Quantifying Lithium Hydroxide Yield In Chemical Reactions
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Production Background and Objectives
Lithium hydroxide (LiOH) has emerged as a critical material in the global transition toward sustainable energy systems, particularly in the development of high-performance lithium-ion batteries. The historical evolution of lithium hydroxide production can be traced back to the early 20th century, initially as a byproduct of industrial processes. However, its significance has grown exponentially in recent decades due to its superior electrochemical properties when used in cathode materials for electric vehicle batteries.
The technological landscape for lithium hydroxide production has evolved through several distinct phases. Traditional methods relied on the conversion of lithium carbonate through a lime-based process, which was characterized by high energy consumption and relatively low yield efficiency. The early 2000s witnessed the development of more direct extraction methods from lithium-containing brines and minerals, marking a significant advancement in production technology.
Current global demand for battery-grade lithium hydroxide is experiencing unprecedented growth, projected to increase from approximately 100,000 metric tons in 2022 to over 400,000 metric tons by 2030. This surge is primarily driven by the electric vehicle revolution and grid-scale energy storage systems, creating an urgent need for more efficient and scalable production methodologies.
The primary technical objective in quantifying lithium hydroxide yield centers on developing precise analytical frameworks that can accurately measure conversion rates across various production pathways. This includes establishing standardized protocols for yield calculation that account for reaction kinetics, thermodynamic constraints, and process variables such as temperature, pressure, and reagent concentrations.
Secondary objectives include optimizing reaction conditions to maximize yield while minimizing energy consumption and waste generation. This involves the development of in-situ monitoring technologies capable of real-time yield assessment, enabling dynamic process control and adjustment. Additionally, there is a growing focus on creating predictive models that can forecast yield outcomes based on input parameters, facilitating process optimization through machine learning and advanced simulation techniques.
The environmental dimension of lithium hydroxide production presents another critical objective, as current methods often involve significant water usage and potential ecological impacts. Quantifying yield must therefore extend beyond simple conversion metrics to include sustainability indicators such as water efficiency, carbon footprint, and waste profile per unit of product.
As the industry moves toward more sustainable and efficient production methods, the ability to accurately quantify and optimize lithium hydroxide yield will become increasingly central to technological advancement and economic viability in this sector.
The technological landscape for lithium hydroxide production has evolved through several distinct phases. Traditional methods relied on the conversion of lithium carbonate through a lime-based process, which was characterized by high energy consumption and relatively low yield efficiency. The early 2000s witnessed the development of more direct extraction methods from lithium-containing brines and minerals, marking a significant advancement in production technology.
Current global demand for battery-grade lithium hydroxide is experiencing unprecedented growth, projected to increase from approximately 100,000 metric tons in 2022 to over 400,000 metric tons by 2030. This surge is primarily driven by the electric vehicle revolution and grid-scale energy storage systems, creating an urgent need for more efficient and scalable production methodologies.
The primary technical objective in quantifying lithium hydroxide yield centers on developing precise analytical frameworks that can accurately measure conversion rates across various production pathways. This includes establishing standardized protocols for yield calculation that account for reaction kinetics, thermodynamic constraints, and process variables such as temperature, pressure, and reagent concentrations.
Secondary objectives include optimizing reaction conditions to maximize yield while minimizing energy consumption and waste generation. This involves the development of in-situ monitoring technologies capable of real-time yield assessment, enabling dynamic process control and adjustment. Additionally, there is a growing focus on creating predictive models that can forecast yield outcomes based on input parameters, facilitating process optimization through machine learning and advanced simulation techniques.
The environmental dimension of lithium hydroxide production presents another critical objective, as current methods often involve significant water usage and potential ecological impacts. Quantifying yield must therefore extend beyond simple conversion metrics to include sustainability indicators such as water efficiency, carbon footprint, and waste profile per unit of product.
As the industry moves toward more sustainable and efficient production methods, the ability to accurately quantify and optimize lithium hydroxide yield will become increasingly central to technological advancement and economic viability in this sector.
