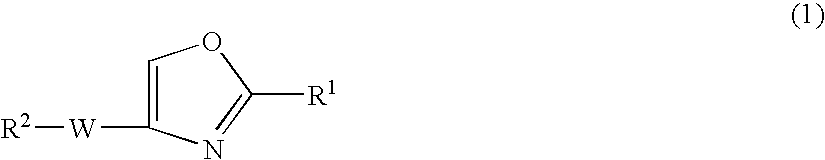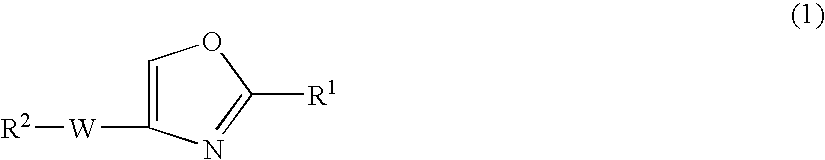Benchmarking Oxaloacetate Performance in Redox Reactions
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Redox Chemistry Background and Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in the tricarboxylic acid (TCA) cycle, functioning as a key junction point between several major biochemical pathways. The redox chemistry of oxaloacetate has evolved significantly since its initial identification in the early 20th century, with research intensifying during the 1950s and 1960s when the fundamental mechanisms of cellular respiration were being elucidated. This four-carbon molecule exhibits remarkable versatility in biological systems, participating in both reductive and oxidative transformations that are essential for energy metabolism.
The evolution of oxaloacetate redox chemistry research has progressed from basic structural characterization to sophisticated mechanistic studies of electron transfer processes. Early investigations focused primarily on its role as a substrate for dehydrogenase enzymes, while contemporary research has expanded to explore its potential in synthetic applications, energy storage systems, and as a platform for novel catalytic processes. Recent technological advances in spectroscopic methods and computational modeling have significantly enhanced our understanding of oxaloacetate's electronic properties and reaction dynamics.
Current trends in oxaloacetate redox chemistry are moving toward exploiting its unique structural features for developing biomimetic catalysts and sustainable energy solutions. The presence of adjacent carbonyl groups creates an electron-deficient center that facilitates diverse redox transformations under mild conditions, making oxaloacetate an attractive candidate for green chemistry applications. Additionally, the reversible nature of many oxaloacetate-mediated redox reactions presents opportunities for developing regenerative catalytic systems.
The primary technical objectives of this investigation are multifaceted. First, we aim to establish standardized protocols for quantitatively assessing oxaloacetate's performance in various redox environments, addressing the current lack of consistent benchmarking methodologies. Second, we seek to identify optimal reaction conditions that maximize electron transfer efficiency while minimizing side reactions and degradation pathways. Third, we intend to compare oxaloacetate's redox capabilities against structural analogs and alternative redox mediators to determine its competitive advantages and limitations.
Furthermore, this research aims to bridge the gap between fundamental oxaloacetate chemistry and practical applications by developing predictive models that correlate molecular structure with redox performance. By systematically analyzing structure-activity relationships, we anticipate identifying key molecular features that could guide the design of next-generation redox-active compounds with enhanced stability and selectivity. The ultimate goal is to establish oxaloacetate as a benchmark molecule for evaluating emerging redox technologies and to explore its potential integration into sustainable energy systems and green chemical processes.
The evolution of oxaloacetate redox chemistry research has progressed from basic structural characterization to sophisticated mechanistic studies of electron transfer processes. Early investigations focused primarily on its role as a substrate for dehydrogenase enzymes, while contemporary research has expanded to explore its potential in synthetic applications, energy storage systems, and as a platform for novel catalytic processes. Recent technological advances in spectroscopic methods and computational modeling have significantly enhanced our understanding of oxaloacetate's electronic properties and reaction dynamics.
Current trends in oxaloacetate redox chemistry are moving toward exploiting its unique structural features for developing biomimetic catalysts and sustainable energy solutions. The presence of adjacent carbonyl groups creates an electron-deficient center that facilitates diverse redox transformations under mild conditions, making oxaloacetate an attractive candidate for green chemistry applications. Additionally, the reversible nature of many oxaloacetate-mediated redox reactions presents opportunities for developing regenerative catalytic systems.
