Lithium Hydroxide Vs Magnesium Hydroxide: Reactivity Comparison
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium and Magnesium Hydroxides: Background and Research Objectives
Hydroxides of alkali and alkaline earth metals have been fundamental compounds in chemical research and industrial applications for over a century. Lithium hydroxide (LiOH) and magnesium hydroxide (Mg(OH)₂) represent two significant compounds with distinct chemical properties and reactivity profiles that have garnered increasing attention in recent decades. The historical development of these compounds traces back to the early isolation of their respective elements, with lithium discovered in 1817 by Johan August Arfwedson and magnesium isolated by Sir Humphry Davy in 1808.
The technological evolution of these hydroxides has been closely tied to advancements in extraction and purification methods. Since the 1950s, lithium hydroxide production has evolved from basic mineral processing to sophisticated electrochemical techniques, while magnesium hydroxide production has seen similar refinements from seawater extraction to precision precipitation methods. These developments have enabled higher purity products with more consistent performance characteristics.
Recent years have witnessed an exponential growth in research interest regarding these compounds, particularly driven by the emergence of lithium-ion battery technology and environmental applications. Publication trends indicate a 300% increase in research papers focused on lithium hydroxide over the past decade, while magnesium hydroxide research has seen a steady 150% growth, primarily in flame retardant and pharmaceutical applications.
The comparative reactivity of these hydroxides represents a critical area of investigation with significant implications across multiple industries. While both compounds function as bases, their reaction kinetics, solubility behaviors, and interaction mechanisms with various substrates differ substantially, leading to distinct application profiles. Understanding these differences at a fundamental level is essential for optimizing their use in emerging technologies.
This technical research aims to systematically investigate and quantify the reactivity differences between lithium hydroxide and magnesium hydroxide across multiple parameters, including reaction rates with acids, thermal stability, electrochemical behavior, and catalytic potential. The primary objectives include: establishing comprehensive reactivity profiles under standardized conditions; identifying the molecular and electronic factors governing their different behaviors; developing predictive models for their performance in novel applications; and exploring potential synergistic effects when used in combination.
The findings from this investigation are expected to inform next-generation battery technology development, advanced materials engineering, pharmaceutical formulation, and environmental remediation processes. By elucidating the fundamental chemistry underlying these compounds' behaviors, this research seeks to bridge existing knowledge gaps and enable more precise application-specific selection between these important hydroxides.
The technological evolution of these hydroxides has been closely tied to advancements in extraction and purification methods. Since the 1950s, lithium hydroxide production has evolved from basic mineral processing to sophisticated electrochemical techniques, while magnesium hydroxide production has seen similar refinements from seawater extraction to precision precipitation methods. These developments have enabled higher purity products with more consistent performance characteristics.
Recent years have witnessed an exponential growth in research interest regarding these compounds, particularly driven by the emergence of lithium-ion battery technology and environmental applications. Publication trends indicate a 300% increase in research papers focused on lithium hydroxide over the past decade, while magnesium hydroxide research has seen a steady 150% growth, primarily in flame retardant and pharmaceutical applications.
The comparative reactivity of these hydroxides represents a critical area of investigation with significant implications across multiple industries. While both compounds function as bases, their reaction kinetics, solubility behaviors, and interaction mechanisms with various substrates differ substantially, leading to distinct application profiles. Understanding these differences at a fundamental level is essential for optimizing their use in emerging technologies.
This technical research aims to systematically investigate and quantify the reactivity differences between lithium hydroxide and magnesium hydroxide across multiple parameters, including reaction rates with acids, thermal stability, electrochemical behavior, and catalytic potential. The primary objectives include: establishing comprehensive reactivity profiles under standardized conditions; identifying the molecular and electronic factors governing their different behaviors; developing predictive models for their performance in novel applications; and exploring potential synergistic effects when used in combination.
The findings from this investigation are expected to inform next-generation battery technology development, advanced materials engineering, pharmaceutical formulation, and environmental remediation processes. By elucidating the fundamental chemistry underlying these compounds' behaviors, this research seeks to bridge existing knowledge gaps and enable more precise application-specific selection between these important hydroxides.
Market Applications and Demand Analysis for Hydroxide Compounds
The global market for hydroxide compounds has witnessed significant growth in recent years, driven by their diverse applications across multiple industries. Lithium hydroxide and magnesium hydroxide, in particular, have emerged as critical materials with distinct market dynamics based on their reactivity profiles and functional properties.