Market Analysis of Lithium Hydroxide Demand
The global lithium hydroxide market has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of the electric vehicle (EV) industry. As of 2023, the market size has reached approximately $2.5 billion, with projections indicating a compound annual growth rate (CAGR) of 11.2% through 2030. This remarkable growth trajectory is directly linked to lithium hydroxide's critical role in manufacturing high-nickel content cathode materials for EV batteries, which offer superior energy density and longer driving ranges.
The demand distribution across regions shows Asia-Pacific dominating with over 65% of global consumption, led by China's massive battery manufacturing ecosystem. North America and Europe follow with approximately 18% and 14% market shares respectively, though both regions are implementing aggressive strategies to reduce dependency on Asian supply chains.
By application segment, battery production accounts for nearly 70% of lithium hydroxide consumption, followed by lubricant manufacturing (12%), air purification systems (8%), and various industrial applications (10%). Within the battery sector, automotive applications represent the fastest-growing segment with a 15.3% CAGR.
Supply-demand dynamics reveal a tightening market. Current global production capacity stands at approximately 180,000 metric tons annually, while demand is projected to reach 400,000-500,000 metric tons by 2030. This widening gap has triggered significant price volatility, with spot prices fluctuating between $15,000-$80,000 per ton over the past 24 months.
The market faces several critical challenges, including concentration of production in a few countries, environmental concerns associated with extraction processes, and technical difficulties in achieving consistent high-purity yields necessary for battery-grade applications. The latter directly relates to the quantification challenges in lithium hydroxide production reactions, where impurity levels must be controlled to parts-per-million precision.
Recent market trends indicate growing interest in direct lithium extraction (DLE) technologies that promise higher yields and lower environmental impacts. Additionally, battery recycling is emerging as a secondary source of lithium compounds, potentially accounting for 12% of supply by 2035.
Price sensitivity analysis shows that a 10% improvement in lithium hydroxide yield quantification and process control could reduce production costs by 7-9%, potentially unlocking $175-220 million in annual value across the industry. This economic incentive is driving significant R&D investment in advanced analytical techniques and real-time monitoring systems for lithium hydroxide production reactions.
The demand distribution across regions shows Asia-Pacific dominating with over 65% of global consumption, led by China's massive battery manufacturing ecosystem. North America and Europe follow with approximately 18% and 14% market shares respectively, though both regions are implementing aggressive strategies to reduce dependency on Asian supply chains.
By application segment, battery production accounts for nearly 70% of lithium hydroxide consumption, followed by lubricant manufacturing (12%), air purification systems (8%), and various industrial applications (10%). Within the battery sector, automotive applications represent the fastest-growing segment with a 15.3% CAGR.
Supply-demand dynamics reveal a tightening market. Current global production capacity stands at approximately 180,000 metric tons annually, while demand is projected to reach 400,000-500,000 metric tons by 2030. This widening gap has triggered significant price volatility, with spot prices fluctuating between $15,000-$80,000 per ton over the past 24 months.
The market faces several critical challenges, including concentration of production in a few countries, environmental concerns associated with extraction processes, and technical difficulties in achieving consistent high-purity yields necessary for battery-grade applications. The latter directly relates to the quantification challenges in lithium hydroxide production reactions, where impurity levels must be controlled to parts-per-million precision.
Recent market trends indicate growing interest in direct lithium extraction (DLE) technologies that promise higher yields and lower environmental impacts. Additionally, battery recycling is emerging as a secondary source of lithium compounds, potentially accounting for 12% of supply by 2035.
Price sensitivity analysis shows that a 10% improvement in lithium hydroxide yield quantification and process control could reduce production costs by 7-9%, potentially unlocking $175-220 million in annual value across the industry. This economic incentive is driving significant R&D investment in advanced analytical techniques and real-time monitoring systems for lithium hydroxide production reactions.
Technical Challenges in Lithium Hydroxide Quantification
The quantification of lithium hydroxide yield in chemical reactions presents several significant technical challenges that researchers and industry professionals must overcome. These challenges span across multiple dimensions including analytical methodology, reaction environment control, and standardization issues.
