How To Optimize Lithium Hydroxide's Role In pH Adjustment Processes
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide pH Adjustment Technology Background
Lithium hydroxide (LiOH) has emerged as a significant chemical compound in pH adjustment processes across various industries over the past several decades. Initially gaining prominence in the 1950s primarily for specialized applications in aerospace and nuclear industries, lithium hydroxide has evolved to become an increasingly important alkaline agent in contemporary industrial processes.
The compound's unique properties distinguish it from other commonly used bases such as sodium hydroxide (NaOH) and potassium hydroxide (KOH). Lithium hydroxide offers a higher alkalinity per unit mass, making it particularly efficient in applications where weight considerations are critical. Additionally, its lower solubility compared to other alkali metal hydroxides provides more controlled release characteristics in certain pH adjustment scenarios.
From a chemical perspective, lithium hydroxide dissociates in water to form lithium ions (Li⁺) and hydroxide ions (OH⁻), with the latter being responsible for increasing pH levels. The compound's relatively high pKb value of approximately 0.18 indicates its strong basic nature, allowing for effective neutralization of acidic solutions even at lower concentrations.
The technological evolution of lithium hydroxide in pH adjustment has been marked by significant improvements in production methods, purity levels, and application techniques. Early production relied primarily on the electrolysis of lithium chloride solutions, while modern methods incorporate more efficient processes including the reaction of lithium carbonate with calcium hydroxide, yielding higher purity products suitable for sensitive applications.
Recent advancements have focused on developing specialized formulations of lithium hydroxide for specific pH adjustment requirements. These include monohydrate and anhydrous forms, each offering distinct advantages depending on the application environment. The monohydrate form (LiOH·H₂O) provides more stable handling characteristics, while the anhydrous form offers higher hydroxide concentration per unit mass.
The growing importance of lithium-ion battery technology has significantly influenced lithium hydroxide production and availability, creating both challenges and opportunities for its application in pH adjustment processes. As battery manufacturing demands increase, production capacity has expanded, potentially benefiting other application areas through economies of scale and technological spillovers.
Environmental considerations have also shaped the technological trajectory of lithium hydroxide in pH adjustment applications. Research has increasingly focused on minimizing environmental impacts through more efficient dosing strategies, recovery methods, and the development of closed-loop systems that reduce waste and maximize resource utilization.
The compound's unique properties distinguish it from other commonly used bases such as sodium hydroxide (NaOH) and potassium hydroxide (KOH). Lithium hydroxide offers a higher alkalinity per unit mass, making it particularly efficient in applications where weight considerations are critical. Additionally, its lower solubility compared to other alkali metal hydroxides provides more controlled release characteristics in certain pH adjustment scenarios.
From a chemical perspective, lithium hydroxide dissociates in water to form lithium ions (Li⁺) and hydroxide ions (OH⁻), with the latter being responsible for increasing pH levels. The compound's relatively high pKb value of approximately 0.18 indicates its strong basic nature, allowing for effective neutralization of acidic solutions even at lower concentrations.
The technological evolution of lithium hydroxide in pH adjustment has been marked by significant improvements in production methods, purity levels, and application techniques. Early production relied primarily on the electrolysis of lithium chloride solutions, while modern methods incorporate more efficient processes including the reaction of lithium carbonate with calcium hydroxide, yielding higher purity products suitable for sensitive applications.
Recent advancements have focused on developing specialized formulations of lithium hydroxide for specific pH adjustment requirements. These include monohydrate and anhydrous forms, each offering distinct advantages depending on the application environment. The monohydrate form (LiOH·H₂O) provides more stable handling characteristics, while the anhydrous form offers higher hydroxide concentration per unit mass.
The growing importance of lithium-ion battery technology has significantly influenced lithium hydroxide production and availability, creating both challenges and opportunities for its application in pH adjustment processes. As battery manufacturing demands increase, production capacity has expanded, potentially benefiting other application areas through economies of scale and technological spillovers.
Environmental considerations have also shaped the technological trajectory of lithium hydroxide in pH adjustment applications. Research has increasingly focused on minimizing environmental impacts through more efficient dosing strategies, recovery methods, and the development of closed-loop systems that reduce waste and maximize resource utilization.