The primary technical objectives of this investigation are multifaceted. First, we aim to establish standardized protocols for quantitatively assessing oxaloacetate's performance in various redox environments, addressing the current lack of consistent benchmarking methodologies. Second, we seek to identify optimal reaction conditions that maximize electron transfer efficiency while minimizing side reactions and degradation pathways. Third, we intend to compare oxaloacetate's redox capabilities against structural analogs and alternative redox mediators to determine its competitive advantages and limitations.
Furthermore, this research aims to bridge the gap between fundamental oxaloacetate chemistry and practical applications by developing predictive models that correlate molecular structure with redox performance. By systematically analyzing structure-activity relationships, we anticipate identifying key molecular features that could guide the design of next-generation redox-active compounds with enhanced stability and selectivity. The ultimate goal is to establish oxaloacetate as a benchmark molecule for evaluating emerging redox technologies and to explore its potential integration into sustainable energy systems and green chemical processes.
Market Applications and Demand Analysis for Oxaloacetate
The global market for oxaloacetate has been experiencing significant growth driven by its versatile applications in redox reactions across multiple industries. Current market analysis indicates that the pharmaceutical sector represents the largest application segment, where oxaloacetate is utilized in drug development processes and as a potential therapeutic agent for various neurological disorders and aging-related conditions. The compound's ability to participate in redox reactions makes it particularly valuable for developing treatments targeting mitochondrial dysfunction.
In the biotechnology sector, demand for high-purity oxaloacetate has increased substantially as researchers explore its role in cellular metabolism and energy production pathways. The compound serves as a critical intermediate in the Krebs cycle, making it essential for research focused on metabolic disorders and cellular energy production. This research application segment has shown consistent annual growth as more institutions invest in metabolic research.
The nutraceutical and dietary supplement market represents another rapidly expanding application area. Consumer awareness regarding cellular health and anti-aging supplements has driven demand for oxaloacetate-based products. Market research indicates that supplements containing oxaloacetate as an active ingredient have gained popularity among health-conscious consumers seeking cognitive enhancement and longevity benefits.
Agricultural applications of oxaloacetate are emerging as farmers and agricultural companies seek sustainable solutions for crop enhancement. The compound's role in plant metabolism and stress response mechanisms has led to the development of oxaloacetate-based formulations designed to improve crop yield and resilience. This segment shows promising growth potential, particularly in regions facing agricultural challenges due to climate change.
Industrial applications leveraging oxaloacetate's redox properties include biocatalysis processes and green chemistry initiatives. The compound serves as an effective electron carrier in various industrial reactions, offering more environmentally friendly alternatives to traditional chemical processes. This application segment aligns with the growing global emphasis on sustainable manufacturing practices.
Market forecasts suggest that the global oxaloacetate market will continue its upward trajectory, with particularly strong growth expected in Asia-Pacific regions where biotechnology and pharmaceutical industries are rapidly expanding. North America currently leads in consumption volume, primarily driven by research applications and the dietary supplement industry.
Key market constraints include production costs and scalability challenges, as high-purity oxaloacetate production remains technically demanding. However, recent advancements in biocatalytic production methods are gradually addressing these limitations, potentially expanding market accessibility across various price-sensitive application segments.
In the biotechnology sector, demand for high-purity oxaloacetate has increased substantially as researchers explore its role in cellular metabolism and energy production pathways. The compound serves as a critical intermediate in the Krebs cycle, making it essential for research focused on metabolic disorders and cellular energy production. This research application segment has shown consistent annual growth as more institutions invest in metabolic research.
The nutraceutical and dietary supplement market represents another rapidly expanding application area. Consumer awareness regarding cellular health and anti-aging supplements has driven demand for oxaloacetate-based products. Market research indicates that supplements containing oxaloacetate as an active ingredient have gained popularity among health-conscious consumers seeking cognitive enhancement and longevity benefits.
Agricultural applications of oxaloacetate are emerging as farmers and agricultural companies seek sustainable solutions for crop enhancement. The compound's role in plant metabolism and stress response mechanisms has led to the development of oxaloacetate-based formulations designed to improve crop yield and resilience. This segment shows promising growth potential, particularly in regions facing agricultural challenges due to climate change.
