroxide from spent batteries, preserving its electrochemical properties for reuse in new battery production. These methods involve selective extraction techniques, purification steps, and crystallization processes that yield high-purity lithium hydroxide suitable for battery applications. The recycled lithium hydroxide demonstrates comparable performance to virgin material in terms of cycle life enhancement, supporting sustainable battery manufacturing and reducing environmental impact.Expand Specific Solutions05 Lithium hydroxide in solid-state battery systems
Incorporating lithium hydroxide into solid-state electrolyte formulations improves ionic conductivity and interfacial stability between electrodes and electrolytes. The presence of lithium hydroxide facilitates the formation of beneficial grain boundary phases that enhance lithium-ion transport while suppressing dendrite growth. This approach leads to solid-state batteries with superior cycle life, improved safety characteristics, and better performance at wider temperature ranges compared to conventional liquid electrolyte systems.Expand Specific Solutions
Key Industry Players in Lithium Hydroxide Battery Production
The lithium hydroxide battery cycle life technology landscape is currently in a growth phase, with the market expanding rapidly due to increasing demand for high-performance batteries in electric vehicles and energy storage systems. Major players like Samsung SDI, CATL, LG Energy Solution, and Toyota are leading innovation in this space, with significant R&D investments to enhance battery longevity and performance. Emerging companies such as Sion Power and Form Energy are developing breakthrough technologies, while established chemical manufacturers like Solvay and LG Chem are focusing on advanced lithium hydroxide formulations. The technology is approaching maturity in consumer electronics applications but remains in development for long-duration energy storage and extreme temperature applications, with companies competing to achieve optimal balance between cycle life extension and cost-effectiveness.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has pioneered a "Dual-Layer Concentration Gradient" (DCG) technology that leverages lithium hydroxide's properties to enhance battery cycle life. Their approach involves creating cathode materials with a radially distributed concentration of lithium hydroxide, with higher concentrations at the particle surface[1]. This gradient structure significantly reduces surface reactivity with the electrolyte, a primary cause of capacity fade. Samsung's research indicates that their DCG cathodes show approximately 40% less capacity degradation after 500 cycles compared to conventional cathodes[3]. Additionally, Samsung has developed a proprietary "low-temperature lithium hydroxide reaction" process that creates cathode materials with fewer structural defects, resulting in more stable performance during cycling. Their latest generation batteries incorporating this technology demonstrate exceptional cycle life, maintaining over 85% capacity after 1,000 cycles at 1C charge/discharge rates[6].
Strengths: Dual-Layer Concentration Gradient technology provides exceptional protection against surface-related degradation; low-temperature synthesis process creates structurally superior cathode materials. Weaknesses: Complex manufacturing process increases production costs; technology requires specialized equipment that limits manufacturing flexibility.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an advanced lithium hydroxide processing technique that significantly enhances battery cycle life. Their method involves precise control of lithium hydroxide particle size (typically between 5-10μm) and purity (>99.5%) to optimize cathode material performance[1]. CATL's proprietary "crystal structure stabilization" technology uses lithium hydroxide to create more stable cathode materials that resist structural degradation during repeated charge-discharge cycles. Their research shows that optimized lithium hydroxide integration can extend cycle life by up to 30% compared to conventional formulations[3]. Additionally, CATL has pioneered a low-temperature lithium hydroxide synthesis process that reduces impurities and creates more uniform particles, resulting in cathode materials with superior electrochemical stability and longer cycle life in their flagship battery products[5].
Strengths: Superior purity control and particle size optimization leads to exceptional cycle stability; proprietary crystal structure stabilization technology provides competitive advantage. Weaknesses: Higher production costs associated with ultra-high purity lithium hydroxide; process requires precise environmental controls that may limit manufacturing scalability.