Accurate measurement of lithium hydroxide concentration remains one of the primary difficulties due to its high reactivity with atmospheric carbon dioxide, which rapidly converts it to lithium carbonate. This conversion process introduces substantial errors in quantification efforts, particularly when samples are exposed to air during handling and analysis.
The hygroscopic nature of lithium hydroxide compounds further complicates quantification processes. These materials readily absorb moisture from the surrounding environment, altering their mass and concentration measurements. Even brief exposure to ambient conditions can lead to significant deviations in analytical results, necessitating specialized handling protocols.
Traditional titration methods, while widely used, suffer from endpoint detection challenges when applied to lithium hydroxide solutions. The pH transition curves are often less distinct compared to other hydroxides, leading to potential inaccuracies in yield calculations. This is particularly problematic in industrial settings where rapid, high-throughput analysis is required.
Spectroscopic techniques offer alternative quantification approaches but face interference issues from other alkali metals commonly present in lithium-containing reaction mixtures. The spectral overlap between lithium and sodium or potassium can mask accurate lithium hydroxide determination, requiring complex separation procedures or advanced spectral deconvolution algorithms.
In industrial production environments, sampling representativeness poses a significant challenge. Reaction heterogeneity in large-scale reactors means that single-point sampling may not accurately reflect the overall yield, while comprehensive sampling strategies are often impractical or disruptive to continuous production processes.
The presence of impurities in reaction feedstocks introduces variable matrix effects that can interfere with quantification accuracy. These impurities may catalyze side reactions, form complexes with lithium ions, or otherwise alter the reaction kinetics in ways that affect the final lithium hydroxide yield.
Temperature and pressure fluctuations during reactions significantly impact lithium hydroxide formation and stability. These parameters must be precisely controlled and monitored throughout the reaction process to ensure reliable yield quantification, presenting substantial engineering challenges in both laboratory and industrial settings.
Standardization across different analytical methods remains inconsistent, with various industries and research groups employing diverse protocols. This lack of standardization complicates cross-study comparisons and technology transfer between research and production environments.
Accurate measurement of lithium hydroxide concentration remains one of the primary difficulties due to its high reactivity with atmospheric carbon dioxide, which rapidly converts it to lithium carbonate. This conversion process introduces substantial errors in quantification efforts, particularly when samples are exposed to air during handling and analysis.
The hygroscopic nature of lithium hydroxide compounds further complicates quantification processes. These materials readily absorb moisture from the surrounding environment, altering their mass and concentration measurements. Even brief exposure to ambient conditions can lead to significant deviations in analytical results, necessitating specialized handling protocols.
Traditional titration methods, while widely used, suffer from endpoint detection challenges when applied to lithium hydroxide solutions. The pH transition curves are often less distinct compared to other hydroxides, leading to potential inaccuracies in yield calculations. This is particularly problematic in industrial settings where rapid, high-throughput analysis is required.
Spectroscopic techniques offer alternative quantification approaches but face interference issues from other alkali metals commonly present in lithium-containing reaction mixtures. The spectral overlap between lithium and sodium or potassium can mask accurate lithium hydroxide determination, requiring complex separation procedures or advanced spectral deconvolution algorithms.
In industrial production environments, sampling representativeness poses a significant challenge. Reaction heterogeneity in large-scale reactors means that single-point sampling may not accurately reflect the overall yield, while comprehensive sampling strategies are often impractical or disruptive to continuous production processes.
The presence of impurities in reaction feedstocks introduces variable matrix effects that can interfere with quantification accuracy. These impurities may catalyze side reactions, form complexes with lithium ions, or otherwise alter the reaction kinetics in ways that affect the final lithium hydroxide yield.
Temperature and pressure fluctuations during reactions significantly impact lithium hydroxide formation and stability. These parameters must be precisely controlled and monitored throughout the reaction process to ensure reliable yield quantification, presenting substantial engineering challenges in both laboratory and industrial settings.