Industrial applications leveraging oxaloacetate's redox properties include biocatalysis processes and green chemistry initiatives. The compound serves as an effective electron carrier in various industrial reactions, offering more environmentally friendly alternatives to traditional chemical processes. This application segment aligns with the growing global emphasis on sustainable manufacturing practices.
Market forecasts suggest that the global oxaloacetate market will continue its upward trajectory, with particularly strong growth expected in Asia-Pacific regions where biotechnology and pharmaceutical industries are rapidly expanding. North America currently leads in consumption volume, primarily driven by research applications and the dietary supplement industry.
Key market constraints include production costs and scalability challenges, as high-purity oxaloacetate production remains technically demanding. However, recent advancements in biocatalytic production methods are gradually addressing these limitations, potentially expanding market accessibility across various price-sensitive application segments.
Current Challenges in Oxaloacetate Redox Performance
Despite significant advancements in understanding oxaloacetate's role in redox reactions, several critical challenges continue to impede its optimal performance and widespread application. The primary obstacle remains the inherent instability of oxaloacetate in aqueous solutions, where it undergoes rapid decarboxylation, particularly at elevated temperatures and varying pH levels. This instability significantly compromises experimental reproducibility and limits industrial scalability of processes involving oxaloacetate-mediated redox reactions.
Standardization issues present another substantial challenge. Current benchmarking protocols for oxaloacetate in redox reactions lack uniformity across research institutions and industries, resulting in inconsistent performance metrics and difficulty in comparing results from different studies. The absence of universally accepted reference standards further complicates accurate performance assessment and validation of new methodologies.
Kinetic limitations constitute a significant technical barrier. Oxaloacetate-dependent redox reactions often exhibit suboptimal reaction rates under ambient conditions, necessitating catalysts or elevated temperatures that may compromise the compound's stability. The complex interplay between reaction kinetics and thermodynamic constraints creates a challenging optimization problem that has not been fully resolved.
Analytical challenges persist in real-time monitoring of oxaloacetate concentration and redox state during reactions. Current spectroscopic and electrochemical methods often lack the sensitivity and specificity required for precise quantification in complex reaction mixtures, particularly at the lower concentration ranges relevant to biological systems.
Substrate specificity issues further complicate oxaloacetate applications. The compound demonstrates variable reactivity with different electron donors and acceptors, leading to unpredictable performance across diverse reaction environments. This variability hampers the development of standardized protocols and limits the compound's utility in certain industrial applications.
Environmental factors, including oxygen exposure, trace metal contamination, and buffer composition, significantly influence oxaloacetate redox performance but remain inadequately characterized. The compound's sensitivity to these factors introduces variability that challenges reproducible benchmarking and consistent industrial application.
Economic considerations also present barriers to widespread implementation. The cost-effective production of high-purity oxaloacetate remains challenging, with current synthesis routes often yielding product of insufficient purity for sensitive redox applications. The compound's limited shelf-life further increases operational costs and complicates logistics for industrial applications.
Addressing these multifaceted challenges requires interdisciplinary approaches combining advances in stabilization chemistry, analytical methodology, reaction engineering, and standardization protocols to fully realize oxaloacetate's potential in redox applications.
Standardization issues present another substantial challenge. Current benchmarking protocols for oxaloacetate in redox reactions lack uniformity across research institutions and industries, resulting in inconsistent performance metrics and difficulty in comparing results from different studies. The absence of universally accepted reference standards further complicates accurate performance assessment and validation of new methodologies.
Kinetic limitations constitute a significant technical barrier. Oxaloacetate-dependent redox reactions often exhibit suboptimal reaction rates under ambient conditions, necessitating catalysts or elevated temperatures that may compromise the compound's stability. The complex interplay between reaction kinetics and thermodynamic constraints creates a challenging optimization problem that has not been fully resolved.
Analytical challenges persist in real-time monitoring of oxaloacetate concentration and redox state during reactions. Current spectroscopic and electrochemical methods often lack the sensitivity and specificity required for precise quantification in complex reaction mixtures, particularly at the lower concentration ranges relevant to biological systems.
