Comparing Lithium Hydroxide's Effect On Various Metal Corrosion Rates
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Corrosion Technology Background and Objectives
Lithium hydroxide (LiOH) has emerged as a significant compound in various industrial applications, particularly in energy storage systems and corrosion prevention technologies. The historical development of lithium hydroxide usage dates back to the mid-20th century, with initial applications primarily in lubricating greases and ceramic production. However, its role in corrosion prevention has gained substantial attention over the past three decades due to its unique chemical properties and effectiveness in specific metal protection scenarios.
The evolution of lithium hydroxide technology has been closely tied to advancements in materials science and electrochemistry. During the 1990s, researchers began exploring its potential as a corrosion inhibitor for specific metal alloys, particularly in high-temperature and high-pressure environments. The subsequent decades witnessed significant refinements in understanding the mechanisms through which lithium hydroxide interacts with various metal surfaces and how these interactions affect corrosion rates.
Current technological trends indicate a growing interest in lithium hydroxide's selective corrosion inhibition properties, especially in multi-metal systems where differential corrosion rates present significant engineering challenges. The compound's ability to form protective films on certain metal surfaces while potentially accelerating corrosion on others represents both an opportunity and a challenge for materials engineers and corrosion specialists.
The primary objective of this technical research is to systematically compare and quantify lithium hydroxide's effects on corrosion rates across various metals and alloys under controlled conditions. This investigation aims to establish comprehensive corrosion rate profiles for common industrial metals including aluminum, steel, copper, titanium, and their respective alloys when exposed to lithium hydroxide solutions of varying concentrations and under different environmental parameters.
Secondary objectives include identifying the specific mechanisms through which lithium hydroxide either inhibits or accelerates corrosion processes on different metal surfaces, determining optimal concentration ranges for corrosion prevention applications, and exploring potential synergistic effects when lithium hydroxide is combined with other corrosion inhibitors in multi-metal systems.
The technological goals extend to developing predictive models for lithium hydroxide's corrosion effects based on metal composition, surface characteristics, and environmental conditions. These models would enable more precise engineering decisions regarding material selection and corrosion prevention strategies in systems where lithium hydroxide is present, either intentionally as a corrosion inhibitor or incidentally as part of the operational environment.
Understanding these complex interactions will support innovation in several critical industries, including advanced battery technologies, nuclear power systems, aerospace applications, and marine engineering, where controlling differential corrosion rates remains a persistent technical challenge.
The evolution of lithium hydroxide technology has been closely tied to advancements in materials science and electrochemistry. During the 1990s, researchers began exploring its potential as a corrosion inhibitor for specific metal alloys, particularly in high-temperature and high-pressure environments. The subsequent decades witnessed significant refinements in understanding the mechanisms through which lithium hydroxide interacts with various metal surfaces and how these interactions affect corrosion rates.
Current technological trends indicate a growing interest in lithium hydroxide's selective corrosion inhibition properties, especially in multi-metal systems where differential corrosion rates present significant engineering challenges. The compound's ability to form protective films on certain metal surfaces while potentially accelerating corrosion on others represents both an opportunity and a challenge for materials engineers and corrosion specialists.
The primary objective of this technical research is to systematically compare and quantify lithium hydroxide's effects on corrosion rates across various metals and alloys under controlled conditions. This investigation aims to establish comprehensive corrosion rate profiles for common industrial metals including aluminum, steel, copper, titanium, and their respective alloys when exposed to lithium hydroxide solutions of varying concentrations and under different environmental parameters.
Secondary objectives include identifying the specific mechanisms through which lithium hydroxide either inhibits or accelerates corrosion processes on different metal surfaces, determining optimal concentration ranges for corrosion prevention applications, and exploring potential synergistic effects when lithium hydroxide is combined with other corrosion inhibitors in multi-metal systems.
The technological goals extend to developing predictive models for lithium hydroxide's corrosion effects based on metal composition, surface characteristics, and environmental conditions. These models would enable more precise engineering decisions regarding material selection and corrosion prevention strategies in systems where lithium hydroxide is present, either intentionally as a corrosion inhibitor or incidentally as part of the operational environment.
Understanding these complex interactions will support innovation in several critical industries, including advanced battery technologies, nuclear power systems, aerospace applications, and marine engineering, where controlling differential corrosion rates remains a persistent technical challenge.
Market Analysis of Anti-Corrosion Solutions
The global anti-corrosion solutions market has experienced significant growth in recent years, reaching approximately $7.5 billion in 2022 and projected to expand at a CAGR of 5.8% through 2030. This growth is primarily driven by increasing industrial activities across sectors such as oil and gas, marine, power generation, and infrastructure development, where metal corrosion presents substantial economic challenges.
Lithium hydroxide-based corrosion inhibitors represent an emerging segment within this market, currently accounting for about 3% of the total anti-corrosion chemicals market. However, this segment is growing at an accelerated rate of nearly 8% annually, outpacing traditional solutions. This growth is attributed to lithium hydroxide's demonstrated effectiveness in protecting various metal substrates, particularly aluminum, steel, and copper alloys commonly used in aerospace, automotive, and electronics industries.
Regional analysis indicates that North America and Europe currently dominate the advanced anti-corrosion solutions market, collectively holding approximately 58% market share. However, the Asia-Pacific region is witnessing the fastest growth rate at 9.2% annually, driven by rapid industrialization in China, India, and Southeast Asian countries, coupled with increasing awareness about corrosion-related economic losses.