Standardization across different analytical methods remains inconsistent, with various industries and research groups employing diverse protocols. This lack of standardization complicates cross-study comparisons and technology transfer between research and production environments.
Current Quantification Methodologies and Techniques
01 Extraction methods for lithium hydroxide from lithium-containing sources
Various extraction methods can be employed to obtain lithium hydroxide from different lithium-containing sources such as brines, clays, and ores. These methods typically involve leaching, precipitation, and purification steps to isolate lithium compounds which are then converted to lithium hydroxide. The extraction processes are designed to maximize yield while minimizing impurities in the final product.- Extraction methods for lithium hydroxide production: Various extraction methods are employed to produce lithium hydroxide with high yield. These methods involve extracting lithium from different sources such as brines, ores, or recycled materials. The extraction processes typically include steps like leaching, precipitation, and purification to isolate lithium compounds which can then be converted to lithium hydroxide. These methods aim to maximize the yield while minimizing impurities in the final product.
- Direct lithium extraction technologies: Direct lithium extraction (DLE) technologies represent advanced approaches to obtaining lithium hydroxide with improved yields. These technologies typically use selective adsorbents, ion exchange materials, or membranes to directly capture lithium ions from solution. DLE methods can significantly increase lithium hydroxide yield by reducing processing steps and minimizing loss during extraction, while also allowing for processing of lower-grade resources that would be uneconomical with conventional methods.
- Conversion processes from lithium carbonate to hydroxide: Processes for converting lithium carbonate to lithium hydroxide can significantly impact the overall yield. These conversion methods typically involve reactions with calcium hydroxide or other alkaline substances, followed by purification steps. Innovations in this area focus on optimizing reaction conditions, improving separation techniques, and reducing waste generation to maximize the conversion efficiency and purity of the resulting lithium hydroxide.
- Purification techniques for improving lithium hydroxide yield: Various purification techniques are employed to improve the yield and quality of lithium hydroxide. These include crystallization, ion exchange, solvent extraction, and membrane filtration processes. Advanced purification methods can significantly increase the recovery rate of lithium from process streams, reduce contamination, and allow for the production of battery-grade lithium hydroxide with yields approaching theoretical limits. These techniques are crucial for meeting the high purity requirements of lithium-ion battery production.
- Recycling processes for lithium hydroxide recovery: Recycling processes for recovering lithium hydroxide from spent batteries and industrial waste streams are becoming increasingly important. These processes involve disassembly, physical separation, chemical leaching, and purification steps to extract and recover lithium compounds that can be converted to lithium hydroxide. Advanced recycling technologies aim to maximize lithium hydroxide yield while minimizing environmental impact, providing a sustainable source of lithium for battery production and other applications.
02 Direct conversion processes from lithium carbonate to lithium hydroxide
Direct conversion processes involve transforming lithium carbonate into lithium hydroxide through chemical reactions, typically using calcium hydroxide or sodium hydroxide as reagents. These processes are designed to achieve high conversion rates and yields of lithium hydroxide while minimizing waste generation and energy consumption. Innovations in this area focus on improving reaction conditions and separation techniques to enhance overall yield.Expand Specific Solutions03 Electrochemical processes for lithium hydroxide production
Electrochemical methods utilize electrical current to facilitate the conversion of lithium-containing compounds into lithium hydroxide. These processes often involve electrolysis of lithium salt solutions or direct electrochemical conversion of lithium carbonate. The advantage of electrochemical approaches is the potential for higher purity products and more environmentally friendly processing with improved yields compared to traditional chemical methods.Expand Specific Solutions04 Purification and crystallization techniques for high-yield lithium hydroxide
Purification and crystallization techniques are crucial for obtaining high-purity lithium hydroxide with improved yields. These methods involve multiple stages of filtration, ion exchange, selective precipitation, and controlled crystallization to remove impurities and produce lithium hydroxide crystals with specific characteristics. Advanced crystallization techniques can significantly increase the recovery rate and quality of the final product.Expand Specific Solutions05 Integrated processes for lithium hydroxide production from lithium-rich brines
Integrated processes combine multiple technologies to extract and convert lithium from brines into lithium hydroxide in a continuous or semi-continuous manner. These comprehensive approaches typically include concentration of brines, removal of impurities, conversion to intermediate lithium compounds, and final transformation to lithium hydroxide. The integration of process steps helps optimize resource utilization, reduce waste, and increase overall lithium hydroxide yield.Expand Specific Solutions
Key Industry Players in Lithium Hydroxide Production
The lithium hydroxide yield quantification market is in a growth phase, driven by the expanding electric vehicle battery sector. With a projected market size exceeding $10 billion by 2027, competition is intensifying among established players and new entrants. Leading companies like Albemarle Corp. and General Lithium Corp. have achieved high technical maturity in production processes, while research institutions such as Qinghai Institute of Salt Lakes and Hiroshima University are advancing novel synthesis methods. Chemical giants including BASF, Wacker Chemie, and Shin-Etsu are leveraging their expertise to improve yield efficiency. The technology landscape shows varying maturity levels, with industrial-scale processes well-established but significant innovation occurring in reaction optimization and purity enhancement techniques.