Market Analysis for Lithium Hydroxide in pH Control Applications
The global market for lithium hydroxide in pH control applications has been experiencing steady growth, driven by increasing industrial applications across multiple sectors. The compound's unique properties make it particularly valuable for precise pH adjustment in environments where sodium or potassium alternatives may be unsuitable. Current market valuation for lithium hydroxide in pH adjustment applications stands at approximately $320 million, with projections indicating a compound annual growth rate of 4.7% through 2028.
Water treatment represents the largest application segment, accounting for nearly 38% of market share. The pharmaceutical industry follows at 27%, where lithium hydroxide's high purity grades are essential for maintaining strict pH parameters in drug formulation processes. Food processing applications constitute about 18% of the market, while specialty chemical manufacturing accounts for 12%. The remaining market share is distributed among various niche applications including cosmetics and electronics manufacturing.
Regionally, North America dominates the market with approximately 35% share, followed by Europe (28%) and Asia-Pacific (25%). The Asia-Pacific region, however, is demonstrating the fastest growth rate at 6.3% annually, primarily driven by expanding industrial activities in China and India. Latin America, despite being a major lithium producer, currently represents only 8% of consumption in pH control applications, indicating potential for future market development.
Demand drivers include increasingly stringent water quality regulations worldwide, growing pharmaceutical manufacturing, and the expansion of food processing industries in developing economies. The compound's effectiveness in precise pH control, particularly in applications requiring minimal sodium content, positions it favorably against alternatives like sodium hydroxide or calcium hydroxide in specific high-value applications.
Price sensitivity remains a significant market factor, with lithium hydroxide commanding a premium over traditional pH adjustment chemicals. This has limited penetration in cost-sensitive bulk applications but has created defensible market positions in high-value, precision-critical processes. Recent supply chain disruptions in the broader lithium market have introduced price volatility, with average prices increasing by 22% between 2020 and 2022.
Market segmentation by grade shows technical grade lithium hydroxide dominating industrial pH applications (68%), while pharmaceutical and food-grade materials command higher margins but represent smaller volume segments. The emergence of battery-grade lithium hydroxide production has created competition for refined material, occasionally impacting availability for pH control applications during supply constraints.
Customer purchasing patterns indicate a trend toward long-term supply agreements to mitigate price volatility, particularly among pharmaceutical and specialty chemical manufacturers where process validation requirements make switching costs high.
Water treatment represents the largest application segment, accounting for nearly 38% of market share. The pharmaceutical industry follows at 27%, where lithium hydroxide's high purity grades are essential for maintaining strict pH parameters in drug formulation processes. Food processing applications constitute about 18% of the market, while specialty chemical manufacturing accounts for 12%. The remaining market share is distributed among various niche applications including cosmetics and electronics manufacturing.
Regionally, North America dominates the market with approximately 35% share, followed by Europe (28%) and Asia-Pacific (25%). The Asia-Pacific region, however, is demonstrating the fastest growth rate at 6.3% annually, primarily driven by expanding industrial activities in China and India. Latin America, despite being a major lithium producer, currently represents only 8% of consumption in pH control applications, indicating potential for future market development.
Demand drivers include increasingly stringent water quality regulations worldwide, growing pharmaceutical manufacturing, and the expansion of food processing industries in developing economies. The compound's effectiveness in precise pH control, particularly in applications requiring minimal sodium content, positions it favorably against alternatives like sodium hydroxide or calcium hydroxide in specific high-value applications.
Price sensitivity remains a significant market factor, with lithium hydroxide commanding a premium over traditional pH adjustment chemicals. This has limited penetration in cost-sensitive bulk applications but has created defensible market positions in high-value, precision-critical processes. Recent supply chain disruptions in the broader lithium market have introduced price volatility, with average prices increasing by 22% between 2020 and 2022.
Market segmentation by grade shows technical grade lithium hydroxide dominating industrial pH applications (68%), while pharmaceutical and food-grade materials command higher margins but represent smaller volume segments. The emergence of battery-grade lithium hydroxide production has created competition for refined material, occasionally impacting availability for pH control applications during supply constraints.
Customer purchasing patterns indicate a trend toward long-term supply agreements to mitigate price volatility, particularly among pharmaceutical and specialty chemical manufacturers where process validation requirements make switching costs high.