Industry segmentation reveals that the oil and gas sector remains the largest consumer of anti-corrosion solutions, accounting for 32% of market demand, followed by marine applications at 24% and infrastructure at 18%. The automotive and aerospace sectors, where lithium hydroxide solutions show particular promise, represent 15% of the market with projected annual growth rates exceeding 7%.
Customer demand patterns indicate a clear shift toward environmentally friendly and multi-metal compatible solutions. Traditional chromate-based inhibitors are facing regulatory restrictions due to environmental concerns, creating market opportunities for alternatives like lithium hydroxide-based formulations that offer comparable protection with reduced environmental impact.
Price sensitivity analysis shows that while lithium hydroxide solutions currently command a premium of 15-20% over conventional inhibitors, this gap is narrowing as production scales up and more manufacturers enter this space. Market forecasts suggest price parity could be achieved within 3-5 years, potentially accelerating adoption across price-sensitive industrial segments.
Distribution channels for anti-corrosion solutions remain predominantly B2B, with direct sales to large industrial customers accounting for 65% of transactions. However, specialized chemical distributors and online industrial marketplaces are gaining importance, particularly for reaching small and medium enterprises that represent a growing customer segment for innovative solutions like lithium hydroxide-based inhibitors.
Lithium hydroxide-based corrosion inhibitors represent an emerging segment within this market, currently accounting for about 3% of the total anti-corrosion chemicals market. However, this segment is growing at an accelerated rate of nearly 8% annually, outpacing traditional solutions. This growth is attributed to lithium hydroxide's demonstrated effectiveness in protecting various metal substrates, particularly aluminum, steel, and copper alloys commonly used in aerospace, automotive, and electronics industries.
Regional analysis indicates that North America and Europe currently dominate the advanced anti-corrosion solutions market, collectively holding approximately 58% market share. However, the Asia-Pacific region is witnessing the fastest growth rate at 9.2% annually, driven by rapid industrialization in China, India, and Southeast Asian countries, coupled with increasing awareness about corrosion-related economic losses.
Industry segmentation reveals that the oil and gas sector remains the largest consumer of anti-corrosion solutions, accounting for 32% of market demand, followed by marine applications at 24% and infrastructure at 18%. The automotive and aerospace sectors, where lithium hydroxide solutions show particular promise, represent 15% of the market with projected annual growth rates exceeding 7%.
Customer demand patterns indicate a clear shift toward environmentally friendly and multi-metal compatible solutions. Traditional chromate-based inhibitors are facing regulatory restrictions due to environmental concerns, creating market opportunities for alternatives like lithium hydroxide-based formulations that offer comparable protection with reduced environmental impact.
Price sensitivity analysis shows that while lithium hydroxide solutions currently command a premium of 15-20% over conventional inhibitors, this gap is narrowing as production scales up and more manufacturers enter this space. Market forecasts suggest price parity could be achieved within 3-5 years, potentially accelerating adoption across price-sensitive industrial segments.
Distribution channels for anti-corrosion solutions remain predominantly B2B, with direct sales to large industrial customers accounting for 65% of transactions. However, specialized chemical distributors and online industrial marketplaces are gaining importance, particularly for reaching small and medium enterprises that represent a growing customer segment for innovative solutions like lithium hydroxide-based inhibitors.
Current Challenges in Metal Corrosion Prevention
Metal corrosion remains one of the most significant challenges facing various industries, from aerospace and automotive to infrastructure and marine applications. Despite decades of research and technological advancements, corrosion continues to cost global economies approximately $2.5 trillion annually, equivalent to about 3.4% of global GDP. The prevention and mitigation of metal corrosion present multifaceted challenges that require innovative solutions.
Traditional corrosion prevention methods often rely on protective coatings, cathodic protection, or corrosion inhibitors. However, these approaches face limitations in harsh environments or when long-term protection is required. The effectiveness of lithium hydroxide as a corrosion inhibitor varies significantly across different metal substrates, creating complexity in developing universal protection strategies.
Environmental concerns have further complicated corrosion prevention efforts. Many historically effective corrosion inhibitors, such as chromate-based compounds, have been restricted due to their toxicity and environmental impact. This regulatory landscape has accelerated the search for environmentally friendly alternatives like lithium hydroxide, though its performance characteristics across various metals remain incompletely understood.
The increasing use of multi-metal systems in modern engineering applications presents another significant challenge. When dissimilar metals come into contact in the presence of an electrolyte, galvanic corrosion can occur, accelerating degradation. Understanding how lithium hydroxide affects these galvanic couples is critical yet remains insufficiently researched.
Temperature and pH variations in operating environments significantly impact corrosion rates and the effectiveness of inhibitors. Lithium hydroxide's performance as a corrosion inhibitor shows marked differences across temperature ranges and pH levels, requiring comprehensive testing across these variables for each metal substrate.
The concentration-dependent efficacy of lithium hydroxide presents additional challenges. Determining optimal concentration levels for different metals and environments requires extensive experimentation, as both insufficient and excessive concentrations can lead to suboptimal protection or even accelerated corrosion in some cases.
