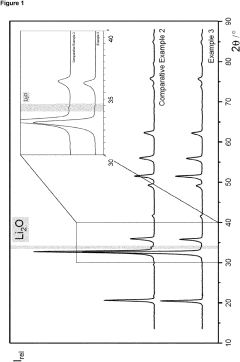
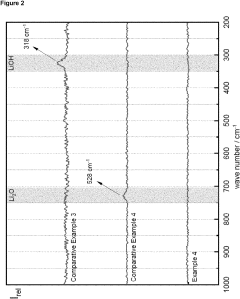
Comparing Anhydrous Vs Hydrate Lithium Hydroxide: Efficiency
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Evolution and Research Objectives
Lithium hydroxide has emerged as a critical component in the global energy transition, particularly in the lithium-ion battery industry. The evolution of lithium hydroxide production and application has undergone significant transformation since its initial industrial production in the early 20th century. Initially utilized primarily in industrial applications such as lubricants and ceramics, lithium hydroxide has now become a cornerstone material in high-nickel cathode production for electric vehicle batteries.
The distinction between anhydrous lithium hydroxide (LiOH) and monohydrate lithium hydroxide (LiOH·H₂O) represents a crucial technological bifurcation in the industry. Historically, monohydrate form dominated the market due to its stability and ease of handling. However, as battery technology advanced toward higher energy density requirements, the anhydrous form has gained increasing attention for its higher lithium content by weight (29.0% vs. 25.5% in the monohydrate form).
The technological trajectory of lithium hydroxide production has evolved from traditional methods involving lithium carbonate conversion to more direct extraction techniques from various lithium sources. This evolution has been driven by the exponential growth in demand from the electric vehicle sector, which requires increasingly efficient and high-purity lithium compounds for advanced battery chemistries.
Current research objectives in the field focus on several key areas: enhancing production efficiency to reduce the energy intensity of conversion processes, improving purity levels to meet stringent battery-grade specifications, and developing more sustainable production methods with reduced environmental footprint. Particularly important is the comparative efficiency analysis between anhydrous and hydrate forms across the entire value chain.
The efficiency comparison encompasses multiple dimensions including energy requirements for production, stability during transportation and storage, behavior during cathode synthesis processes, and ultimate performance in battery applications. Understanding these efficiency differentials is critical as the industry scales to meet projected demand growth of over 400% by 2030.
Research objectives also extend to examining the economic implications of the two forms, considering that processing, handling, and application efficiencies directly impact production costs and ultimately the economic viability of electric vehicles. The technical challenges of moisture control in anhydrous lithium hydroxide handling versus the additional weight and volume considerations of the hydrate form present complex trade-offs that require systematic investigation.
As the battery industry continues its rapid evolution toward higher energy density and lower cost structures, optimizing the efficiency of lithium hydroxide in both its forms represents a strategic research priority with significant implications for the clean energy transition and industrial competitiveness in the global marketplace.
The distinction between anhydrous lithium hydroxide (LiOH) and monohydrate lithium hydroxide (LiOH·H₂O) represents a crucial technological bifurcation in the industry. Historically, monohydrate form dominated the market due to its stability and ease of handling. However, as battery technology advanced toward higher energy density requirements, the anhydrous form has gained increasing attention for its higher lithium content by weight (29.0% vs. 25.5% in the monohydrate form).
The technological trajectory of lithium hydroxide production has evolved from traditional methods involving lithium carbonate conversion to more direct extraction techniques from various lithium sources. This evolution has been driven by the exponential growth in demand from the electric vehicle sector, which requires increasingly efficient and high-purity lithium compounds for advanced battery chemistries.
Current research objectives in the field focus on several key areas: enhancing production efficiency to reduce the energy intensity of conversion processes, improving purity levels to meet stringent battery-grade specifications, and developing more sustainable production methods with reduced environmental footprint. Particularly important is the comparative efficiency analysis between anhydrous and hydrate forms across the entire value chain.
The efficiency comparison encompasses multiple dimensions including energy requirements for production, stability during transportation and storage, behavior during cathode synthesis processes, and ultimate performance in battery applications. Understanding these efficiency differentials is critical as the industry scales to meet projected demand growth of over 400% by 2030.