Technical Challenges in Lithium Hydroxide pH Adjustment
Despite its effectiveness as a pH adjustment agent, lithium hydroxide presents several significant technical challenges in practical applications. The primary issue stems from its relatively high cost compared to alternative alkaline agents such as sodium hydroxide or calcium hydroxide. This economic constraint often limits its widespread adoption in industrial settings where large volumes of pH adjustment chemicals are required, despite its superior performance characteristics.
The handling of lithium hydroxide poses considerable safety concerns due to its highly caustic nature. It can cause severe chemical burns upon skin contact and presents serious respiratory hazards if inhaled as dust. These safety risks necessitate specialized handling protocols, personal protective equipment, and storage facilities, adding complexity and cost to operational procedures.
Solubility limitations represent another technical hurdle. While lithium hydroxide is more soluble than many other metal hydroxides, its solubility is still relatively limited at approximately 12.8 g/100 mL at 20°C. This constraint affects dosing strategies and may require more sophisticated delivery systems to achieve precise pH control in certain applications.
The reactivity of lithium hydroxide with atmospheric carbon dioxide presents a significant challenge for long-term storage and handling. When exposed to air, lithium hydroxide readily absorbs CO2, forming lithium carbonate, which alters its chemical properties and reduces its effectiveness as a pH adjustment agent. This necessitates sealed storage systems and careful handling protocols to maintain product integrity.
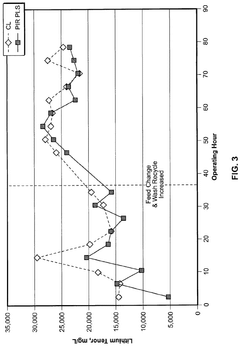
Dosing precision represents a critical technical challenge, particularly in applications requiring tight pH control. The high alkalinity of lithium hydroxide means that small variations in dosage can result in significant pH fluctuations, potentially compromising process outcomes or product quality. This necessitates sophisticated monitoring and control systems to maintain optimal pH levels.
The environmental impact of lithium compounds has emerged as an increasing concern. The growing demand for lithium in battery applications has heightened awareness of its environmental footprint, including water usage in extraction processes and potential ecological impacts. This creates additional pressure to optimize lithium hydroxide usage and develop recovery systems to minimize waste.
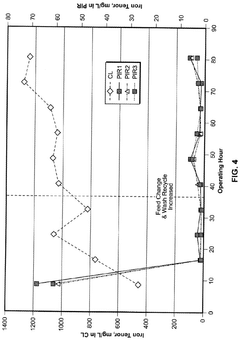
Compatibility issues with other process chemicals or materials can also arise, particularly in complex industrial processes where multiple chemical interactions occur simultaneously. Lithium hydroxide may form precipitates or unwanted byproducts with certain compounds, potentially affecting process efficiency or product quality.
The handling of lithium hydroxide poses considerable safety concerns due to its highly caustic nature. It can cause severe chemical burns upon skin contact and presents serious respiratory hazards if inhaled as dust. These safety risks necessitate specialized handling protocols, personal protective equipment, and storage facilities, adding complexity and cost to operational procedures.
Solubility limitations represent another technical hurdle. While lithium hydroxide is more soluble than many other metal hydroxides, its solubility is still relatively limited at approximately 12.8 g/100 mL at 20°C. This constraint affects dosing strategies and may require more sophisticated delivery systems to achieve precise pH control in certain applications.
The reactivity of lithium hydroxide with atmospheric carbon dioxide presents a significant challenge for long-term storage and handling. When exposed to air, lithium hydroxide readily absorbs CO2, forming lithium carbonate, which alters its chemical properties and reduces its effectiveness as a pH adjustment agent. This necessitates sealed storage systems and careful handling protocols to maintain product integrity.
Dosing precision represents a critical technical challenge, particularly in applications requiring tight pH control. The high alkalinity of lithium hydroxide means that small variations in dosage can result in significant pH fluctuations, potentially compromising process outcomes or product quality. This necessitates sophisticated monitoring and control systems to maintain optimal pH levels.
The environmental impact of lithium compounds has emerged as an increasing concern. The growing demand for lithium in battery applications has heightened awareness of its environmental footprint, including water usage in extraction processes and potential ecological impacts. This creates additional pressure to optimize lithium hydroxide usage and develop recovery systems to minimize waste.