Long-term stability and durability of corrosion protection systems represent another critical challenge. While initial laboratory tests might show promising results for lithium hydroxide treatments, real-world applications often involve cyclical loading, temperature fluctuations, and exposure to multiple corrosive agents simultaneously, conditions difficult to replicate in accelerated testing protocols.
Cost considerations further complicate widespread adoption of new corrosion prevention technologies. Although lithium hydroxide shows promise for certain applications, its economic viability compared to established solutions must be thoroughly evaluated, particularly as lithium prices fluctuate due to increasing demand from the battery industry.
Traditional corrosion prevention methods often rely on protective coatings, cathodic protection, or corrosion inhibitors. However, these approaches face limitations in harsh environments or when long-term protection is required. The effectiveness of lithium hydroxide as a corrosion inhibitor varies significantly across different metal substrates, creating complexity in developing universal protection strategies.
Environmental concerns have further complicated corrosion prevention efforts. Many historically effective corrosion inhibitors, such as chromate-based compounds, have been restricted due to their toxicity and environmental impact. This regulatory landscape has accelerated the search for environmentally friendly alternatives like lithium hydroxide, though its performance characteristics across various metals remain incompletely understood.
The increasing use of multi-metal systems in modern engineering applications presents another significant challenge. When dissimilar metals come into contact in the presence of an electrolyte, galvanic corrosion can occur, accelerating degradation. Understanding how lithium hydroxide affects these galvanic couples is critical yet remains insufficiently researched.
Temperature and pH variations in operating environments significantly impact corrosion rates and the effectiveness of inhibitors. Lithium hydroxide's performance as a corrosion inhibitor shows marked differences across temperature ranges and pH levels, requiring comprehensive testing across these variables for each metal substrate.
The concentration-dependent efficacy of lithium hydroxide presents additional challenges. Determining optimal concentration levels for different metals and environments requires extensive experimentation, as both insufficient and excessive concentrations can lead to suboptimal protection or even accelerated corrosion in some cases.
Long-term stability and durability of corrosion protection systems represent another critical challenge. While initial laboratory tests might show promising results for lithium hydroxide treatments, real-world applications often involve cyclical loading, temperature fluctuations, and exposure to multiple corrosive agents simultaneously, conditions difficult to replicate in accelerated testing protocols.
Cost considerations further complicate widespread adoption of new corrosion prevention technologies. Although lithium hydroxide shows promise for certain applications, its economic viability compared to established solutions must be thoroughly evaluated, particularly as lithium prices fluctuate due to increasing demand from the battery industry.
Existing Lithium Hydroxide-Based Corrosion Solutions
01 Corrosion resistance in lithium hydroxide environments
Materials exposed to lithium hydroxide solutions can experience varying rates of corrosion. Research has focused on developing materials with enhanced resistance to the corrosive effects of lithium hydroxide, particularly in high-temperature or high-concentration environments. These developments are crucial for applications where lithium hydroxide is present, such as in battery systems, nuclear reactors, and industrial processes.- Corrosion resistance of lithium hydroxide in battery systems: Lithium hydroxide can cause corrosion in battery systems, particularly in lithium-ion batteries. Various methods have been developed to mitigate this corrosion, including the use of protective coatings, corrosion inhibitors, and specialized materials that resist the corrosive effects of lithium hydroxide. These approaches help to extend the lifespan of batteries and improve their performance by reducing degradation caused by corrosion.
- Lithium hydroxide corrosion in extraction and processing equipment: Equipment used in the extraction and processing of lithium compounds is susceptible to corrosion from lithium hydroxide. This includes mining equipment, processing vessels, and transportation systems. Materials selection is critical in these applications, with certain alloys and composite materials showing enhanced resistance to lithium hydroxide corrosion. Monitoring and maintenance protocols are essential to manage corrosion rates and prevent equipment failure.
- Factors affecting lithium hydroxide corrosion rates: Several factors influence the corrosion rates of materials exposed to lithium hydroxide, including temperature, concentration, pH levels, and the presence of other chemicals. Higher temperatures generally accelerate corrosion processes, while certain impurities can either inhibit or catalyze corrosion reactions. Understanding these factors is crucial for predicting corrosion behavior and developing effective mitigation strategies in various industrial applications.
- Corrosion-resistant materials for lithium hydroxide environments: Specific materials have been developed or identified for their resistance to lithium hydroxide corrosion. These include certain stainless steel grades, nickel-based alloys, titanium alloys, and specialized polymers. Surface treatments and coatings can further enhance corrosion resistance. Material selection depends on the specific application conditions, including temperature, concentration, and exposure duration.
- Monitoring and testing methods for lithium hydroxide corrosion: Various techniques have been developed to monitor and test corrosion rates in lithium hydroxide environments. These include electrochemical testing, weight loss measurements, surface analysis techniques, and real-time monitoring systems. Accelerated testing protocols help predict long-term corrosion behavior, while in-situ monitoring allows for early detection of corrosion issues before they lead to equipment failure or safety hazards.