Critical Patents and Research on Lithium Hydroxide Battery Chemistry
Method for determining the deterioration of a battery
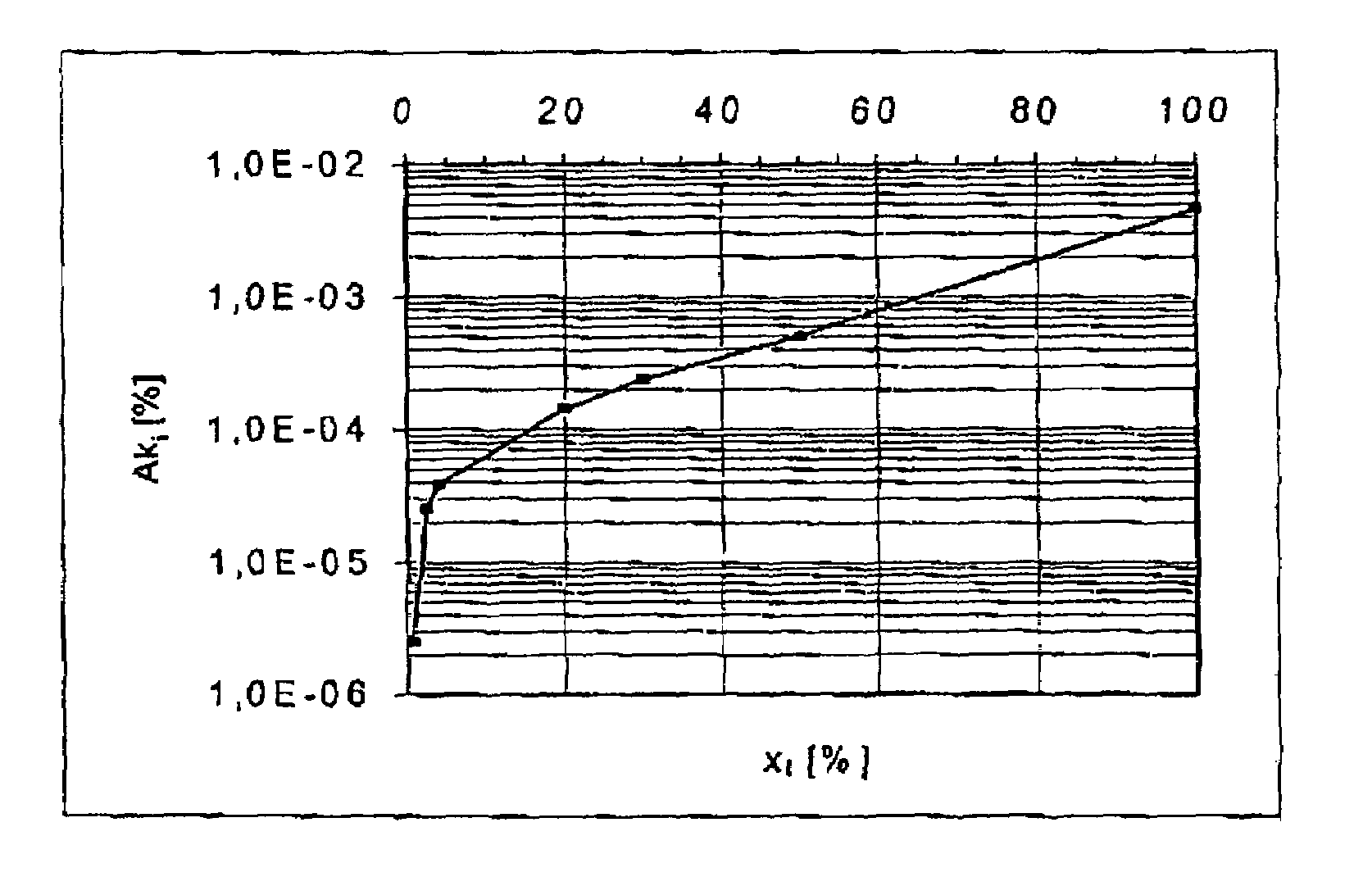
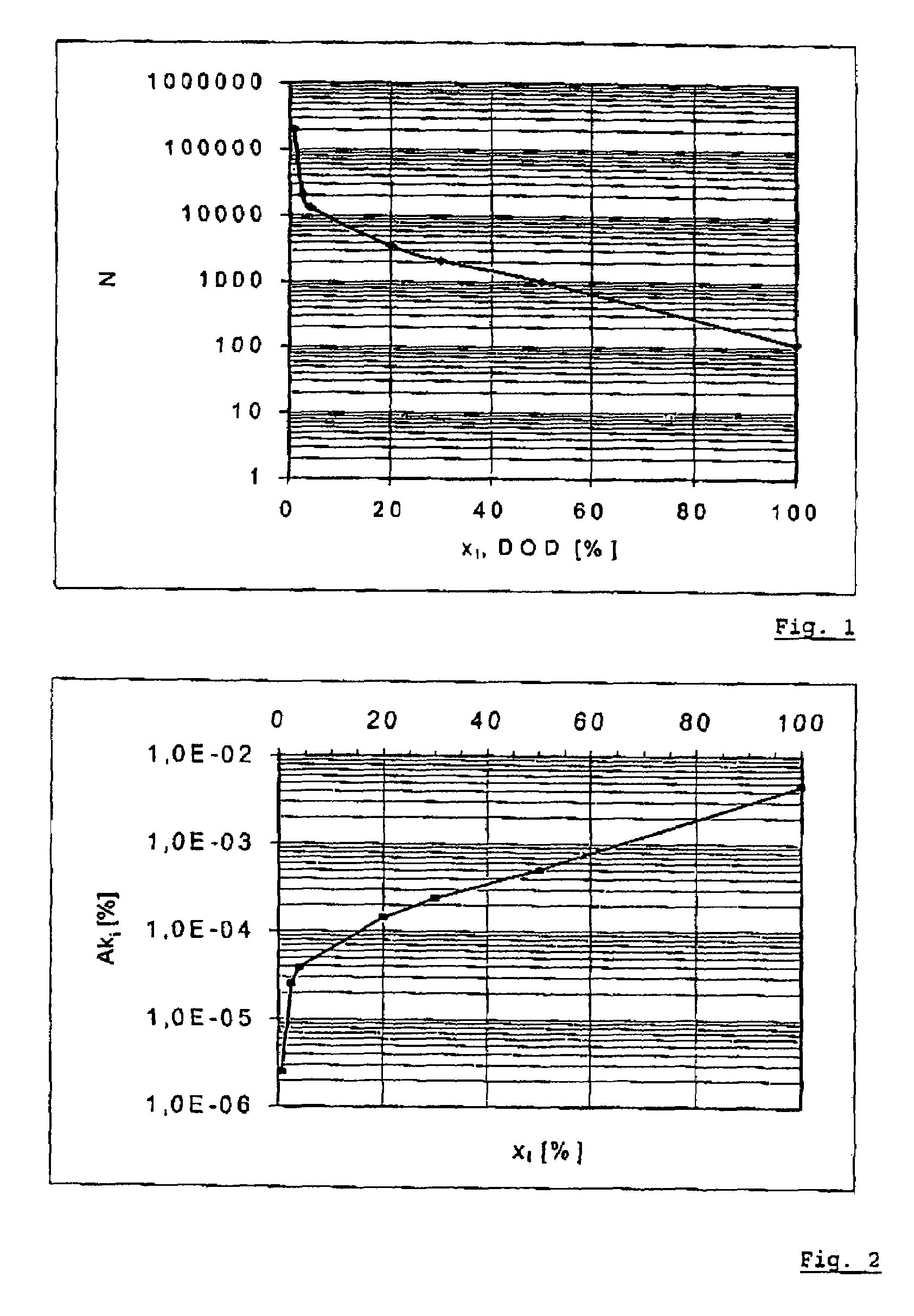
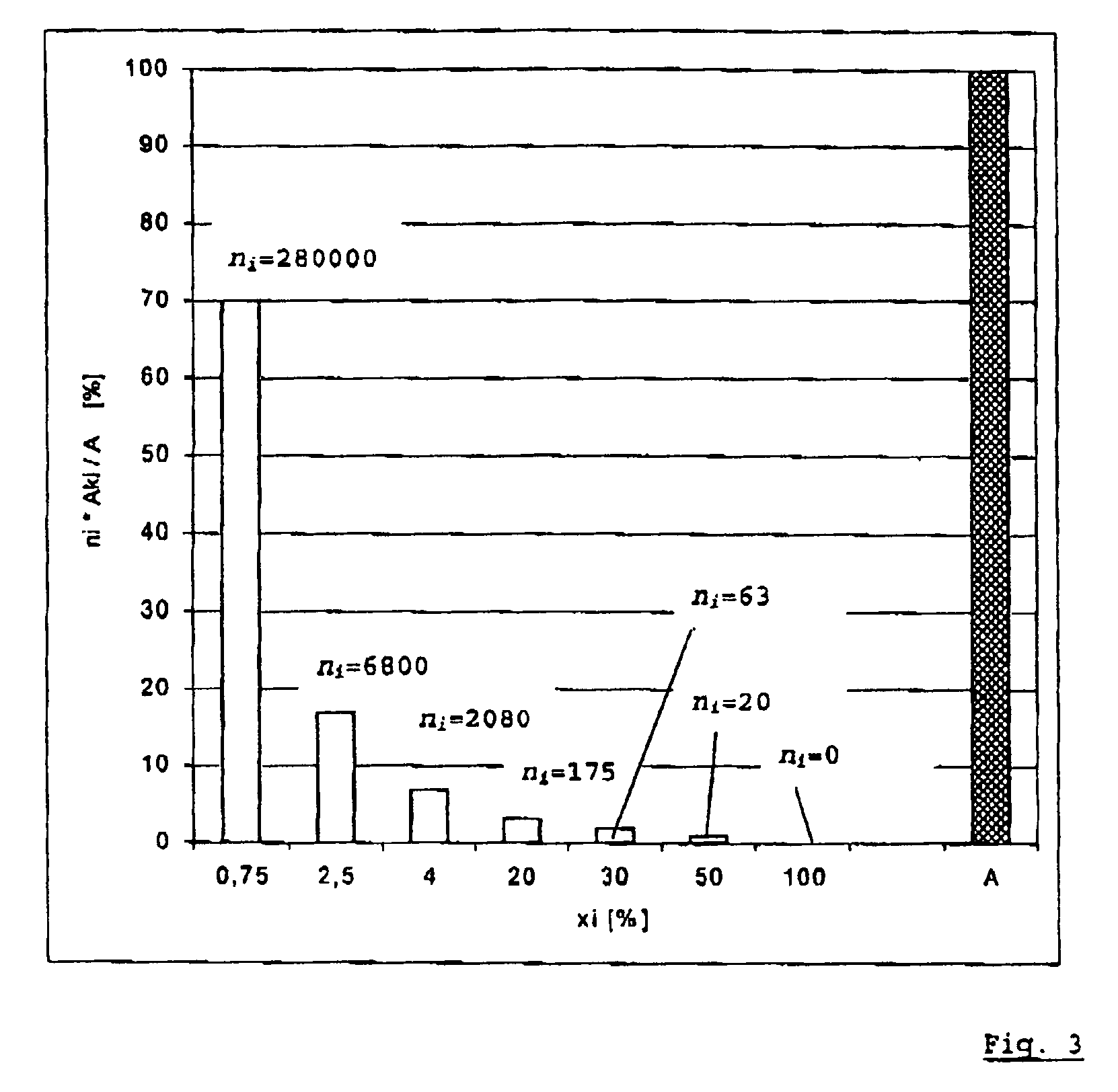
PatentInactiveUS7554330B2
Innovation
- A method that measures charge and discharge cycles by number and depth, using a characteristic deterioration curve specific to each battery type to calculate a summation of characteristic deterioration values, which simplifies the determination of battery deterioration and remaining life by representing it as a percentage.
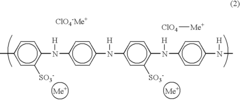
Negative electrode for lithium battery and lithium battery comprising same
PatentInactiveUS20040058232A1
Innovation
- A protective layer comprising an organosulfur compound, an electron conductive polymer, and an ionic conductive polymer is applied to the lithium metal layer to prevent dendrite formation and oxidation, enhancing cycle life and capacity characteristics.
Environmental Impact Assessment of Lithium Hydroxide Battery Production
The environmental impact of lithium hydroxide production for batteries represents a critical consideration in the sustainable development of energy storage technologies. The extraction and processing of lithium compounds, particularly lithium hydroxide, involves significant environmental implications that must be thoroughly assessed to ensure responsible manufacturing practices.