Compatibility issues with other process chemicals or materials can also arise, particularly in complex industrial processes where multiple chemical interactions occur simultaneously. Lithium hydroxide may form precipitates or unwanted byproducts with certain compounds, potentially affecting process efficiency or product quality.
Current pH Adjustment Solutions Using Lithium Hydroxide
01 Lithium hydroxide for pH adjustment in battery electrolytes
Lithium hydroxide is used as a pH adjusting agent in battery electrolyte formulations to optimize battery performance and stability. By controlling the pH level of the electrolyte solution, lithium hydroxide helps to prevent unwanted side reactions, extend battery life, and improve overall efficiency. This approach is particularly important in lithium-ion batteries where maintaining proper pH levels can significantly impact the electrochemical processes and prevent degradation of electrode materials.- Lithium hydroxide for pH adjustment in lithium extraction processes: Lithium hydroxide is used as a pH adjustment agent in various lithium extraction and processing methods. By controlling the pH level with lithium hydroxide, the efficiency of lithium extraction from brines, ores, or other sources can be improved. The pH adjustment helps in selective precipitation, ion exchange, or adsorption processes, enhancing the purity and recovery rate of lithium compounds.
- pH control in lithium battery manufacturing: In lithium battery manufacturing processes, lithium hydroxide is utilized for pH adjustment to optimize electrode material synthesis and electrolyte preparation. Controlling the pH with lithium hydroxide affects the crystallization, particle size, and morphology of cathode materials, which directly impacts battery performance characteristics such as capacity, cycling stability, and rate capability.
- Lithium hydroxide in environmental applications: Lithium hydroxide is employed for pH adjustment in environmental applications such as carbon capture, water treatment, and soil remediation. The alkaline properties of lithium hydroxide help neutralize acidic compounds, precipitate heavy metals, or enhance the absorption of carbon dioxide. These applications leverage the high alkalinity and specific chemical properties of lithium hydroxide compared to other basic compounds.
- Precise pH control systems using lithium hydroxide: Advanced systems and methods have been developed for precise pH control using lithium hydroxide in various industrial processes. These systems may include automated dosing equipment, real-time pH monitoring, feedback control mechanisms, and specialized mixing technologies to ensure optimal pH levels are maintained. The precision of pH adjustment is critical in applications requiring tight control of reaction conditions or product specifications.
- Lithium hydroxide in combination with other pH adjusters: Lithium hydroxide is often used in combination with other pH adjusting agents to achieve specific pH profiles or buffering effects. These combinations may include other alkali metal hydroxides, carbonates, or organic bases that work synergistically with lithium hydroxide. The selection of complementary pH adjusters depends on the specific application requirements, such as solubility constraints, temperature stability, or ion interference considerations.
02 pH adjustment in lithium extraction processes
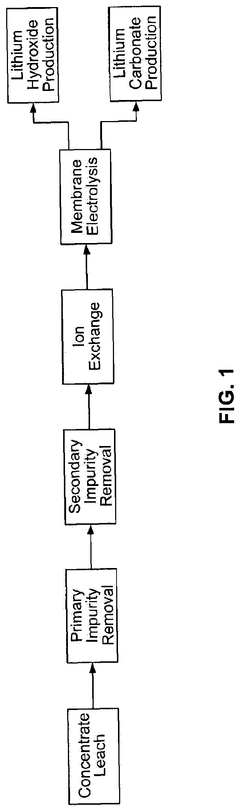
Lithium hydroxide is utilized for pH adjustment in various lithium extraction and recovery processes from brines, ores, and other sources. The controlled addition of lithium hydroxide helps to maintain optimal pH conditions during precipitation, crystallization, and separation stages. This pH adjustment is critical for maximizing lithium recovery rates, improving purity of the extracted lithium compounds, and enhancing the efficiency of the overall extraction process.Expand Specific Solutions03 pH control in lithium hydroxide production methods
Various production methods for lithium hydroxide incorporate pH adjustment steps to control reaction conditions and product quality. The precise control of pH during manufacturing processes helps to optimize yield, purity, and crystal morphology of the lithium hydroxide product. These methods often involve careful monitoring and adjustment of pH at different stages of the production process, including precipitation, crystallization, and purification steps.Expand Specific Solutions04 Lithium hydroxide for pH adjustment in industrial applications