Research objectives also extend to examining the economic implications of the two forms, considering that processing, handling, and application efficiencies directly impact production costs and ultimately the economic viability of electric vehicles. The technical challenges of moisture control in anhydrous lithium hydroxide handling versus the additional weight and volume considerations of the hydrate form present complex trade-offs that require systematic investigation.
As the battery industry continues its rapid evolution toward higher energy density and lower cost structures, optimizing the efficiency of lithium hydroxide in both its forms represents a strategic research priority with significant implications for the clean energy transition and industrial competitiveness in the global marketplace.
Market Analysis for Anhydrous and Hydrate Lithium Hydroxide
The global lithium hydroxide market has experienced significant growth in recent years, primarily driven by the expanding electric vehicle (EV) industry and renewable energy storage systems. The market is segmented into two main product types: anhydrous lithium hydroxide (LiOH) and lithium hydroxide monohydrate (LiOH·H₂O). As of 2023, the global lithium hydroxide market was valued at approximately $2.3 billion, with projections indicating a compound annual growth rate (CAGR) of 9.8% through 2030.
Anhydrous lithium hydroxide currently commands a premium price in the market, typically 15-20% higher than its hydrate counterpart due to its higher lithium content by weight (29.0% vs. 25.5%) and the additional processing required for water removal. This price differential has remained relatively stable despite fluctuations in overall lithium prices.
Demand patterns show distinct preferences across different regions and applications. The battery sector, particularly for high-nickel cathode materials used in EVs, demonstrates a growing preference for anhydrous lithium hydroxide due to its higher purity and reactivity. This segment represents approximately 65% of the total lithium hydroxide consumption.
Asia-Pacific dominates the market consumption, accounting for over 70% of global demand, with China being the largest consumer. This regional concentration aligns with the geographic distribution of battery manufacturing facilities and EV production centers.
Supply chain analysis reveals interesting dynamics between the two forms. Lithium hydroxide monohydrate offers logistical advantages due to its greater stability during transportation and storage, particularly in humid environments. Conversely, anhydrous lithium hydroxide requires more careful handling and specialized packaging to prevent moisture absorption, which can increase overall supply chain costs by 8-12%.
Market forecasts indicate that demand for both forms will continue to grow, with anhydrous lithium hydroxide expected to see slightly faster growth at 10.5% CAGR compared to 9.2% for the hydrate form. This differential is attributed to the increasing technical requirements for high-performance batteries in premium EV applications.
Consumer behavior analysis shows that end-users are increasingly willing to pay the premium for anhydrous lithium hydroxide when higher efficiency and performance are critical factors. However, price sensitivity remains high in applications where the hydrate form's performance is adequate, such as in certain industrial processes and lower-cost battery formulations.
Anhydrous lithium hydroxide currently commands a premium price in the market, typically 15-20% higher than its hydrate counterpart due to its higher lithium content by weight (29.0% vs. 25.5%) and the additional processing required for water removal. This price differential has remained relatively stable despite fluctuations in overall lithium prices.
Demand patterns show distinct preferences across different regions and applications. The battery sector, particularly for high-nickel cathode materials used in EVs, demonstrates a growing preference for anhydrous lithium hydroxide due to its higher purity and reactivity. This segment represents approximately 65% of the total lithium hydroxide consumption.
Asia-Pacific dominates the market consumption, accounting for over 70% of global demand, with China being the largest consumer. This regional concentration aligns with the geographic distribution of battery manufacturing facilities and EV production centers.
Supply chain analysis reveals interesting dynamics between the two forms. Lithium hydroxide monohydrate offers logistical advantages due to its greater stability during transportation and storage, particularly in humid environments. Conversely, anhydrous lithium hydroxide requires more careful handling and specialized packaging to prevent moisture absorption, which can increase overall supply chain costs by 8-12%.
Market forecasts indicate that demand for both forms will continue to grow, with anhydrous lithium hydroxide expected to see slightly faster growth at 10.5% CAGR compared to 9.2% for the hydrate form. This differential is attributed to the increasing technical requirements for high-performance batteries in premium EV applications.