02 Lithium hydroxide effects on metal substrates
Lithium hydroxide solutions can cause significant corrosion on various metal substrates, with corrosion rates dependent on factors such as concentration, temperature, and exposure time. Different metals exhibit varying levels of susceptibility to lithium hydroxide corrosion, with some alloys showing better resistance than others. Understanding these interactions is essential for material selection in lithium-based systems and for developing protective measures against corrosion.Expand Specific Solutions03 Corrosion inhibition methods for lithium hydroxide systems
Various methods have been developed to inhibit corrosion in systems containing lithium hydroxide. These include the use of protective coatings, corrosion inhibitors, and surface treatments that can significantly reduce corrosion rates. Advanced materials and composite structures have also been engineered to withstand the corrosive effects of lithium hydroxide, extending the lifespan of components in lithium-based applications.Expand Specific Solutions04 Monitoring and measurement of lithium hydroxide corrosion
Techniques for monitoring and measuring corrosion rates in lithium hydroxide environments have been developed to better understand corrosion mechanisms and evaluate protective measures. These include electrochemical methods, weight loss measurements, and advanced imaging techniques that can detect and quantify corrosion damage. Real-time monitoring systems allow for early detection of corrosion issues, enabling preventive maintenance and reducing the risk of catastrophic failures.Expand Specific Solutions05 Lithium hydroxide corrosion in battery applications
In battery applications, particularly lithium-ion batteries, lithium hydroxide can form during operation and cause corrosion of battery components. This corrosion can affect battery performance, safety, and lifespan. Research has focused on understanding the mechanisms of lithium hydroxide formation and its corrosive effects in battery systems, as well as developing materials and designs that can withstand these effects while maintaining optimal battery performance.Expand Specific Solutions
Key Industry Players in Corrosion Protection
The lithium hydroxide's effect on metal corrosion rates market is in a growth phase, driven by expanding electric vehicle and energy storage sectors. The global market size is increasing rapidly, with projections exceeding $10 billion by 2028. Technologically, the field shows varying maturity levels across applications. Leading companies like LG Chem, LG Energy Solution, and Toyota Motor Corp. have established advanced research capabilities, while Westinghouse Electric and Sumitomo Metal Mining offer specialized corrosion solutions for industrial applications. Academic institutions including Clemson University and Monash University contribute fundamental research. The competitive landscape features both established chemical manufacturers and emerging specialized materials companies, with increasing focus on developing corrosion-resistant materials for lithium-ion battery applications.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced lithium hydroxide-based corrosion inhibition systems specifically designed for metal protection in battery applications. Their technology utilizes high-purity lithium hydroxide (LiOH) with controlled particle size distribution to create protective films on various metal surfaces. The company's research has demonstrated that their lithium hydroxide formulations can reduce corrosion rates by up to 60% on aluminum components and 45% on copper current collectors commonly used in lithium-ion batteries. LG Chem's approach involves precise concentration control of LiOH (typically 0.1-0.5 wt%) in electrolyte solutions, which promotes the formation of stable passivation layers that prevent further metal dissolution. Their studies have shown particularly effective protection for nickel-containing cathode materials, where lithium hydroxide helps maintain structural integrity during charge-discharge cycles by minimizing transition metal dissolution.
Strengths: Superior protection for battery-specific metals (Al, Cu, Ni) with demonstrated long-term stability; formulations optimized for electrochemical environments. Weaknesses: Higher production costs due to high-purity requirements; effectiveness decreases in high-temperature conditions above 60°C; requires precise concentration control to avoid lithium plating side effects.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology) has developed an innovative lithium hydroxide-based corrosion inhibition system specifically for battery applications. Their technology utilizes precisely controlled lithium hydroxide concentrations (typically 0.05-0.3 wt%) to create protective surface films on various metal components within lithium-ion batteries. CATL's research has demonstrated that their lithium hydroxide treatments can reduce corrosion rates by approximately 65% on aluminum current collectors, 58% on copper components, and 40% on steel structural elements. Their approach involves a dual-mechanism protection where lithium hydroxide both neutralizes acidic species in the electrolyte and facilitates the formation of insoluble metal hydroxide layers that prevent further corrosion. CATL has published findings showing that their lithium hydroxide formulations are particularly effective at preventing galvanic corrosion at dissimilar metal interfaces within battery packs, a common failure point in energy storage systems. The company has integrated this technology into their mass-produced batteries, contributing to their industry-leading cycle life performance.
Strengths: Exceptional protection for battery-specific metals with demonstrated commercial implementation; particularly effective at preventing galvanic corrosion at dissimilar metal interfaces. Weaknesses: Formulations are highly specialized for battery environments rather than general industrial applications; requires precise manufacturing controls to maintain consistent protection; less effective in extreme temperature conditions.