Beyond battery applications, lithium hydroxide is used as a pH adjusting agent in various industrial processes including water treatment, lubricant formulation, and chemical synthesis. Its strong alkaline properties make it effective for neutralizing acidic solutions and maintaining desired pH levels in manufacturing processes. The controlled use of lithium hydroxide for pH adjustment can improve process efficiency, product quality, and reduce corrosion in industrial equipment.Expand Specific Solutions05 pH monitoring and control systems for lithium processes
Advanced monitoring and control systems are employed to precisely manage pH levels in processes involving lithium hydroxide. These systems utilize sensors, automated dosing equipment, and feedback control mechanisms to maintain optimal pH conditions. Real-time pH monitoring allows for immediate adjustments to lithium hydroxide addition rates, ensuring consistent process conditions and product quality while minimizing chemical consumption and environmental impact.Expand Specific Solutions
Key Industry Players in Lithium Hydroxide Production
The lithium hydroxide pH adjustment market is currently in a growth phase, with increasing demand driven by battery manufacturing, water treatment, and industrial applications. The market size is expanding rapidly, projected to reach significant value by 2030 due to electric vehicle proliferation. Technologically, companies like Nemaska Lithium, BASF, and Toyota are advancing optimization techniques, while Lilac Solutions and Guangdong Bangpu are developing innovative extraction and recycling methods. Asian players including Sumitomo Metal Mining, Tinci Materials, and Samsung Electronics are focusing on high-purity production processes. The competitive landscape features established chemical companies alongside specialized lithium producers, with increasing emphasis on sustainable production methods and process efficiency to meet growing industrial demands.
Nemaska Lithium, Inc.
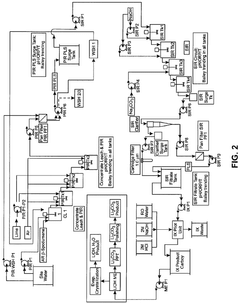
Technical Solution: Nemaska Lithium has developed a proprietary process for optimizing lithium hydroxide's role in pH adjustment through their patented electrolysis method. Their approach involves producing high-purity lithium hydroxide directly from spodumene concentrate using a combination of hydrometallurgical and electrolysis techniques. The process carefully controls the electrolysis parameters to produce lithium hydroxide with precise alkalinity profiles, allowing for more accurate pH adjustment in various applications. Their technology includes a membrane-based electrolysis system that minimizes impurities and ensures consistent concentration levels, which is critical for precise pH control. The company has also implemented advanced process control systems that monitor and adjust the production parameters in real-time to maintain optimal lithium hydroxide quality for pH adjustment applications.
Strengths: Produces exceptionally high-purity lithium hydroxide (>99.5%) with consistent alkalinity profiles, enabling precise pH control in sensitive applications. Their direct production method reduces processing steps and associated contamination risks. Weaknesses: The electrolysis process requires significant energy input, potentially increasing operational costs compared to traditional methods. The technology also requires specialized equipment and expertise to maintain optimal production conditions.
Eurodia Industrie SA
Technical Solution: Eurodia Industrie SA has pioneered advanced membrane-based separation technologies for optimizing lithium hydroxide in pH adjustment processes. Their approach centers on electrodialysis with bipolar membranes (EDBM) that enable precise control over lithium hydroxide concentration and purity. The technology separates lithium salts into highly pure lithium hydroxide through a series of ion-selective membranes, allowing for customized alkalinity profiles tailored to specific pH adjustment requirements. Eurodia's system incorporates sophisticated monitoring equipment that continuously analyzes solution conductivity, pH, and ion concentration to maintain optimal lithium hydroxide quality. Their process includes proprietary membrane configurations that minimize energy consumption while maximizing lithium hydroxide yield and purity. Additionally, Eurodia has developed specialized cleaning protocols that extend membrane life and maintain separation efficiency, ensuring consistent lithium hydroxide performance in pH adjustment applications across various industries including pharmaceuticals, food processing, and water treatment.
Strengths: Their membrane technology produces exceptionally pure lithium hydroxide with minimal contaminants, ideal for sensitive pH adjustment applications. The system offers excellent energy efficiency compared to traditional electrolysis methods. Weaknesses: The membrane systems require careful maintenance and periodic replacement, potentially increasing operational complexity. The technology demands specialized technical expertise for optimal operation and troubleshooting.