Consumer behavior analysis shows that end-users are increasingly willing to pay the premium for anhydrous lithium hydroxide when higher efficiency and performance are critical factors. However, price sensitivity remains high in applications where the hydrate form's performance is adequate, such as in certain industrial processes and lower-cost battery formulations.
Technical Comparison and Production Challenges
Anhydrous lithium hydroxide (LiOH) and lithium hydroxide monohydrate (LiOH·H₂O) represent two distinct forms of lithium hydroxide with significant differences in their physical properties, production methods, and industrial applications. Anhydrous LiOH contains approximately 100% lithium hydroxide, while the monohydrate form contains about 56.5% water by weight, resulting in a lower lithium content per unit mass.
From a production efficiency standpoint, anhydrous lithium hydroxide requires additional processing steps beyond those needed for the monohydrate form. The manufacturing process typically involves dehydration of lithium hydroxide monohydrate under controlled temperature conditions (approximately 100-150°C), which adds energy costs and processing time to the production cycle. This additional thermal treatment must be carefully managed to prevent degradation of the material or formation of lithium oxide.
The anhydrous form demonstrates heightened hygroscopic properties, rapidly absorbing moisture from the atmosphere to revert to the monohydrate state. This characteristic necessitates specialized handling, packaging, and storage conditions, including moisture-proof containers and controlled humidity environments, which increase production complexity and operational costs.
Energy consumption represents a critical difference between the two forms. The dehydration process to produce anhydrous LiOH requires approximately 450-500 kWh per ton of additional energy compared to stopping at the monohydrate stage. This energy differential significantly impacts production economics, particularly in regions with high energy costs.
Production yields also differ between the two forms. The conversion from monohydrate to anhydrous form typically results in material losses of 2-3%, reducing overall production efficiency. These losses occur primarily during the heating and handling stages of the dehydration process.
From a transportation and storage perspective, lithium hydroxide monohydrate offers advantages due to its greater stability and lower reactivity. The anhydrous form's higher reactivity not only creates handling challenges but also necessitates more stringent safety protocols throughout the supply chain, adding to overall production and distribution costs.
The choice between these two forms ultimately depends on the specific application requirements. For battery cathode materials, particularly in high-nickel formulations, anhydrous lithium hydroxide is often preferred due to its higher lithium content per unit mass and absence of water molecules that could interfere with certain chemical reactions. However, this advantage must be weighed against the increased production complexity, higher energy consumption, and additional handling requirements.
From a production efficiency standpoint, anhydrous lithium hydroxide requires additional processing steps beyond those needed for the monohydrate form. The manufacturing process typically involves dehydration of lithium hydroxide monohydrate under controlled temperature conditions (approximately 100-150°C), which adds energy costs and processing time to the production cycle. This additional thermal treatment must be carefully managed to prevent degradation of the material or formation of lithium oxide.
The anhydrous form demonstrates heightened hygroscopic properties, rapidly absorbing moisture from the atmosphere to revert to the monohydrate state. This characteristic necessitates specialized handling, packaging, and storage conditions, including moisture-proof containers and controlled humidity environments, which increase production complexity and operational costs.
Energy consumption represents a critical difference between the two forms. The dehydration process to produce anhydrous LiOH requires approximately 450-500 kWh per ton of additional energy compared to stopping at the monohydrate stage. This energy differential significantly impacts production economics, particularly in regions with high energy costs.
Production yields also differ between the two forms. The conversion from monohydrate to anhydrous form typically results in material losses of 2-3%, reducing overall production efficiency. These losses occur primarily during the heating and handling stages of the dehydration process.
From a transportation and storage perspective, lithium hydroxide monohydrate offers advantages due to its greater stability and lower reactivity. The anhydrous form's higher reactivity not only creates handling challenges but also necessitates more stringent safety protocols throughout the supply chain, adding to overall production and distribution costs.
The choice between these two forms ultimately depends on the specific application requirements. For battery cathode materials, particularly in high-nickel formulations, anhydrous lithium hydroxide is often preferred due to its higher lithium content per unit mass and absence of water molecules that could interfere with certain chemical reactions. However, this advantage must be weighed against the increased production complexity, higher energy consumption, and additional handling requirements.