In the battery sector, lithium hydroxide demand has experienced exponential growth due to its essential role in manufacturing high-nickel content cathode materials for electric vehicle (EV) batteries. The global lithium hydroxide market reached approximately 67,000 metric tons in 2021 and is projected to grow at a compound annual growth rate of 25-30% through 2030. This surge is primarily attributed to the superior thermal stability that lithium hydroxide provides to nickel-rich cathodes, enabling longer battery life and improved safety characteristics.
Magnesium hydroxide, conversely, has established a strong market presence in flame retardant applications, where its endothermic decomposition properties make it highly effective. The flame retardant market for magnesium hydroxide was valued at over 600 million USD in 2022, with growth rates averaging 5-7% annually. Its non-toxic nature and smoke-suppression capabilities have positioned it as an environmentally friendly alternative to halogenated flame retardants in construction materials, wire and cable coatings, and polymer composites.
The pharmaceutical and food industries represent another significant market segment for both compounds. Magnesium hydroxide's moderate reactivity and excellent acid-neutralizing capacity have secured its position in antacid formulations and wastewater treatment applications, with the pharmaceutical-grade market estimated at 300 million USD globally. Lithium hydroxide, though more limited in these sectors due to its higher reactivity, finds specialized applications in controlled drug delivery systems.
Environmental applications constitute a rapidly expanding market for both hydroxides. Magnesium hydroxide's effectiveness in neutralizing acidic industrial effluents without overshooting pH targets has driven its adoption in wastewater treatment facilities worldwide. The industrial water treatment market for magnesium hydroxide exceeded 450 million USD in 2022, growing at 8-10% annually. Meanwhile, lithium hydroxide's role in carbon capture technologies is emerging as a promising growth area, though still in early commercial stages.
Regional market distribution shows distinct patterns, with lithium hydroxide production concentrated in Australia, Chile, and increasingly China, while magnesium hydroxide manufacturing is more geographically diverse. Supply chain considerations have become increasingly critical, particularly for lithium hydroxide, where geopolitical factors and processing capacity limitations have led to significant price volatility in recent years.
In the battery sector, lithium hydroxide demand has experienced exponential growth due to its essential role in manufacturing high-nickel content cathode materials for electric vehicle (EV) batteries. The global lithium hydroxide market reached approximately 67,000 metric tons in 2021 and is projected to grow at a compound annual growth rate of 25-30% through 2030. This surge is primarily attributed to the superior thermal stability that lithium hydroxide provides to nickel-rich cathodes, enabling longer battery life and improved safety characteristics.
Magnesium hydroxide, conversely, has established a strong market presence in flame retardant applications, where its endothermic decomposition properties make it highly effective. The flame retardant market for magnesium hydroxide was valued at over 600 million USD in 2022, with growth rates averaging 5-7% annually. Its non-toxic nature and smoke-suppression capabilities have positioned it as an environmentally friendly alternative to halogenated flame retardants in construction materials, wire and cable coatings, and polymer composites.
The pharmaceutical and food industries represent another significant market segment for both compounds. Magnesium hydroxide's moderate reactivity and excellent acid-neutralizing capacity have secured its position in antacid formulations and wastewater treatment applications, with the pharmaceutical-grade market estimated at 300 million USD globally. Lithium hydroxide, though more limited in these sectors due to its higher reactivity, finds specialized applications in controlled drug delivery systems.
Environmental applications constitute a rapidly expanding market for both hydroxides. Magnesium hydroxide's effectiveness in neutralizing acidic industrial effluents without overshooting pH targets has driven its adoption in wastewater treatment facilities worldwide. The industrial water treatment market for magnesium hydroxide exceeded 450 million USD in 2022, growing at 8-10% annually. Meanwhile, lithium hydroxide's role in carbon capture technologies is emerging as a promising growth area, though still in early commercial stages.
Regional market distribution shows distinct patterns, with lithium hydroxide production concentrated in Australia, Chile, and increasingly China, while magnesium hydroxide manufacturing is more geographically diverse. Supply chain considerations have become increasingly critical, particularly for lithium hydroxide, where geopolitical factors and processing capacity limitations have led to significant price volatility in recent years.