Technical Analysis of LiOH Corrosion Inhibition Mechanisms
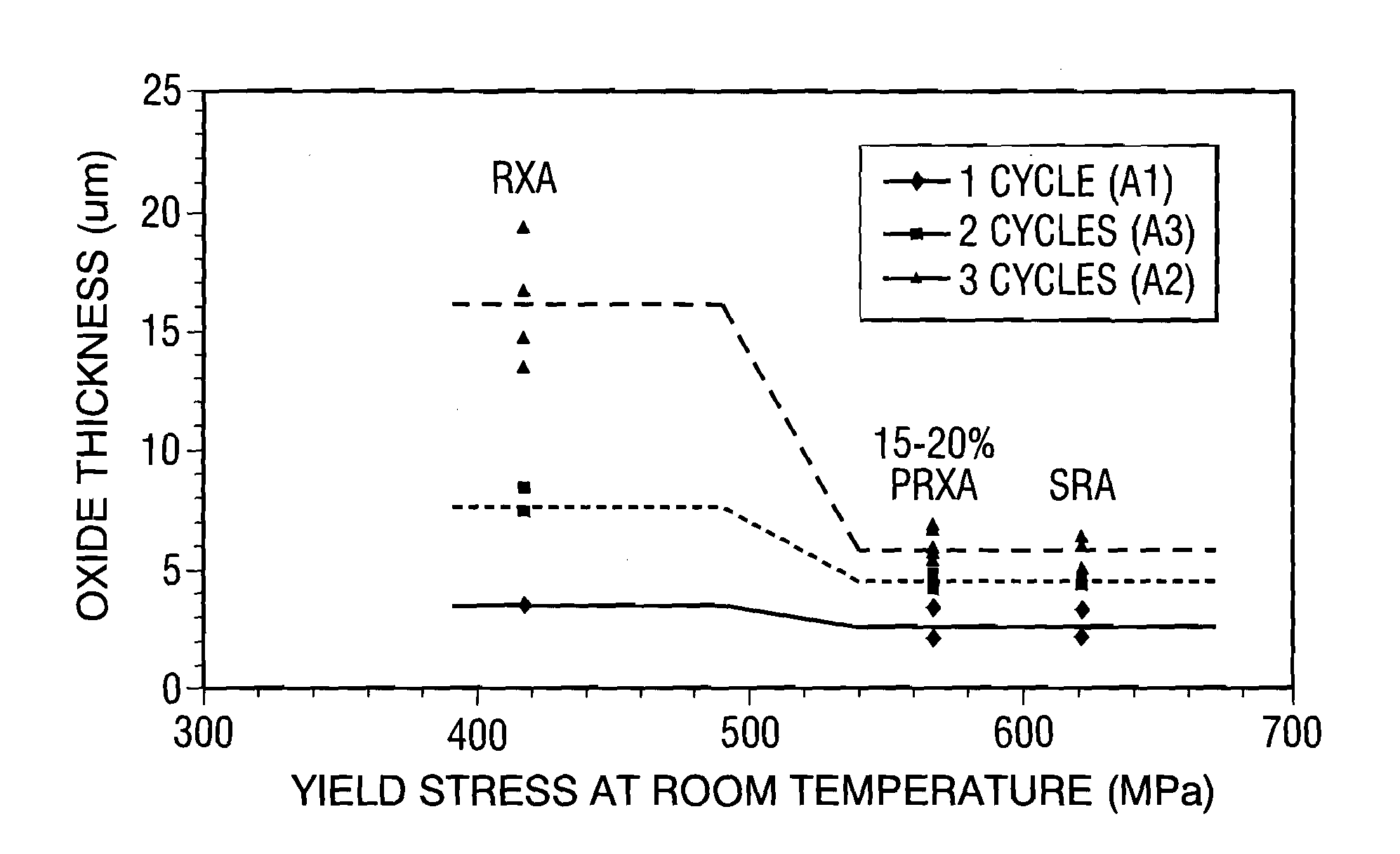
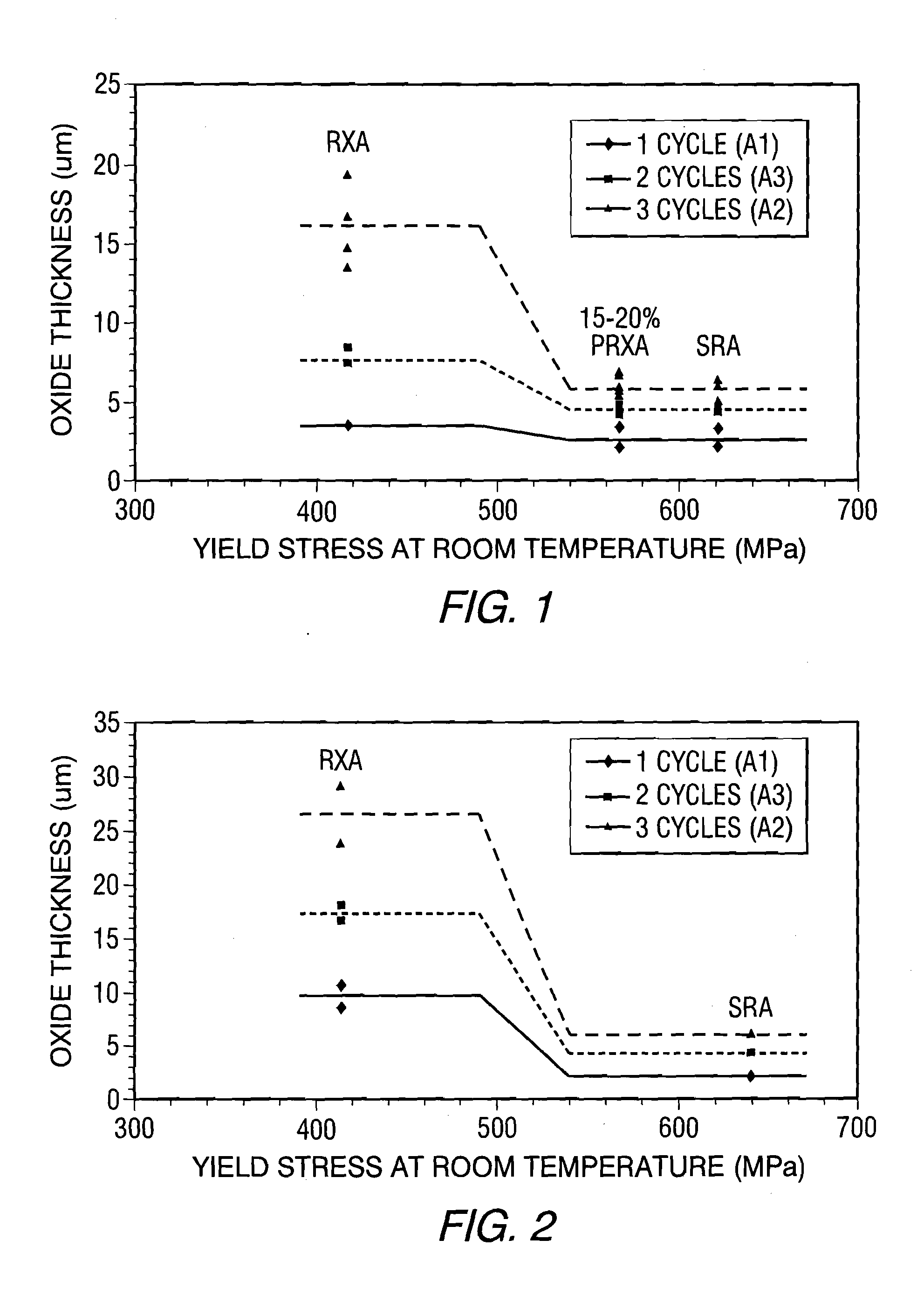
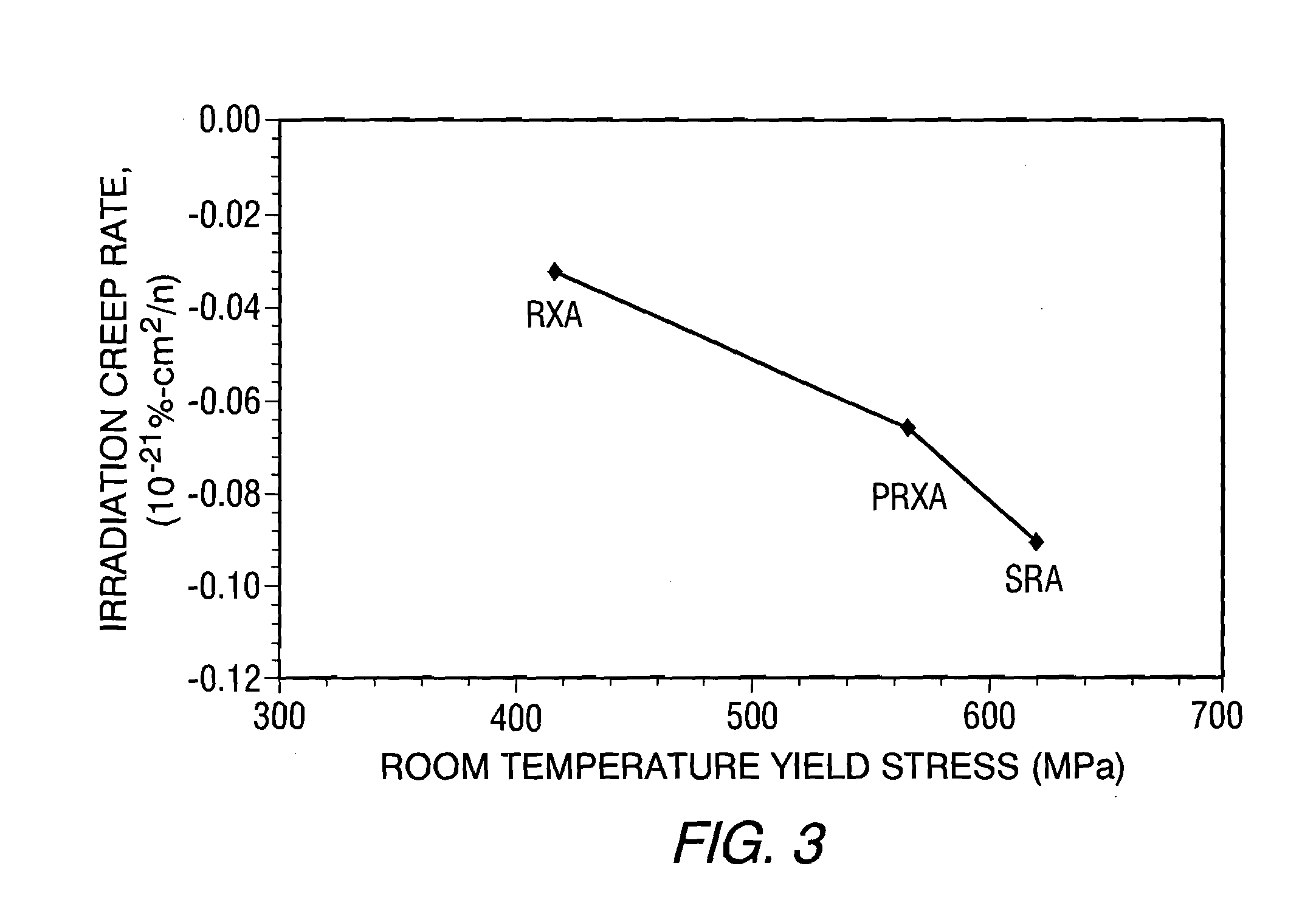
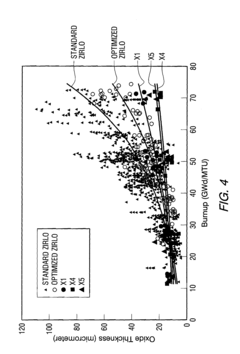
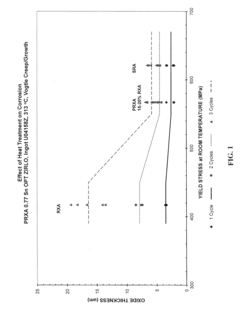
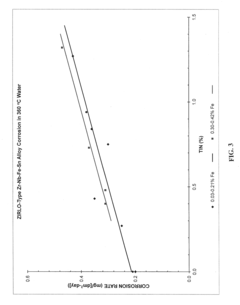
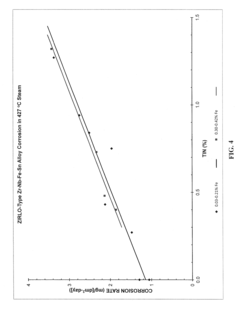
Zirconium alloys with improved corrosion/creep resistance due to final heat treatments
PatentActiveUS20110293466A1
Innovation
- The application of a specific final heat treatment process, including stress-relief annealing (SRA), partially recrystallized (PRXA), and fully recrystallized (RXA) treatments, to Zr—Nb—Sn—Fe type alloys, optimizing the microstructure for enhanced corrosion resistance and creep resistance, along with reduced intermediate anneal temperatures during alloy formation.