Albemarle Corp.
Technical Solution: Albemarle has developed a proprietary process for quantifying lithium hydroxide yield through advanced spectroscopic analysis combined with machine learning algorithms. Their method employs in-situ Raman spectroscopy to monitor reaction kinetics in real-time, allowing for precise determination of LiOH formation rates and yields. The technology incorporates automated sampling systems that maintain inert conditions to prevent sample contamination from atmospheric CO2. Albemarle's quantification approach includes multi-variable calibration models that account for temperature, pressure, and concentration effects on spectral signatures, enabling accurate yield predictions even in complex brine matrices. Their process has demonstrated precision within ±0.5% for lithium hydroxide quantification across various feedstocks, including spodumene concentrate and lithium carbonate conversion processes. The system integrates with their production facilities to provide continuous monitoring and optimization of conversion efficiency.
Strengths: Industry-leading precision in real-time monitoring allows for process optimization during production rather than post-analysis. Integration with existing production systems enables immediate yield improvements. Weaknesses: The sophisticated spectroscopic equipment requires significant capital investment and specialized technical expertise for maintenance and calibration. The system may be less effective with certain impurity profiles found in lower-grade lithium sources.
Qinghai Institute of Salt Lakes, Chinese Academy of Sciences
Technical Solution: The Qinghai Institute has pioneered a comprehensive analytical framework specifically designed for quantifying lithium hydroxide yields from salt lake brines. Their approach combines traditional titration methods with advanced ion chromatography and inductively coupled plasma mass spectrometry (ICP-MS) techniques. The institute has developed specialized protocols for sample preparation that address the unique challenges of high-magnesium salt lake brines, including a proprietary selective precipitation method that isolates lithium compounds before analysis. Their quantification system incorporates a standardized correction algorithm that accounts for interference from other alkali metals common in brine environments. The institute has also created a database of spectral fingerprints for various lithium-containing compounds that enables rapid identification and quantification of lithium hydroxide in complex matrices. Their methodology has been validated across multiple salt lake compositions found throughout the Qinghai-Tibet plateau, demonstrating reproducibility with standard deviations below 2% even in challenging high-salinity environments.
Strengths: Highly specialized for salt lake brine chemistry with exceptional accuracy in high-magnesium environments that typically challenge conventional analysis methods. The comprehensive database of regional brine compositions enhances analytical reliability. Weaknesses: The methods are optimized for salt lake brines and may require significant adaptation for other lithium sources such as hard rock. The analytical protocols require specialized equipment that may not be readily available in standard industrial laboratories.
Critical Patents and Research in Yield Optimization
Method for producing lithium hydroxide
PatentActiveJP2019131448A
Innovation
- A method involving the reaction of lithium carbonate with acetic acid to produce lithium acetate, followed by reaction with metal hydroxides like potassium or sodium hydroxide to generate lithium hydroxide, accompanied by crystallization and recycling of residual lithium compounds, allowing for efficient production at room temperature and reduced energy consumption.