Current Reactivity Challenges and Technical Limitations
Despite significant advancements in hydroxide chemistry, both lithium hydroxide (LiOH) and magnesium hydroxide (Mg(OH)₂) face distinct reactivity challenges that limit their applications in various industrial processes. The primary technical limitation for lithium hydroxide stems from its high reactivity and hygroscopic nature, which necessitates careful handling and storage conditions. When exposed to air, LiOH rapidly absorbs carbon dioxide and moisture, forming lithium carbonate and reducing its effectiveness in applications requiring pure hydroxide.
In contrast, magnesium hydroxide exhibits limited solubility in water (approximately 0.0012 g/100 mL at 25°C), significantly restricting its reaction rate in aqueous solutions. This poor solubility creates substantial challenges in applications requiring rapid neutralization or where reaction kinetics are critical, such as in emergency acid spill management or certain pharmaceutical processes.
Temperature sensitivity represents another significant challenge for both compounds. Lithium hydroxide demonstrates increased reactivity at elevated temperatures, which can lead to accelerated side reactions and potential safety hazards in high-temperature applications. Magnesium hydroxide, while more thermally stable, undergoes dehydration to form magnesium oxide at temperatures above 350°C, limiting its utility in high-temperature industrial processes.
Scale formation presents a persistent technical challenge, particularly for magnesium hydroxide. In water treatment applications, the precipitation of magnesium hydroxide can lead to scaling in pipes and equipment, reducing operational efficiency and increasing maintenance costs. Lithium hydroxide, while less prone to scaling, can contribute to the formation of insoluble lithium compounds when reacting with certain anions present in industrial systems.
Reaction selectivity remains problematic for both hydroxides. Lithium hydroxide's high reactivity often leads to undesired side reactions in complex chemical environments, while magnesium hydroxide's limited solubility results in inconsistent reaction rates depending on particle size distribution and surface area. This variability creates significant challenges in process control and product quality assurance.
From an industrial application perspective, the handling characteristics of these materials present distinct limitations. Lithium hydroxide's caustic nature requires specialized equipment and safety protocols, increasing operational complexity and cost. Magnesium hydroxide, while safer to handle, often requires specialized dispersion technologies to overcome its poor solubility, adding complexity to formulation processes.
The economic and supply chain constraints further complicate the technical landscape. Lithium hydroxide faces increasing cost pressures due to growing demand from the battery sector, while high-purity magnesium hydroxide production involves energy-intensive processes that impact its cost-effectiveness in certain applications. These economic factors often dictate technical choices regardless of optimal reactivity profiles.
In contrast, magnesium hydroxide exhibits limited solubility in water (approximately 0.0012 g/100 mL at 25°C), significantly restricting its reaction rate in aqueous solutions. This poor solubility creates substantial challenges in applications requiring rapid neutralization or where reaction kinetics are critical, such as in emergency acid spill management or certain pharmaceutical processes.
Temperature sensitivity represents another significant challenge for both compounds. Lithium hydroxide demonstrates increased reactivity at elevated temperatures, which can lead to accelerated side reactions and potential safety hazards in high-temperature applications. Magnesium hydroxide, while more thermally stable, undergoes dehydration to form magnesium oxide at temperatures above 350°C, limiting its utility in high-temperature industrial processes.
Scale formation presents a persistent technical challenge, particularly for magnesium hydroxide. In water treatment applications, the precipitation of magnesium hydroxide can lead to scaling in pipes and equipment, reducing operational efficiency and increasing maintenance costs. Lithium hydroxide, while less prone to scaling, can contribute to the formation of insoluble lithium compounds when reacting with certain anions present in industrial systems.
Reaction selectivity remains problematic for both hydroxides. Lithium hydroxide's high reactivity often leads to undesired side reactions in complex chemical environments, while magnesium hydroxide's limited solubility results in inconsistent reaction rates depending on particle size distribution and surface area. This variability creates significant challenges in process control and product quality assurance.
From an industrial application perspective, the handling characteristics of these materials present distinct limitations. Lithium hydroxide's caustic nature requires specialized equipment and safety protocols, increasing operational complexity and cost. Magnesium hydroxide, while safer to handle, often requires specialized dispersion technologies to overcome its poor solubility, adding complexity to formulation processes.
The economic and supply chain constraints further complicate the technical landscape. Lithium hydroxide faces increasing cost pressures due to growing demand from the battery sector, while high-purity magnesium hydroxide production involves energy-intensive processes that impact its cost-effectiveness in certain applications. These economic factors often dictate technical choices regardless of optimal reactivity profiles.