Technical Innovations in Lithium Hydroxide Application
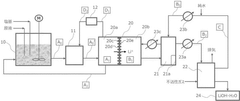
Processes for preparing lithium hydroxide
PatentPendingEP4424408A9
Innovation
- A process involving electrolysis or electrodialysis of an aqueous lithium compound composition, maintaining a pH of about 1 to 4, and subsequent steps such as leaching, precipitation, and ion exchange to produce high-purity lithium hydroxide, reducing impurity content and optimizing production efficiency.
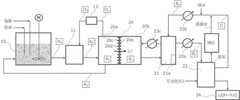
Method for producing lithium hydroxide
PatentWO2022203055A1
Innovation
- A method involving a two-stage pH adjustment process in mixing lithium-containing aqueous solutions with a base, followed by lithium ion extraction using an electrochemical device with a Li selectively permeable membrane, and subsequent concentration and separation of lithium ions through adsorption and crystallization, allowing for the production of high-purity lithium hydroxide from diverse aqueous solutions.
Environmental Impact Assessment
The environmental implications of lithium hydroxide usage in pH adjustment processes warrant careful consideration, particularly as industrial applications expand. Lithium hydroxide's production involves significant resource extraction, primarily from salt flats and hard rock mining operations, which can lead to habitat disruption and biodiversity loss in sensitive ecosystems. These extraction processes typically consume substantial water resources—approximately 500,000 gallons per ton of lithium—creating potential conflicts in water-scarce regions where lithium deposits are often located.
Energy consumption represents another critical environmental concern, as lithium hydroxide production requires approximately 15-20 MWh of electricity per ton produced. The carbon footprint varies significantly depending on the energy sources utilized, with renewable-powered operations demonstrating up to 30% lower emissions compared to fossil fuel-dependent production facilities.
Wastewater management presents ongoing challenges, as lithium hydroxide processing generates effluent containing various contaminants including dissolved metals, sulfates, and process chemicals. Advanced treatment systems can mitigate these impacts, with reverse osmosis and ion exchange technologies achieving removal efficiencies exceeding 95% for most contaminants, though at considerable operational cost.
The optimization of lithium hydroxide in pH adjustment processes offers several environmental benefits when properly implemented. Compared to traditional alkaline agents like sodium hydroxide, lithium hydroxide's higher efficiency means smaller quantities are required to achieve equivalent pH adjustments, potentially reducing transportation emissions by 15-25% and decreasing overall chemical usage. Additionally, its precise pH control capabilities can minimize chemical waste from overcorrection scenarios.
End-of-life considerations must address the fate of lithium-containing waste streams. Recovery technologies have advanced significantly, with hydrometallurgical processes now capable of reclaiming up to 90% of lithium from industrial waste streams for reuse. This circular approach substantially reduces the need for virgin material extraction while minimizing disposal impacts.
Regulatory frameworks governing lithium hydroxide usage continue to evolve globally, with particular attention to water quality standards and waste classification. The implementation of life cycle assessment methodologies has become increasingly important for industrial users seeking to quantify and minimize environmental footprints across the entire value chain, from production through application to ultimate disposal or recovery.
Energy consumption represents another critical environmental concern, as lithium hydroxide production requires approximately 15-20 MWh of electricity per ton produced. The carbon footprint varies significantly depending on the energy sources utilized, with renewable-powered operations demonstrating up to 30% lower emissions compared to fossil fuel-dependent production facilities.
Wastewater management presents ongoing challenges, as lithium hydroxide processing generates effluent containing various contaminants including dissolved metals, sulfates, and process chemicals. Advanced treatment systems can mitigate these impacts, with reverse osmosis and ion exchange technologies achieving removal efficiencies exceeding 95% for most contaminants, though at considerable operational cost.
The optimization of lithium hydroxide in pH adjustment processes offers several environmental benefits when properly implemented. Compared to traditional alkaline agents like sodium hydroxide, lithium hydroxide's higher efficiency means smaller quantities are required to achieve equivalent pH adjustments, potentially reducing transportation emissions by 15-25% and decreasing overall chemical usage. Additionally, its precise pH control capabilities can minimize chemical waste from overcorrection scenarios.
End-of-life considerations must address the fate of lithium-containing waste streams. Recovery technologies have advanced significantly, with hydrometallurgical processes now capable of reclaiming up to 90% of lithium from industrial waste streams for reuse. This circular approach substantially reduces the need for virgin material extraction while minimizing disposal impacts.