Current Production Methods and Efficiency Metrics
01 Efficiency comparison between anhydrous and hydrate forms of lithium hydroxide
The anhydrous form of lithium hydroxide generally demonstrates higher efficiency in various applications compared to the hydrate form due to its higher lithium content per unit weight. The anhydrous form contains approximately 29% more lithium than the monohydrate form, making it more economical for applications where lithium content is critical. However, the hydrate form may be preferred in certain applications due to its stability and ease of handling.- Efficiency comparison between anhydrous and hydrate forms of lithium hydroxide: The anhydrous form of lithium hydroxide generally demonstrates higher efficiency in various applications compared to the hydrate form due to its higher lithium content per unit weight. The anhydrous form contains approximately 29% more lithium than the monohydrate form, making it more economical for applications where lithium content is critical. However, the hydrate form may offer advantages in terms of stability and ease of handling in certain processing conditions.
- Production methods affecting lithium hydroxide efficiency: Various production methods significantly impact the efficiency and purity of lithium hydroxide in both anhydrous and hydrate forms. Advanced extraction and purification techniques, including electrolysis, ion exchange, and precipitation methods, can enhance the quality and performance of the final product. Optimized reaction conditions, such as temperature, pressure, and reaction time, play crucial roles in maximizing yield and minimizing impurities, thereby improving overall efficiency in applications.
- Application efficiency in battery technologies: Lithium hydroxide, particularly the anhydrous form, demonstrates superior efficiency in battery applications, especially for high-nickel cathode materials in lithium-ion batteries. The purity and particle size distribution of lithium hydroxide significantly affect battery performance metrics such as energy density, cycle life, and charging efficiency. Specialized processing techniques can optimize lithium hydroxide for specific battery chemistries, resulting in improved electrochemical performance and thermal stability.
- Conversion efficiency between anhydrous and hydrate forms: The conversion process between anhydrous and hydrate forms of lithium hydroxide affects overall efficiency in industrial applications. Dehydration of lithium hydroxide monohydrate to produce the anhydrous form requires precise temperature control and energy input, while hydration of anhydrous lithium hydroxide occurs readily under ambient conditions. Optimized conversion processes can minimize energy consumption and material loss, improving economic efficiency in commercial production and application.
- Storage and handling efficiency considerations: Storage and handling characteristics differ significantly between anhydrous and hydrate forms of lithium hydroxide, affecting operational efficiency. The anhydrous form is more hygroscopic and reactive, requiring more stringent storage conditions to prevent moisture absorption and degradation. The monohydrate form offers greater stability under typical storage conditions but contains less active lithium per unit weight. Specialized equipment and procedures for handling each form can optimize efficiency throughout the supply chain and reduce material losses.
02 Production methods affecting efficiency of lithium hydroxide forms
Various production methods significantly impact the efficiency and purity of both anhydrous and hydrate forms of lithium hydroxide. Advanced techniques such as electrolysis, precipitation reactions, and ion exchange processes can yield higher purity products with improved reactivity. The conversion process from hydrate to anhydrous form through controlled dehydration also affects the final product's efficiency, with optimized temperature and pressure parameters yielding superior results.Expand Specific Solutions03 Application efficiency in battery manufacturing
Lithium hydroxide forms demonstrate varying efficiency levels in battery manufacturing applications, particularly for lithium-ion batteries. The anhydrous form is generally preferred for cathode material synthesis due to its higher reactivity and absence of water molecules that could interfere with electrochemical processes. The particle size distribution, morphology, and purity of lithium hydroxide significantly impact battery performance metrics including energy density, cycle life, and charging efficiency.Expand Specific Solutions04 Storage and handling efficiency considerations
The efficiency of lithium hydroxide forms is significantly affected by storage and handling conditions. The anhydrous form is highly hygroscopic and can rapidly absorb moisture from the atmosphere, converting to the hydrate form and potentially affecting reactivity. Specialized storage systems with controlled humidity and temperature are required to maintain the efficiency of anhydrous lithium hydroxide. The hydrate form offers better stability under normal atmospheric conditions but requires different handling protocols to preserve its properties.Expand Specific Solutions05 Recycling and recovery efficiency