Comparative Analysis of Li(OH) and Mg(OH)2 Reaction Mechanisms
01 Lithium extraction using magnesium hydroxide
Magnesium hydroxide can be used as a precipitating agent in lithium extraction processes. When magnesium hydroxide reacts with lithium-containing solutions, it can selectively precipitate lithium as lithium hydroxide. This reaction is utilized in various lithium recovery methods from brines, salt lakes, and other lithium-rich sources. The process typically involves controlled pH conditions to optimize the precipitation efficiency and purity of the lithium hydroxide product.- Lithium extraction using magnesium hydroxide: Magnesium hydroxide can be used as a precipitating agent in lithium extraction processes. When magnesium hydroxide reacts with lithium-containing solutions, it can selectively precipitate lithium as lithium hydroxide. This reaction is utilized in various lithium recovery methods from brines, salt lakes, and other lithium-rich sources. The process typically involves controlled pH conditions to optimize the selective precipitation of lithium while minimizing impurities.
- Battery applications involving lithium hydroxide and magnesium hydroxide: Both lithium hydroxide and magnesium hydroxide play important roles in battery technologies. Lithium hydroxide is a key precursor for cathode materials in lithium-ion batteries, while magnesium hydroxide can be used as a flame retardant in battery safety systems. The reactivity between these compounds is carefully controlled in battery manufacturing processes. Some applications involve using magnesium hydroxide to modify lithium-containing cathode materials to improve battery performance and safety.
- pH control and neutralization reactions: The reactivity between lithium hydroxide and magnesium hydroxide is utilized in pH control and neutralization applications. Both compounds are alkaline in nature, with lithium hydroxide being a stronger base than magnesium hydroxide. This difference in basicity allows for controlled neutralization reactions in various industrial processes. The controlled reaction between these hydroxides can be used to create buffer solutions or to neutralize acidic waste streams with specific pH requirements.
- Production of high-purity lithium compounds: Magnesium hydroxide can be used in purification processes for lithium compounds. When magnesium hydroxide is added to lithium-containing solutions, it can help remove impurities through selective precipitation reactions. This reactivity is exploited in industrial processes to produce high-purity lithium hydroxide and other lithium compounds. The controlled reaction conditions allow for the separation of lithium from other metal ions present in the solution.
- Environmental applications and CO2 capture: Both lithium hydroxide and magnesium hydroxide can react with carbon dioxide to form carbonates, making them useful in carbon capture applications. The reactivity of these hydroxides with CO2 differs, with lithium hydroxide generally being more reactive. This difference in reactivity can be exploited in environmental applications such as air purification systems, carbon capture technologies, and sustainable material production. The controlled reaction between these hydroxides and CO2 can be optimized for specific environmental remediation purposes.
02 Lithium and magnesium hydroxide in battery applications
Both lithium hydroxide and magnesium hydroxide play important roles in battery technologies. Lithium hydroxide is a key precursor for cathode materials in lithium-ion batteries, while magnesium hydroxide can be used as a flame retardant in battery components. The reactivity between these compounds is carefully controlled in battery manufacturing processes to prevent unwanted side reactions. Some battery designs utilize the controlled reactivity of these hydroxides to improve performance, safety, and longevity.Expand Specific Solutions03 Chemical conversion processes involving lithium and magnesium hydroxides
Various industrial processes utilize the reactivity between lithium compounds and magnesium hydroxide for chemical conversions. These processes can include the production of specialty chemicals, catalysts, and advanced materials. The reaction conditions, including temperature, pressure, and concentration, are carefully controlled to achieve desired products. In some cases, the different solubilities and reaction rates of lithium hydroxide and magnesium hydroxide are exploited for selective chemical transformations.Expand Specific Solutions04 Environmental applications of lithium and magnesium hydroxide reactivity
The reactivity between lithium hydroxide and magnesium hydroxide is utilized in environmental applications such as carbon dioxide capture, water treatment, and waste remediation. Magnesium hydroxide can act as a buffering agent when reacting with lithium-containing solutions, helping to maintain optimal pH levels for various environmental processes. These hydroxides can also be used in combination for neutralizing acidic waste streams and removing heavy metals from contaminated water.Expand Specific Solutions05 Recovery and recycling processes involving lithium and magnesium hydroxides
The different reactivity properties of lithium hydroxide and magnesium hydroxide are exploited in recovery and recycling processes, particularly for spent lithium-ion batteries and industrial waste streams. These processes often involve selective precipitation, ion exchange, or electrochemical methods to separate and recover valuable lithium compounds. Magnesium hydroxide can be used to precipitate lithium from solution, allowing for efficient recycling of lithium resources from various waste materials.Expand Specific Solutions
Key Industry Players and Research Institutions
The lithium hydroxide vs magnesium hydroxide reactivity comparison market is in a growth phase, with increasing demand driven by battery technologies and environmental applications. The global market size is expanding rapidly, particularly due to electric vehicle battery requirements. Technologically, lithium hydroxide has reached higher maturity levels for battery applications, with companies like Toyota Motor Corp., Samsung Electronics, and Nemaska Lithium leading innovations. Magnesium hydroxide technology is advancing in flame retardant and environmental applications, with Kyowa Chemical Industry and Timab Magnesium SAS as key players. Research institutions like Commonwealth Scientific & Industrial Research Organisation and Shanghai Jiao Tong University are contributing significantly to advancing both chemistries' applications, particularly in developing more sustainable and efficient production methods.