Primary environmental concerns include water consumption and contamination, especially in lithium-rich regions such as the "Lithium Triangle" spanning Chile, Argentina, and Bolivia. Traditional lithium extraction methods require approximately 500,000 gallons of water per ton of lithium, potentially depleting local water resources in often arid regions. This water-intensive process can disrupt ecosystems and affect agricultural activities in surrounding communities.
Carbon emissions associated with lithium hydroxide production vary significantly based on production methods. The conversion from lithium carbonate to lithium hydroxide—a process increasingly favored for high-nickel cathode materials that enhance cycle life—generates an estimated 5-15 tons of CO2 equivalent per ton of lithium hydroxide. These emissions contribute to the overall carbon footprint of battery manufacturing, though they remain substantially lower than the lifecycle emissions of fossil fuel alternatives.
Land use changes and habitat disruption constitute another environmental dimension requiring assessment. Open-pit mining operations for lithium-bearing minerals can transform landscapes and disrupt local biodiversity. Brine extraction operations, while less visibly disruptive, may alter subsurface hydrology and affect sensitive salt flat ecosystems.
Chemical waste management presents ongoing challenges in lithium hydroxide production. The process generates various byproducts including sodium sulfate, calcium carbonate, and magnesium hydroxide, which require proper disposal or valorization to prevent environmental contamination. Advanced production facilities increasingly implement closed-loop systems to minimize waste discharge and recover valuable materials.
Energy consumption in lithium hydroxide refinement processes represents another significant environmental factor. The transformation from raw lithium to battery-grade lithium hydroxide requires substantial thermal and electrical energy inputs, though technological improvements have gradually reduced these requirements. Facilities powered by renewable energy can substantially mitigate this impact, with several major producers now implementing solar and wind power integration.
Regulatory frameworks governing environmental impacts vary considerably across lithium-producing regions, creating inconsistent standards for impact assessment and mitigation. Leading battery manufacturers increasingly demand comprehensive environmental impact documentation from their lithium hydroxide suppliers, driving improvements in production practices throughout the supply chain.
Primary environmental concerns include water consumption and contamination, especially in lithium-rich regions such as the "Lithium Triangle" spanning Chile, Argentina, and Bolivia. Traditional lithium extraction methods require approximately 500,000 gallons of water per ton of lithium, potentially depleting local water resources in often arid regions. This water-intensive process can disrupt ecosystems and affect agricultural activities in surrounding communities.
Carbon emissions associated with lithium hydroxide production vary significantly based on production methods. The conversion from lithium carbonate to lithium hydroxide—a process increasingly favored for high-nickel cathode materials that enhance cycle life—generates an estimated 5-15 tons of CO2 equivalent per ton of lithium hydroxide. These emissions contribute to the overall carbon footprint of battery manufacturing, though they remain substantially lower than the lifecycle emissions of fossil fuel alternatives.
Land use changes and habitat disruption constitute another environmental dimension requiring assessment. Open-pit mining operations for lithium-bearing minerals can transform landscapes and disrupt local biodiversity. Brine extraction operations, while less visibly disruptive, may alter subsurface hydrology and affect sensitive salt flat ecosystems.
Chemical waste management presents ongoing challenges in lithium hydroxide production. The process generates various byproducts including sodium sulfate, calcium carbonate, and magnesium hydroxide, which require proper disposal or valorization to prevent environmental contamination. Advanced production facilities increasingly implement closed-loop systems to minimize waste discharge and recover valuable materials.
Energy consumption in lithium hydroxide refinement processes represents another significant environmental factor. The transformation from raw lithium to battery-grade lithium hydroxide requires substantial thermal and electrical energy inputs, though technological improvements have gradually reduced these requirements. Facilities powered by renewable energy can substantially mitigate this impact, with several major producers now implementing solar and wind power integration.
Regulatory frameworks governing environmental impacts vary considerably across lithium-producing regions, creating inconsistent standards for impact assessment and mitigation. Leading battery manufacturers increasingly demand comprehensive environmental impact documentation from their lithium hydroxide suppliers, driving improvements in production practices throughout the supply chain.