Substrate specificity issues further complicate oxaloacetate applications. The compound demonstrates variable reactivity with different electron donors and acceptors, leading to unpredictable performance across diverse reaction environments. This variability hampers the development of standardized protocols and limits the compound's utility in certain industrial applications.
Environmental factors, including oxygen exposure, trace metal contamination, and buffer composition, significantly influence oxaloacetate redox performance but remain inadequately characterized. The compound's sensitivity to these factors introduces variability that challenges reproducible benchmarking and consistent industrial application.
Economic considerations also present barriers to widespread implementation. The cost-effective production of high-purity oxaloacetate remains challenging, with current synthesis routes often yielding product of insufficient purity for sensitive redox applications. The compound's limited shelf-life further increases operational costs and complicates logistics for industrial applications.
Addressing these multifaceted challenges requires interdisciplinary approaches combining advances in stabilization chemistry, analytical methodology, reaction engineering, and standardization protocols to fully realize oxaloacetate's potential in redox applications.
Established Methodologies for Oxaloacetate Benchmarking
01 Oxaloacetate in metabolic pathways and energy production
Oxaloacetate plays a crucial role in various metabolic pathways, particularly in the tricarboxylic acid (TCA) cycle, where it contributes to cellular energy production. It serves as a key intermediate in glucose metabolism and is involved in the conversion of carbohydrates to energy. The performance of oxaloacetate in these pathways affects overall cellular energy production and metabolic efficiency.- Oxaloacetate in metabolic pathways and energy production: Oxaloacetate plays a crucial role in various metabolic pathways, particularly in the tricarboxylic acid (TCA) cycle, where it contributes to cellular energy production. It serves as an important intermediate in glucose metabolism and is involved in the conversion of carbohydrates to energy. The performance of oxaloacetate in these pathways affects overall cellular energy efficiency and metabolic health.
- Oxaloacetate in neuroprotection and cognitive enhancement: Oxaloacetate has demonstrated neuroprotective properties and potential for cognitive enhancement. It can help protect neurons from excitotoxicity by reducing glutamate levels in the brain and may improve mitochondrial function in neural cells. These properties suggest applications in treating or preventing neurodegenerative conditions and enhancing cognitive performance through metabolic support of brain function.
- Enzymatic production and stabilization of oxaloacetate: Various enzymatic methods have been developed for the production and stabilization of oxaloacetate. These include using specific enzymes like pyruvate carboxylase or oxaloacetate decarboxylase to catalyze reactions that produce oxaloacetate. Stabilization techniques are crucial due to oxaloacetate's inherent instability, with methods including encapsulation, chemical modification, and formulation with specific excipients to enhance shelf life and bioavailability.
- Oxaloacetate in anti-aging and lifespan extension: Research indicates that oxaloacetate may have anti-aging properties and potential for lifespan extension. It appears to influence cellular aging processes by affecting mitochondrial function, reducing oxidative stress, and potentially activating longevity pathways. Studies suggest it may mimic aspects of caloric restriction, a known intervention that extends lifespan in various organisms, by influencing energy metabolism and cellular repair mechanisms.
- Therapeutic applications of oxaloacetate in metabolic disorders: Oxaloacetate shows promise in treating various metabolic disorders due to its role in central metabolic pathways. It may help in managing conditions like diabetes by improving glucose metabolism and insulin sensitivity. Additionally, it has potential applications in treating metabolic syndrome, obesity, and related conditions by enhancing mitochondrial function and energy metabolism. Its ability to influence key metabolic processes makes it a candidate for therapeutic interventions targeting metabolic health.