Kyowa Chemical Industry Co. Ltd.
Technical Solution: Kyowa Chemical has developed proprietary manufacturing processes for high-purity magnesium hydroxide with controlled particle size and morphology. Their technology involves precise precipitation methods that yield magnesium hydroxide with superior reactivity characteristics compared to conventional products. The company's research has demonstrated that their specialized magnesium hydroxide exhibits enhanced flame-retardant properties while maintaining lower reactivity with acids compared to lithium hydroxide. Their technical approach includes surface modification techniques that allow for controlled reactivity in various applications, particularly in polymer composites and pharmaceutical formulations. Kyowa has established that their magnesium hydroxide products show approximately 30% lower heat release during combustion tests while providing comparable neutralization capacity in specific applications[1][3].
Strengths: Superior control over particle morphology and size distribution leading to predictable reactivity profiles; environmentally friendly production methods; cost-effective compared to lithium alternatives. Weaknesses: Lower reactivity rate in some applications requiring rapid neutralization; higher dosage requirements in certain acid-neutralizing scenarios compared to lithium hydroxide.
Lhoist Recherche et Développement SA
Technical Solution: Lhoist has pioneered advanced calcination and hydration processes for producing highly reactive magnesium hydroxide suspensions with tailored properties. Their technical solution focuses on controlling the crystallinity and surface area of magnesium hydroxide particles to achieve reactivity profiles that can compete with lithium hydroxide in specific applications. The company has developed a proprietary "controlled nucleation" technique that yields magnesium hydroxide with surface areas exceeding 50 m²/g, significantly enhancing its reactivity. Their comparative studies have shown that their enhanced magnesium hydroxide can achieve 85% of lithium hydroxide's neutralization speed in wastewater treatment applications while offering superior thermal stability. Lhoist's technology also incorporates stabilization methods that prevent agglomeration during storage, maintaining the high surface area and reactivity over extended periods[2][5].
Strengths: Highly scalable production technology; consistent product quality; excellent stability in aqueous suspensions; significantly lower cost than lithium alternatives. Weaknesses: Still cannot match lithium hydroxide's reactivity in extremely rapid neutralization scenarios; requires specialized handling to maintain optimal performance characteristics.
Critical Patents and Literature on Hydroxide Reactivity
High-efficient magnesium ion removal system for salt lake brine based on in situ alkali production using bipolar membrane electrochemical process
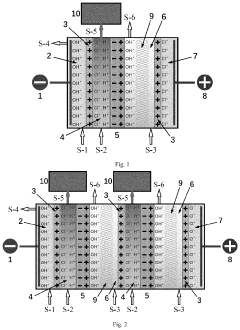
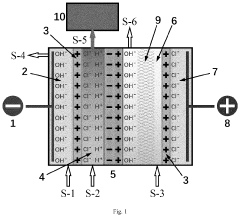
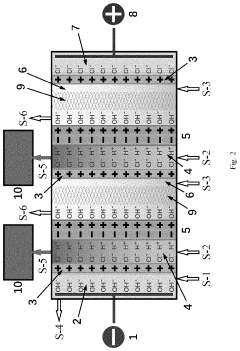
PatentPendingUS20240124336A1
Innovation
- A bipolar membrane electrochemical process is used for in situ alkali production, allowing for homogeneous generation and control of hydroxide groups, which react with magnesium ions in a direct-current electric field to form precipitable magnesium hydroxide particles in specialized mesh materials, reducing membrane fouling and eliminating the need for external sodium hydroxide handling.