Supply Chain Analysis of Lithium Hydroxide for Battery Manufacturing
The global lithium hydroxide supply chain has evolved significantly in response to the growing demand for high-performance lithium-ion batteries. Currently, the supply chain begins with lithium extraction from either brine operations (primarily in South America) or hard rock mining (predominantly in Australia). For battery-grade lithium hydroxide production, hard rock mining of spodumene has become increasingly favored due to its higher purity potential and more straightforward conversion process.
Australia has emerged as the dominant player in lithium raw material supply, accounting for approximately 52% of global production. The extracted spodumene is typically processed into lithium hydroxide in China, which controls about 60% of global lithium hydroxide production capacity. This geographic concentration creates significant supply chain vulnerabilities, as evidenced by recent market disruptions.
The conversion process from spodumene to battery-grade lithium hydroxide involves multiple steps including crushing, calcination, acid leaching, purification, and crystallization. This complex process requires substantial energy input and generates considerable waste streams, presenting both environmental challenges and opportunities for technological improvement.
Recent supply chain disruptions have highlighted the fragility of the current system. COVID-19 pandemic restrictions, geopolitical tensions between major producing nations, and transportation bottlenecks have all contributed to price volatility. In 2021-2022, lithium hydroxide prices increased by over 400% before experiencing a significant correction, demonstrating the market's sensitivity to supply chain disruptions.
Battery manufacturers are increasingly concerned about supply security, leading to vertical integration strategies. Companies like Tesla and CATL have invested directly in mining operations to secure stable lithium hydroxide supplies. Additionally, new production facilities are being developed in North America and Europe to reduce dependency on the China-dominated processing infrastructure.
Sustainability concerns are reshaping the supply chain landscape. The water-intensive nature of brine operations and the energy requirements for hard rock processing have prompted investment in alternative technologies. Direct lithium extraction (DLE) methods and lithium recycling initiatives are gaining traction as potential solutions to reduce environmental impact and diversify supply sources.
The future supply chain is likely to be characterized by greater geographical diversification, increased recycling capacity, and improved production technologies that enhance efficiency while reducing environmental footprint. These developments will be crucial in supporting the growing demand for lithium hydroxide in high-nickel cathode batteries that deliver superior cycle life performance.
Australia has emerged as the dominant player in lithium raw material supply, accounting for approximately 52% of global production. The extracted spodumene is typically processed into lithium hydroxide in China, which controls about 60% of global lithium hydroxide production capacity. This geographic concentration creates significant supply chain vulnerabilities, as evidenced by recent market disruptions.
The conversion process from spodumene to battery-grade lithium hydroxide involves multiple steps including crushing, calcination, acid leaching, purification, and crystallization. This complex process requires substantial energy input and generates considerable waste streams, presenting both environmental challenges and opportunities for technological improvement.
Recent supply chain disruptions have highlighted the fragility of the current system. COVID-19 pandemic restrictions, geopolitical tensions between major producing nations, and transportation bottlenecks have all contributed to price volatility. In 2021-2022, lithium hydroxide prices increased by over 400% before experiencing a significant correction, demonstrating the market's sensitivity to supply chain disruptions.
Battery manufacturers are increasingly concerned about supply security, leading to vertical integration strategies. Companies like Tesla and CATL have invested directly in mining operations to secure stable lithium hydroxide supplies. Additionally, new production facilities are being developed in North America and Europe to reduce dependency on the China-dominated processing infrastructure.
Sustainability concerns are reshaping the supply chain landscape. The water-intensive nature of brine operations and the energy requirements for hard rock processing have prompted investment in alternative technologies. Direct lithium extraction (DLE) methods and lithium recycling initiatives are gaining traction as potential solutions to reduce environmental impact and diversify supply sources.
The future supply chain is likely to be characterized by greater geographical diversification, increased recycling capacity, and improved production technologies that enhance efficiency while reducing environmental footprint. These developments will be crucial in supporting the growing demand for lithium hydroxide in high-nickel cathode batteries that deliver superior cycle life performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!