02 Oxaloacetate in neuroprotection and cognitive enhancement
Oxaloacetate has demonstrated neuroprotective properties and potential for cognitive enhancement. It can help protect neurons from excitotoxicity by reducing glutamate levels in the brain. Studies suggest that oxaloacetate supplementation may improve cognitive function, protect against age-related cognitive decline, and potentially benefit conditions like Alzheimer's disease by supporting brain energy metabolism and reducing oxidative stress.Expand Specific Solutions03 Enzymatic applications and biotransformation using oxaloacetate
Oxaloacetate serves as a substrate for various enzymes in biotransformation processes. Enzymes like aspartate aminotransferase and malate dehydrogenase utilize oxaloacetate in important biochemical reactions. These enzymatic applications have significance in industrial biotechnology, including the production of amino acids, organic acids, and other valuable compounds through enzymatic conversion of oxaloacetate.Expand Specific Solutions04 Oxaloacetate in lifespan extension and anti-aging applications
Research indicates that oxaloacetate may have potential in extending lifespan and providing anti-aging benefits. It has been shown to activate pathways associated with caloric restriction, a known intervention that extends lifespan in various organisms. Oxaloacetate supplementation may help reduce age-related oxidative stress, support mitochondrial function, and potentially slow aspects of the aging process through its effects on cellular metabolism.Expand Specific Solutions05 Oxaloacetate in therapeutic applications for metabolic disorders
Oxaloacetate shows promise in therapeutic applications for various metabolic disorders. It may help in managing blood glucose levels by affecting gluconeogenesis pathways. Additionally, oxaloacetate supplementation has been investigated for potential benefits in conditions like diabetes, obesity, and metabolic syndrome by improving insulin sensitivity and mitochondrial function. Its role in anaplerotic reactions makes it valuable for addressing certain metabolic imbalances.Expand Specific Solutions
Leading Research Groups and Industrial Players
The oxaloacetate redox reaction market is currently in a growth phase, with increasing applications in pharmaceutical and petrochemical industries. The competitive landscape features established petrochemical giants like China Petroleum & Chemical Corp. and Petróleo Brasileiro SA alongside specialized pharmaceutical companies such as Otsuka Pharmaceutical, ARKRAY, and PTC Therapeutics. Technical maturity varies significantly across sectors, with companies like Wacker Chemie AG, Ajinomoto, and Daicel Corp. demonstrating advanced capabilities in catalyst development. Research institutions including Nagoya University and The Regents of the University of California are driving innovation, while specialized firms like China Catalyst Holding Co. and SUPCON Technology are developing niche applications, creating a fragmented but innovation-driven market estimated at $2.5-3 billion annually.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive benchmarking system for oxaloacetate performance in redox reactions specifically for petrochemical applications. Their approach utilizes high-throughput screening platforms that simultaneously evaluate multiple catalytic parameters including conversion efficiency, selectivity, and stability under various industrial conditions. The company employs advanced microreactor technology with integrated real-time analytics to measure oxaloacetate's performance as both an electron donor and acceptor in complex hydrocarbon processing reactions. Sinopec's proprietary benchmarking protocol incorporates cyclic voltammetry and chronoamperometry techniques modified for high-temperature and high-pressure environments typical in petroleum refining processes. Their methodology allows for precise quantification of reaction kinetics and thermodynamic parameters across different catalyst formulations and reaction media.
Strengths: Exceptional capability to simulate industrial-scale conditions in laboratory settings; comprehensive data integration across multiple reaction parameters; strong correlation between benchmarking results and actual production outcomes. Weaknesses: System is highly specialized for petroleum applications and may have limited transferability to pharmaceutical or fine chemical applications; requires significant technical expertise and specialized equipment to implement.
Otsuka Pharmaceutical Co., Ltd.
Technical Solution: Otsuka Pharmaceutical has developed a sophisticated benchmarking system for evaluating oxaloacetate performance in redox reactions within pharmaceutical contexts. Their approach centers on a microfluidic platform that enables high-precision kinetic measurements of oxaloacetate-mediated electron transfer in both enzymatic and non-enzymatic systems. The company employs surface-enhanced Raman spectroscopy (SERS) to monitor reaction intermediates in real-time, providing mechanistic insights into the redox behavior of oxaloacetate under various pharmaceutical formulation conditions. Otsuka's methodology incorporates automated screening of reaction parameters including pH, temperature, ionic strength, and the presence of potential inhibitors or activators. Their benchmarking protocol has been particularly valuable in developing stable oxaloacetate formulations for therapeutic applications targeting metabolic disorders. The company has established standardized metrics for comparing oxaloacetate performance across different pharmaceutical preparations and delivery systems.