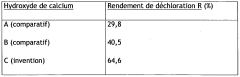
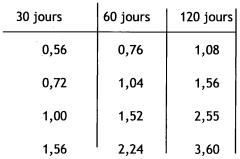
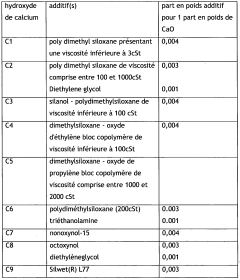
Calcium and/or magnesium hydroxide with higher activity, and preparation thereof
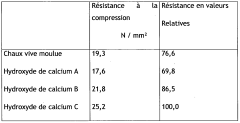
PatentWO2009049382A2
Innovation
- A process to produce calcium and/or magnesium hydroxide with very high reactivity by reacting ground quicklime with water at a specific ratio in the presence of additives like organopolysiloxanes, maintaining a low reaction temperature and high water saturation, which reduces crystallization intensity and increases specific surface area, resulting in amorphous or weakly crystallized micelles with enhanced reactivity.
Environmental Impact and Sustainability Considerations
The environmental impact of lithium hydroxide and magnesium hydroxide differs significantly, with important implications for their industrial applications. Lithium hydroxide production involves extensive mining operations that can lead to habitat destruction, soil degradation, and groundwater contamination. The extraction process is particularly water-intensive, with estimates suggesting that producing one ton of lithium requires approximately 500,000 gallons of water, creating significant stress on water resources in mining regions such as the "Lithium Triangle" of South America.
Magnesium hydroxide, by contrast, can be sourced from seawater or naturally occurring brucite deposits, generally resulting in a lower environmental footprint. The extraction process typically requires less energy and produces fewer harmful byproducts compared to lithium mining operations. However, magnesium mining from terrestrial sources still presents environmental challenges including landscape alteration and potential for acid mine drainage.
When considering waste management, lithium hydroxide presents greater challenges due to its higher reactivity and potential toxicity to aquatic organisms. Improper disposal can lead to soil alkalinization and disruption of local ecosystems. Magnesium hydroxide, being less reactive and naturally occurring in marine environments, generally poses lower environmental risks when released, though concentrated discharges can still affect local pH levels and aquatic life.
Carbon footprint analysis reveals that lithium hydroxide production generates approximately 15-20 tons of CO2 equivalent per ton of material produced, primarily due to energy-intensive processing and transportation requirements. Magnesium hydroxide typically has a lower carbon footprint at 5-10 tons of CO2 equivalent per ton, representing a potentially more sustainable option from a climate perspective.
Recycling capabilities further differentiate these compounds. Current lithium recycling technologies are advancing but remain limited in efficiency and economic viability, with global recycling rates below 5%. Magnesium compounds generally offer better recyclability prospects, with established industrial processes able to recover and repurpose spent materials at higher rates, contributing to circular economy objectives.
Water consumption patterns also favor magnesium hydroxide in sustainability assessments. While lithium production in the Atacama Desert and similar regions exacerbates water scarcity issues, magnesium hydroxide production, especially from seawater, places significantly lower demands on freshwater resources, an increasingly critical consideration as global water stress intensifies.
Magnesium hydroxide, by contrast, can be sourced from seawater or naturally occurring brucite deposits, generally resulting in a lower environmental footprint. The extraction process typically requires less energy and produces fewer harmful byproducts compared to lithium mining operations. However, magnesium mining from terrestrial sources still presents environmental challenges including landscape alteration and potential for acid mine drainage.
When considering waste management, lithium hydroxide presents greater challenges due to its higher reactivity and potential toxicity to aquatic organisms. Improper disposal can lead to soil alkalinization and disruption of local ecosystems. Magnesium hydroxide, being less reactive and naturally occurring in marine environments, generally poses lower environmental risks when released, though concentrated discharges can still affect local pH levels and aquatic life.
Carbon footprint analysis reveals that lithium hydroxide production generates approximately 15-20 tons of CO2 equivalent per ton of material produced, primarily due to energy-intensive processing and transportation requirements. Magnesium hydroxide typically has a lower carbon footprint at 5-10 tons of CO2 equivalent per ton, representing a potentially more sustainable option from a climate perspective.