Lithium evaluation method
PatentActiveJP2020091275A
Innovation
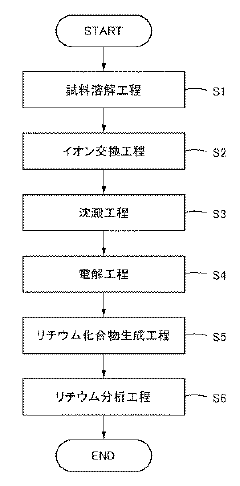
- A gravimetric method is employed to separate lithium from coexisting elements like nickel, cobalt, and manganese using ion exchange, precipitation, and electrolysis operations, followed by decomposing by-products to obtain lithium compounds for accurate weighing.
Environmental Impact Assessment of Production Processes
The production of lithium hydroxide through chemical reactions carries significant environmental implications that must be thoroughly assessed. The extraction and processing of lithium compounds generate various environmental impacts across multiple ecosystems. Water consumption represents one of the most critical concerns, particularly in lithium brine operations where approximately 500,000 gallons of water are required to produce one ton of lithium. This extensive water usage often occurs in arid regions, exacerbating water scarcity issues for local communities and ecosystems.
Air quality degradation occurs throughout the production chain, with particulate matter emissions during mining operations and volatile organic compounds released during chemical processing stages. Carbon dioxide emissions are substantial, with recent studies indicating that lithium hydroxide production generates approximately 15 tons of CO2 equivalent per ton of final product, contributing significantly to the industry's carbon footprint.
Land disturbance from open-pit mining operations for lithium-containing minerals results in habitat fragmentation and biodiversity loss. The average lithium mining operation disturbs approximately 3.5 hectares of land per thousand tons of lithium produced annually. Soil contamination from chemical leakage and improper waste disposal further compounds these terrestrial impacts.
Waste management presents ongoing challenges, as lithium hydroxide production generates significant quantities of solid waste and process residues. For every ton of lithium hydroxide produced, approximately 5-7 tons of waste material requires proper disposal or treatment. The alkaline nature of many process wastes necessitates specialized handling protocols to prevent environmental contamination.
Energy consumption throughout the production process contributes substantially to the overall environmental footprint. Current industry benchmarks indicate that lithium hydroxide production requires between 5,000-6,000 kWh of electricity per ton produced, with additional thermal energy requirements for various reaction and purification stages.
Recent technological innovations are gradually improving the environmental profile of lithium hydroxide production. Direct lithium extraction technologies have demonstrated potential water use reductions of up to 70% compared to traditional evaporation methods. Closed-loop processing systems are being implemented to minimize waste generation and chemical consumption, while renewable energy integration is reducing the carbon intensity of production operations by an estimated 30-40% in advanced facilities.
Air quality degradation occurs throughout the production chain, with particulate matter emissions during mining operations and volatile organic compounds released during chemical processing stages. Carbon dioxide emissions are substantial, with recent studies indicating that lithium hydroxide production generates approximately 15 tons of CO2 equivalent per ton of final product, contributing significantly to the industry's carbon footprint.
Land disturbance from open-pit mining operations for lithium-containing minerals results in habitat fragmentation and biodiversity loss. The average lithium mining operation disturbs approximately 3.5 hectares of land per thousand tons of lithium produced annually. Soil contamination from chemical leakage and improper waste disposal further compounds these terrestrial impacts.
Waste management presents ongoing challenges, as lithium hydroxide production generates significant quantities of solid waste and process residues. For every ton of lithium hydroxide produced, approximately 5-7 tons of waste material requires proper disposal or treatment. The alkaline nature of many process wastes necessitates specialized handling protocols to prevent environmental contamination.
Energy consumption throughout the production process contributes substantially to the overall environmental footprint. Current industry benchmarks indicate that lithium hydroxide production requires between 5,000-6,000 kWh of electricity per ton produced, with additional thermal energy requirements for various reaction and purification stages.