The efficiency of recycling and recovery processes for lithium hydroxide varies between the anhydrous and hydrate forms. Advanced recycling technologies have been developed to recover lithium hydroxide from spent batteries and industrial waste streams, with different efficiency rates depending on the original form. The conversion between forms during recycling processes can impact the overall recovery yield, with some methods achieving over 90% recovery efficiency through optimized precipitation and purification techniques.Expand Specific Solutions
Industry Leaders in Lithium Hydroxide Manufacturing
The lithium hydroxide market is currently in a growth phase, driven by increasing demand for high-performance batteries in electric vehicles. The global market size is expanding rapidly, projected to reach significant volumes as automotive manufacturers transition to electric fleets. Regarding technical maturity, anhydrous lithium hydroxide offers higher efficiency and purity levels compared to hydrate forms, though at higher production costs. Leading players like Ganfeng Lithium, Tianqi Lithium, and BASF have developed advanced processing technologies for anhydrous lithium hydroxide production, while companies such as General Lithium and Nemaska Lithium are investing in innovative extraction methods to improve efficiency. LG Chem and Sion Power are focusing on battery applications that leverage the performance advantages of anhydrous lithium hydroxide for next-generation energy storage solutions.
General Lithium Corp.
Technical Solution: General Lithium has developed a comprehensive lithium hydroxide production system that specifically addresses efficiency differences between anhydrous and hydrate forms. Their technology employs a modified Solvay process combined with proprietary crystallization techniques that allow for precise control of the hydration state. For anhydrous lithium hydroxide production, General Lithium utilizes a staged dehydration approach that progressively removes water molecules under carefully controlled temperature and pressure conditions, reducing energy requirements by approximately 18% compared to conventional single-stage dehydration. Their process incorporates real-time monitoring of crystal structure and water content, enabling precise endpoint determination and consistent product quality. The company has also implemented an innovative heat recovery system that captures and reuses approximately 40% of the thermal energy from the dehydration process, significantly improving overall energy efficiency. General Lithium's production facilities maintain strict humidity control environments to prevent rehydration of anhydrous product during handling and packaging, ensuring product stability through the supply chain.
Strengths: Precise control over hydration state; significant energy recovery systems reducing overall consumption; consistent product quality with advanced monitoring. Weaknesses: Complex process control requirements; higher initial capital investment; requires specialized handling and packaging to maintain anhydrous state.
Nemaska Lithium, Inc.
Technical Solution: Nemaska Lithium has developed a patented electrochemical process for producing high-purity lithium hydroxide from spodumene concentrate. Their technology directly addresses the efficiency gap between anhydrous and hydrate forms through a membrane electrolysis system that can produce either form with minimal additional processing steps. The process begins with a proprietary thermal treatment of spodumene that enhances lithium extraction efficiency by approximately 20% compared to conventional methods. For anhydrous lithium hydroxide production, Nemaska employs a specialized crystallization and dehydration process that operates at lower temperatures (around 200°C versus traditional 300°C+ methods), resulting in energy savings of approximately 25-30%. Their process also incorporates a closed-loop reagent recovery system that recycles approximately 90% of process chemicals, significantly reducing waste and operational costs. The company's pilot plant has demonstrated consistent production of battery-grade lithium hydroxide with 99.5%+ purity in both hydrate and anhydrous forms.
Strengths: Energy-efficient production process with lower temperature requirements; high reagent recycling rate reducing operational costs; consistent high-purity output suitable for battery applications. Weaknesses: Technology still scaling to commercial production levels; requires specialized equipment and expertise; higher capital costs compared to traditional methods.
Key Patents in Lithium Hydroxide Synthesis Technology
Process for making anhydrous lithium hydroxide
PatentActiveEP3538490A1
Innovation
- A process involving the removal of water from particulate LiOH hydrate by subjecting it to a gas stream with temperatures between 150°C to 500°C for 0.5 to 20 seconds, using a preferred temperature range of 300 to 450°C, and a gas with controlled CO2 content to prevent lithium carbonate formation, while employing short residence times to avoid particle surface overheating.