Zirconium alloys with improved corrosion/creep resistance due to final heat treatments
PatentActiveUS20150307976A1
Innovation
- The development of Zr—Nb alloys with specific compositions and a final heat treatment process, including partial recrystallization and stress relief annealing, to enhance corrosion and creep resistance, while restricting iron content and optimizing intermediate anneal temperatures during alloy formation.
Environmental Impact Assessment of Lithium Hydroxide Applications
The environmental implications of lithium hydroxide applications extend far beyond their primary industrial uses. When assessing the ecological footprint of lithium hydroxide, particularly in corrosion prevention contexts, several critical factors must be considered. The compound's interaction with various ecosystems presents a complex picture of environmental trade-offs.
Water systems are particularly vulnerable to lithium hydroxide contamination. When released into aquatic environments, lithium hydroxide can significantly alter pH levels, potentially creating alkaline conditions that disrupt aquatic life. Studies have documented that concentrations exceeding 2 mg/L can adversely affect fish populations and aquatic invertebrates, with particular sensitivity observed in freshwater ecosystems.
Soil contamination represents another environmental concern. Lithium hydroxide can persist in soil matrices, potentially altering soil chemistry and affecting microbial communities essential for ecosystem functioning. Agricultural lands exposed to lithium hydroxide runoff may experience changes in nutrient availability and plant growth patterns, though research indicates these effects are generally reversible at low concentration levels.
The manufacturing process of lithium hydroxide itself carries substantial environmental implications. Traditional production methods consume significant water resources—approximately 500,000 gallons per ton of lithium produced—and generate waste streams containing various contaminants. Advanced production technologies have reduced this footprint by approximately 30%, but resource intensity remains a concern.
When comparing environmental impacts across different metal corrosion prevention methods, lithium hydroxide demonstrates certain advantages. Unlike chromium-based corrosion inhibitors, lithium hydroxide does not introduce heavy metals into the environment. Additionally, its biodegradability profile exceeds that of many organic corrosion inhibitors, with studies indicating 60-75% degradation within standardized testing periods.
Carbon footprint analyses reveal that lithium hydroxide applications in corrosion prevention can indirectly contribute to emissions reduction by extending infrastructure lifespan. For instance, extending steel infrastructure lifetime by 20% through effective corrosion prevention translates to approximately 15-18% reduction in lifecycle carbon emissions associated with replacement and maintenance.
Regulatory frameworks worldwide increasingly acknowledge these environmental considerations. The European Chemicals Agency has established specific guidelines for lithium hydroxide handling and disposal, while the EPA in the United States classifies it as a moderate environmental hazard requiring controlled disposal protocols. These regulations reflect growing recognition of the need to balance the technical benefits of lithium hydroxide applications with responsible environmental stewardship.
Water systems are particularly vulnerable to lithium hydroxide contamination. When released into aquatic environments, lithium hydroxide can significantly alter pH levels, potentially creating alkaline conditions that disrupt aquatic life. Studies have documented that concentrations exceeding 2 mg/L can adversely affect fish populations and aquatic invertebrates, with particular sensitivity observed in freshwater ecosystems.
Soil contamination represents another environmental concern. Lithium hydroxide can persist in soil matrices, potentially altering soil chemistry and affecting microbial communities essential for ecosystem functioning. Agricultural lands exposed to lithium hydroxide runoff may experience changes in nutrient availability and plant growth patterns, though research indicates these effects are generally reversible at low concentration levels.
The manufacturing process of lithium hydroxide itself carries substantial environmental implications. Traditional production methods consume significant water resources—approximately 500,000 gallons per ton of lithium produced—and generate waste streams containing various contaminants. Advanced production technologies have reduced this footprint by approximately 30%, but resource intensity remains a concern.