Recent technological innovations are gradually improving the environmental profile of lithium hydroxide production. Direct lithium extraction technologies have demonstrated potential water use reductions of up to 70% compared to traditional evaporation methods. Closed-loop processing systems are being implemented to minimize waste generation and chemical consumption, while renewable energy integration is reducing the carbon intensity of production operations by an estimated 30-40% in advanced facilities.
Quality Control Standards and Regulatory Compliance
Quality control standards for lithium hydroxide production are governed by stringent regulatory frameworks that vary across global markets. In the United States, the FDA and USP establish specifications for pharmaceutical-grade lithium hydroxide, while industrial applications follow ASTM International standards that specify minimum purity levels of 99.0% for technical grade and 99.9% for battery-grade material. The European Union enforces REACH regulations, requiring comprehensive documentation of chemical properties and safety assessments for lithium compounds.
ISO 9001:2015 certification is essential for lithium hydroxide manufacturers, providing a framework for consistent quality management systems. For battery applications, additional compliance with ISO/TS 16949 automotive quality standards may be required. Quantification methodologies must adhere to these standards, with titration procedures following ASTM E200 guidelines and instrumental analysis methods validated according to ICH Q2(R1) principles.
Regulatory bodies mandate specific analytical techniques for yield quantification, including atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), and high-performance liquid chromatography (HPLC). These methods must demonstrate linearity, accuracy, precision, specificity, and robustness within defined parameters. Documentation requirements include detailed standard operating procedures (SOPs), method validation reports, and calibration certificates for all measuring equipment.
Environmental compliance adds another layer of complexity, with regulations like the Clean Air Act and Clean Water Act in the US imposing limits on emissions and effluents from lithium processing facilities. Similar regulations exist in the EU under the Industrial Emissions Directive. Companies must implement continuous monitoring systems to ensure compliance with these environmental standards during production processes.
Statistical process control (SPC) techniques are increasingly becoming regulatory requirements, with control charts and capability indices used to demonstrate consistent yield quantification. Regulatory inspections focus on verification of these statistical controls, along with review of calibration records and analyst qualification documentation. Non-compliance can result in significant penalties, production shutdowns, or market restrictions.
International harmonization efforts through organizations like the International Conference on Harmonisation (ICH) aim to standardize quality control approaches globally, though regional variations persist. Companies engaged in lithium hydroxide production must maintain comprehensive regulatory intelligence systems to track evolving standards across different markets and ensure continuous compliance with all applicable regulations.
ISO 9001:2015 certification is essential for lithium hydroxide manufacturers, providing a framework for consistent quality management systems. For battery applications, additional compliance with ISO/TS 16949 automotive quality standards may be required. Quantification methodologies must adhere to these standards, with titration procedures following ASTM E200 guidelines and instrumental analysis methods validated according to ICH Q2(R1) principles.
Regulatory bodies mandate specific analytical techniques for yield quantification, including atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), and high-performance liquid chromatography (HPLC). These methods must demonstrate linearity, accuracy, precision, specificity, and robustness within defined parameters. Documentation requirements include detailed standard operating procedures (SOPs), method validation reports, and calibration certificates for all measuring equipment.
Environmental compliance adds another layer of complexity, with regulations like the Clean Air Act and Clean Water Act in the US imposing limits on emissions and effluents from lithium processing facilities. Similar regulations exist in the EU under the Industrial Emissions Directive. Companies must implement continuous monitoring systems to ensure compliance with these environmental standards during production processes.
Statistical process control (SPC) techniques are increasingly becoming regulatory requirements, with control charts and capability indices used to demonstrate consistent yield quantification. Regulatory inspections focus on verification of these statistical controls, along with review of calibration records and analyst qualification documentation. Non-compliance can result in significant penalties, production shutdowns, or market restrictions.
International harmonization efforts through organizations like the International Conference on Harmonisation (ICH) aim to standardize quality control approaches globally, though regional variations persist. Companies engaged in lithium hydroxide production must maintain comprehensive regulatory intelligence systems to track evolving standards across different markets and ensure continuous compliance with all applicable regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!