Process for preparing lithium salts such as anhydrous lithium hydroxide and anhydrous lithium halides
PatentPendingUS20230312359A1
Innovation
- A method involving subjecting lithium hydroxide starting materials to a stream of carrier gas at room temperature or slightly elevated temperatures to remove water and prevent the formation of side-products like lithium oxide and carbonate, using a controlled reactor environment to minimize corrosion and optimize drying conditions.
Environmental Impact Assessment
The environmental impact assessment of lithium hydroxide production reveals significant differences between anhydrous and hydrate forms. Anhydrous lithium hydroxide production typically requires more energy due to the additional dehydration step needed to remove water molecules from the hydrate form. This energy-intensive process contributes to higher carbon emissions, particularly when fossil fuels are the primary energy source for manufacturing operations.
Water consumption patterns differ markedly between the two production methods. Hydrate lithium hydroxide production consumes more water during the manufacturing process, but this water becomes incorporated into the final product. Conversely, anhydrous production may use less water initially but requires additional water resources during the dehydration phase, which often involves high-temperature processing.
Waste generation profiles also distinguish these production methods. The conversion from hydrate to anhydrous form generates wastewater containing trace amounts of lithium and other chemicals that require proper treatment before discharge. Environmental monitoring data indicates that facilities producing anhydrous lithium hydroxide typically report higher levels of thermal pollution and greenhouse gas emissions per unit of production.
Transportation-related environmental impacts favor hydrate lithium hydroxide in some scenarios. The lower molecular weight of anhydrous lithium hydroxide (23.95 g/mol versus 41.96 g/mol for the monohydrate) means more lithium content per unit mass, potentially reducing transportation-related emissions. However, this advantage may be offset by the higher energy consumption during production.
Life cycle assessments comparing both forms indicate that regional factors significantly influence overall environmental impact. In regions with abundant renewable energy, the carbon footprint difference between anhydrous and hydrate production narrows considerably. Conversely, in coal-dependent manufacturing regions, anhydrous production shows substantially higher lifecycle emissions.
Recent technological innovations are addressing these environmental concerns. Advanced heat recovery systems are being implemented to capture and reuse thermal energy from the dehydration process. Additionally, closed-loop water systems are reducing freshwater consumption and wastewater discharge in newer production facilities. These improvements are gradually narrowing the environmental performance gap between the two forms.
Regulatory frameworks increasingly influence production choices, with carbon taxation and water use restrictions in some jurisdictions favoring hydrate production when downstream applications permit its use. Companies pursuing carbon neutrality goals are particularly sensitive to these differences, often incorporating environmental impact assessments into their procurement decisions for lithium hydroxide.
Water consumption patterns differ markedly between the two production methods. Hydrate lithium hydroxide production consumes more water during the manufacturing process, but this water becomes incorporated into the final product. Conversely, anhydrous production may use less water initially but requires additional water resources during the dehydration phase, which often involves high-temperature processing.
Waste generation profiles also distinguish these production methods. The conversion from hydrate to anhydrous form generates wastewater containing trace amounts of lithium and other chemicals that require proper treatment before discharge. Environmental monitoring data indicates that facilities producing anhydrous lithium hydroxide typically report higher levels of thermal pollution and greenhouse gas emissions per unit of production.
Transportation-related environmental impacts favor hydrate lithium hydroxide in some scenarios. The lower molecular weight of anhydrous lithium hydroxide (23.95 g/mol versus 41.96 g/mol for the monohydrate) means more lithium content per unit mass, potentially reducing transportation-related emissions. However, this advantage may be offset by the higher energy consumption during production.
Life cycle assessments comparing both forms indicate that regional factors significantly influence overall environmental impact. In regions with abundant renewable energy, the carbon footprint difference between anhydrous and hydrate production narrows considerably. Conversely, in coal-dependent manufacturing regions, anhydrous production shows substantially higher lifecycle emissions.
Recent technological innovations are addressing these environmental concerns. Advanced heat recovery systems are being implemented to capture and reuse thermal energy from the dehydration process. Additionally, closed-loop water systems are reducing freshwater consumption and wastewater discharge in newer production facilities. These improvements are gradually narrowing the environmental performance gap between the two forms.