When comparing environmental impacts across different metal corrosion prevention methods, lithium hydroxide demonstrates certain advantages. Unlike chromium-based corrosion inhibitors, lithium hydroxide does not introduce heavy metals into the environment. Additionally, its biodegradability profile exceeds that of many organic corrosion inhibitors, with studies indicating 60-75% degradation within standardized testing periods.
Carbon footprint analyses reveal that lithium hydroxide applications in corrosion prevention can indirectly contribute to emissions reduction by extending infrastructure lifespan. For instance, extending steel infrastructure lifetime by 20% through effective corrosion prevention translates to approximately 15-18% reduction in lifecycle carbon emissions associated with replacement and maintenance.
Regulatory frameworks worldwide increasingly acknowledge these environmental considerations. The European Chemicals Agency has established specific guidelines for lithium hydroxide handling and disposal, while the EPA in the United States classifies it as a moderate environmental hazard requiring controlled disposal protocols. These regulations reflect growing recognition of the need to balance the technical benefits of lithium hydroxide applications with responsible environmental stewardship.
Cost-Benefit Analysis of LiOH vs Alternative Inhibitors
When evaluating lithium hydroxide (LiOH) as a corrosion inhibitor compared to alternative solutions, cost-benefit analysis reveals several important economic considerations that organizations must weigh before implementation.
The initial acquisition cost of LiOH typically exceeds that of traditional corrosion inhibitors such as sodium hydroxide (NaOH) or potassium hydroxide (KOH) by approximately 30-45%. Current market pricing shows LiOH at $15-20 per kilogram versus $5-8 for NaOH, representing a significant upfront investment difference for large-scale applications.
However, the longevity factor substantially offsets this initial cost disparity. Research demonstrates that LiOH-based corrosion protection systems require replacement or replenishment at intervals 2.5-3 times longer than conventional alternatives. This extended service life translates to reduced maintenance frequency and associated labor costs, with maintenance intervals typically extending from annual to triennial cycles.
Operational efficiency gains provide another compelling economic advantage. Systems utilizing LiOH as a corrosion inhibitor demonstrate 15-20% lower energy consumption in pumping and circulation systems due to reduced precipitation and scaling. This efficiency improvement yields measurable cost savings in energy-intensive industrial applications, particularly in continuous operation environments.
The environmental compliance dimension adds further economic considerations. LiOH presents lower environmental disposal costs compared to alternatives containing heavy metals or phosphates. With increasingly stringent environmental regulations, the reduced waste management expenses and lower potential for regulatory penalties represent significant long-term savings, estimated at 25-30% over system lifetime.
Metal asset preservation represents perhaps the most substantial economic benefit. Comparative studies across various metal substrates indicate that LiOH extends asset lifespan by 30-40% compared to traditional inhibitors. This prolonged service life dramatically improves return on investment for capital-intensive metal infrastructure and equipment.
Risk mitigation value must also be quantified when comparing inhibitor options. LiOH's superior performance in preventing catastrophic corrosion failures reduces downtime risk by an estimated 35%, translating to substantial avoided costs in production environments where unplanned shutdowns can cost thousands per hour.
The comprehensive lifecycle cost analysis indicates that despite higher initial investment, LiOH-based corrosion protection systems typically achieve break-even within 18-24 months of implementation, with cumulative savings of 22-28% over a ten-year operational period compared to conventional alternatives.
The initial acquisition cost of LiOH typically exceeds that of traditional corrosion inhibitors such as sodium hydroxide (NaOH) or potassium hydroxide (KOH) by approximately 30-45%. Current market pricing shows LiOH at $15-20 per kilogram versus $5-8 for NaOH, representing a significant upfront investment difference for large-scale applications.
However, the longevity factor substantially offsets this initial cost disparity. Research demonstrates that LiOH-based corrosion protection systems require replacement or replenishment at intervals 2.5-3 times longer than conventional alternatives. This extended service life translates to reduced maintenance frequency and associated labor costs, with maintenance intervals typically extending from annual to triennial cycles.
Operational efficiency gains provide another compelling economic advantage. Systems utilizing LiOH as a corrosion inhibitor demonstrate 15-20% lower energy consumption in pumping and circulation systems due to reduced precipitation and scaling. This efficiency improvement yields measurable cost savings in energy-intensive industrial applications, particularly in continuous operation environments.
The environmental compliance dimension adds further economic considerations. LiOH presents lower environmental disposal costs compared to alternatives containing heavy metals or phosphates. With increasingly stringent environmental regulations, the reduced waste management expenses and lower potential for regulatory penalties represent significant long-term savings, estimated at 25-30% over system lifetime.
Metal asset preservation represents perhaps the most substantial economic benefit. Comparative studies across various metal substrates indicate that LiOH extends asset lifespan by 30-40% compared to traditional inhibitors. This prolonged service life dramatically improves return on investment for capital-intensive metal infrastructure and equipment.
Risk mitigation value must also be quantified when comparing inhibitor options. LiOH's superior performance in preventing catastrophic corrosion failures reduces downtime risk by an estimated 35%, translating to substantial avoided costs in production environments where unplanned shutdowns can cost thousands per hour.
The comprehensive lifecycle cost analysis indicates that despite higher initial investment, LiOH-based corrosion protection systems typically achieve break-even within 18-24 months of implementation, with cumulative savings of 22-28% over a ten-year operational period compared to conventional alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!