Recycling capabilities further differentiate these compounds. Current lithium recycling technologies are advancing but remain limited in efficiency and economic viability, with global recycling rates below 5%. Magnesium compounds generally offer better recyclability prospects, with established industrial processes able to recover and repurpose spent materials at higher rates, contributing to circular economy objectives.
Water consumption patterns also favor magnesium hydroxide in sustainability assessments. While lithium production in the Atacama Desert and similar regions exacerbates water scarcity issues, magnesium hydroxide production, especially from seawater, places significantly lower demands on freshwater resources, an increasingly critical consideration as global water stress intensifies.
Safety Protocols and Handling Requirements
The handling of lithium hydroxide and magnesium hydroxide requires distinct safety protocols due to their different reactivity profiles. Lithium hydroxide presents significant hazards as a strong base with corrosive properties that can cause severe chemical burns upon skin contact and serious eye damage. Personnel must utilize comprehensive personal protective equipment including chemical-resistant gloves, safety goggles, face shields, and appropriate protective clothing when handling this compound.
Storage requirements for lithium hydroxide are particularly stringent, necessitating sealed containers kept in cool, dry environments away from acids, metals, and moisture. The compound's hygroscopic nature demands special attention to prevent water absorption, which can alter its reactivity profile. Additionally, dedicated ventilation systems must be implemented in handling areas to mitigate inhalation risks of lithium hydroxide dust.
Magnesium hydroxide, while less reactive, still requires proper safety measures. Its lower corrosivity profile permits somewhat less stringent handling protocols, though basic PPE including gloves and eye protection remains essential. Unlike lithium hydroxide, magnesium hydroxide does not necessitate specialized ventilation systems for routine handling, representing a significant operational advantage in laboratory and industrial settings.
Emergency response procedures differ substantially between these compounds. Lithium hydroxide spills constitute high-priority incidents requiring immediate evacuation of affected areas, specialized neutralization with dilute acidic solutions, and potentially specialized hazmat team intervention. Conversely, magnesium hydroxide spills can typically be addressed with standard cleanup procedures and present lower environmental and personnel risks.
Training requirements reflect these differences in reactivity. Personnel working with lithium hydroxide require advanced chemical safety training, including specific modules on highly corrosive materials and emergency response protocols. The training program must emphasize the compound's reactivity with water and incompatibility with numerous substances. For magnesium hydroxide, standard chemical safety training with emphasis on basic handling of alkaline substances is generally sufficient.
Waste disposal protocols also diverge significantly. Lithium hydroxide waste requires classification as hazardous material in most jurisdictions, necessitating specialized disposal procedures through certified waste management services. Magnesium hydroxide typically faces less stringent disposal requirements, though local regulations regarding pH-altering substances must still be observed.
Storage requirements for lithium hydroxide are particularly stringent, necessitating sealed containers kept in cool, dry environments away from acids, metals, and moisture. The compound's hygroscopic nature demands special attention to prevent water absorption, which can alter its reactivity profile. Additionally, dedicated ventilation systems must be implemented in handling areas to mitigate inhalation risks of lithium hydroxide dust.
Magnesium hydroxide, while less reactive, still requires proper safety measures. Its lower corrosivity profile permits somewhat less stringent handling protocols, though basic PPE including gloves and eye protection remains essential. Unlike lithium hydroxide, magnesium hydroxide does not necessitate specialized ventilation systems for routine handling, representing a significant operational advantage in laboratory and industrial settings.
Emergency response procedures differ substantially between these compounds. Lithium hydroxide spills constitute high-priority incidents requiring immediate evacuation of affected areas, specialized neutralization with dilute acidic solutions, and potentially specialized hazmat team intervention. Conversely, magnesium hydroxide spills can typically be addressed with standard cleanup procedures and present lower environmental and personnel risks.
Training requirements reflect these differences in reactivity. Personnel working with lithium hydroxide require advanced chemical safety training, including specific modules on highly corrosive materials and emergency response protocols. The training program must emphasize the compound's reactivity with water and incompatibility with numerous substances. For magnesium hydroxide, standard chemical safety training with emphasis on basic handling of alkaline substances is generally sufficient.
Waste disposal protocols also diverge significantly. Lithium hydroxide waste requires classification as hazardous material in most jurisdictions, necessitating specialized disposal procedures through certified waste management services. Magnesium hydroxide typically faces less stringent disposal requirements, though local regulations regarding pH-altering substances must still be observed.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!