Regulatory frameworks increasingly influence production choices, with carbon taxation and water use restrictions in some jurisdictions favoring hydrate production when downstream applications permit its use. Companies pursuing carbon neutrality goals are particularly sensitive to these differences, often incorporating environmental impact assessments into their procurement decisions for lithium hydroxide.
Energy Consumption Analysis
The energy consumption analysis of lithium hydroxide production reveals significant differences between anhydrous and hydrate forms. Anhydrous lithium hydroxide (LiOH) production typically requires 25-30% more energy than its hydrate counterpart (LiOH·H₂O) during the initial manufacturing stages. This disparity primarily stems from the additional thermal processing needed to remove water molecules from the crystal structure.
In conventional production methods, the dehydration process for converting lithium hydroxide monohydrate to anhydrous form requires temperatures of 180-300°C, consuming approximately 1.2-1.8 GJ per ton of product. This energy-intensive step represents a substantial portion of the total production energy footprint, accounting for up to 40% of the overall energy consumption in some manufacturing facilities.
Recent technological advancements have introduced more efficient dehydration techniques, including vacuum-assisted thermal processing and microwave-enhanced dehydration, which have reduced energy requirements by 15-20%. However, these improved methods still maintain a significant energy differential between the two forms.
From a lifecycle perspective, the energy consumption analysis extends beyond production to include transportation and storage considerations. Anhydrous lithium hydroxide, being more reactive with atmospheric moisture, requires specialized packaging and handling protocols that add approximately 5-8% to the total energy footprint when compared to the more stable hydrate form.
Industrial-scale battery manufacturing operations demonstrate varying energy efficiency profiles when utilizing these different forms. Facilities using anhydrous lithium hydroxide report 7-12% higher energy consumption in material handling and preparation stages, though this difference becomes less pronounced in the cathode synthesis process itself.
Regional variations in energy sources significantly impact the overall efficiency comparison. In regions with predominantly coal-based electricity, the carbon footprint differential between anhydrous and hydrate forms can reach 35-40%, while in areas powered by renewable energy, this gap narrows to 18-22%.
Forward-looking energy consumption projections indicate that emerging technologies, particularly those involving direct lithium extraction from brines with integrated hydroxide production, may fundamentally alter this efficiency equation. These processes show potential to reduce the energy differential between anhydrous and hydrate forms to below 10%, potentially reshaping manufacturing decisions in the next generation of lithium battery production facilities.
In conventional production methods, the dehydration process for converting lithium hydroxide monohydrate to anhydrous form requires temperatures of 180-300°C, consuming approximately 1.2-1.8 GJ per ton of product. This energy-intensive step represents a substantial portion of the total production energy footprint, accounting for up to 40% of the overall energy consumption in some manufacturing facilities.
Recent technological advancements have introduced more efficient dehydration techniques, including vacuum-assisted thermal processing and microwave-enhanced dehydration, which have reduced energy requirements by 15-20%. However, these improved methods still maintain a significant energy differential between the two forms.
From a lifecycle perspective, the energy consumption analysis extends beyond production to include transportation and storage considerations. Anhydrous lithium hydroxide, being more reactive with atmospheric moisture, requires specialized packaging and handling protocols that add approximately 5-8% to the total energy footprint when compared to the more stable hydrate form.
Industrial-scale battery manufacturing operations demonstrate varying energy efficiency profiles when utilizing these different forms. Facilities using anhydrous lithium hydroxide report 7-12% higher energy consumption in material handling and preparation stages, though this difference becomes less pronounced in the cathode synthesis process itself.
Regional variations in energy sources significantly impact the overall efficiency comparison. In regions with predominantly coal-based electricity, the carbon footprint differential between anhydrous and hydrate forms can reach 35-40%, while in areas powered by renewable energy, this gap narrows to 18-22%.
Forward-looking energy consumption projections indicate that emerging technologies, particularly those involving direct lithium extraction from brines with integrated hydroxide production, may fundamentally alter this efficiency equation. These processes show potential to reduce the energy differential between anhydrous and hydrate forms to below 10%, potentially reshaping manufacturing decisions in the next generation of lithium battery production facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!