Regulatory frameworks governing lithium hydroxide usage continue to evolve globally, with particular attention to water quality standards and waste classification. The implementation of life cycle assessment methodologies has become increasingly important for industrial users seeking to quantify and minimize environmental footprints across the entire value chain, from production through application to ultimate disposal or recovery.
Cost-Benefit Analysis
The economic implications of lithium hydroxide in pH adjustment processes reveal a complex interplay between direct costs and indirect benefits. Initial procurement expenses for lithium hydroxide significantly exceed those of traditional alkaline agents like sodium hydroxide and calcium hydroxide, with market prices ranging from 5-10 times higher depending on purity requirements and market conditions. This substantial cost differential presents an immediate financial barrier to widespread adoption in cost-sensitive industrial applications.
However, a comprehensive cost-benefit analysis must consider the operational efficiencies gained through lithium hydroxide implementation. The superior solubility characteristics of lithium hydroxide translate to reduced dosing requirements, typically 15-25% less volume compared to sodium hydroxide for equivalent pH adjustment. This volumetric efficiency reduces storage requirements, transportation costs, and handling expenses over extended operational periods.
Equipment longevity represents another significant economic advantage. Industrial systems utilizing lithium hydroxide for pH adjustment demonstrate reduced scaling and corrosion rates, extending equipment service life by an estimated 20-30% compared to conventional alkaline agents. The resulting decrease in maintenance frequency and replacement costs contributes substantially to favorable long-term economics despite higher initial investment.
Process stability improvements yield additional economic benefits through reduced production disruptions. Case studies across wastewater treatment facilities indicate that lithium hydroxide's consistent performance reduces pH fluctuation events by approximately 40%, minimizing production downtime and quality control issues. This operational reliability translates to quantifiable productivity gains that offset acquisition costs in continuous processing environments.
Environmental compliance considerations further enhance the economic case for lithium hydroxide in specific applications. Its reduced sludge formation properties decrease waste disposal costs by 15-30% compared to calcium hydroxide alternatives. Additionally, facilities in jurisdictions with stringent environmental regulations may realize significant savings through reduced compliance monitoring requirements and avoidance of potential regulatory penalties.
The return on investment timeline varies considerably across implementation scenarios. High-precision manufacturing processes with substantial downtime costs may achieve ROI within 8-14 months, while general industrial applications typically require 18-36 months to realize net economic benefits. This timeline analysis suggests that lithium hydroxide optimization is most economically viable in high-value production environments where process stability commands premium value.
However, a comprehensive cost-benefit analysis must consider the operational efficiencies gained through lithium hydroxide implementation. The superior solubility characteristics of lithium hydroxide translate to reduced dosing requirements, typically 15-25% less volume compared to sodium hydroxide for equivalent pH adjustment. This volumetric efficiency reduces storage requirements, transportation costs, and handling expenses over extended operational periods.
Equipment longevity represents another significant economic advantage. Industrial systems utilizing lithium hydroxide for pH adjustment demonstrate reduced scaling and corrosion rates, extending equipment service life by an estimated 20-30% compared to conventional alkaline agents. The resulting decrease in maintenance frequency and replacement costs contributes substantially to favorable long-term economics despite higher initial investment.
Process stability improvements yield additional economic benefits through reduced production disruptions. Case studies across wastewater treatment facilities indicate that lithium hydroxide's consistent performance reduces pH fluctuation events by approximately 40%, minimizing production downtime and quality control issues. This operational reliability translates to quantifiable productivity gains that offset acquisition costs in continuous processing environments.
Environmental compliance considerations further enhance the economic case for lithium hydroxide in specific applications. Its reduced sludge formation properties decrease waste disposal costs by 15-30% compared to calcium hydroxide alternatives. Additionally, facilities in jurisdictions with stringent environmental regulations may realize significant savings through reduced compliance monitoring requirements and avoidance of potential regulatory penalties.
The return on investment timeline varies considerably across implementation scenarios. High-precision manufacturing processes with substantial downtime costs may achieve ROI within 8-14 months, while general industrial applications typically require 18-36 months to realize net economic benefits. This timeline analysis suggests that lithium hydroxide optimization is most economically viable in high-value production environments where process stability commands premium value.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!