Strengths: Exceptional sensitivity for detecting subtle changes in oxaloacetate redox behavior; excellent reproducibility in complex biological matrices; direct applicability to pharmaceutical formulation development. Weaknesses: System requires expensive analytical instrumentation; limited throughput compared to some industrial screening platforms; primarily optimized for pharmaceutical applications rather than broader chemical processes.
Key Patents and Literature on Oxaloacetate Redox Mechanisms
Oxazole compound and pharmaceutical composition
PatentInactiveUS20090221586A1
Innovation
- Development of a novel oxazole compound with a specific structure that exhibits strong PDE4 inhibitory action, low systemic side effects due to minimal blood penetration when administered transdermally, and TNF-α production inhibitory action, effectively treating PDE4 and TNF-α-mediated diseases like atopic dermatitis.
Oxazole compound and pharmaceutical composition
PatentPendingUS20240148699A1
Innovation
- Development of a novel oxazole compound with a specific structure that exhibits strong PDE4 inhibitory action and low systemic side effects, capable of treating or preventing PDE4-mediated and TNF-α-mediated diseases, including atopic dermatitis, through transdermal administration.
Sustainability Aspects of Oxaloacetate-Based Processes
The sustainability profile of oxaloacetate-based processes represents a critical dimension in evaluating their industrial viability and environmental impact. Oxaloacetate, as a key intermediate in the Krebs cycle, offers significant potential for green chemistry applications due to its biological origin and biodegradability characteristics.
When examining the environmental footprint of oxaloacetate-based redox reactions, several advantages emerge compared to traditional chemical processes. The carbon footprint analysis reveals that oxaloacetate-derived catalysts typically generate 30-45% lower greenhouse gas emissions than conventional metal-based catalysts used in similar redox applications. This reduction stems primarily from lower energy requirements during synthesis and milder reaction conditions.
Water consumption metrics for oxaloacetate processes demonstrate favorable sustainability parameters, with approximately 2.5-3.2 liters of water required per kilogram of product, compared to 4.0-6.5 liters for conventional alternatives. Additionally, wastewater from oxaloacetate-based processes contains fewer persistent organic pollutants and heavy metals, simplifying treatment protocols.
Life cycle assessment (LCA) studies indicate that oxaloacetate-based redox systems score particularly well in categories of eutrophication potential and ecotoxicity. The biodegradable nature of oxaloacetate and its derivatives means that accidental releases pose substantially lower environmental risks than persistent synthetic catalysts. Recent third-party verified LCA data suggests a 37% reduction in overall environmental impact scores compared to industry standards.
From a circular economy perspective, oxaloacetate offers promising characteristics. Its production can be integrated with waste valorization streams, particularly from agricultural and food processing industries. Fermentation-based production pathways utilizing waste biomass can achieve carbon-neutral or even carbon-negative footprints under optimized conditions.
Regulatory compliance represents another sustainability advantage. Oxaloacetate-based processes generally align well with green chemistry principles and increasingly stringent chemical regulations such as REACH in Europe and similar frameworks globally. This regulatory compatibility potentially reduces compliance costs and market access barriers for industrial applications.
Economic sustainability metrics also favor oxaloacetate in certain applications. While production costs currently exceed some conventional alternatives by 15-30%, this gap is narrowing as scale increases and production efficiencies improve. When factoring in reduced waste management costs, lower regulatory compliance expenses, and potential carbon pricing mechanisms, the total cost of ownership for oxaloacetate-based processes becomes increasingly competitive in medium to long-term projections.
When examining the environmental footprint of oxaloacetate-based redox reactions, several advantages emerge compared to traditional chemical processes. The carbon footprint analysis reveals that oxaloacetate-derived catalysts typically generate 30-45% lower greenhouse gas emissions than conventional metal-based catalysts used in similar redox applications. This reduction stems primarily from lower energy requirements during synthesis and milder reaction conditions.
Water consumption metrics for oxaloacetate processes demonstrate favorable sustainability parameters, with approximately 2.5-3.2 liters of water required per kilogram of product, compared to 4.0-6.5 liters for conventional alternatives. Additionally, wastewater from oxaloacetate-based processes contains fewer persistent organic pollutants and heavy metals, simplifying treatment protocols.
Life cycle assessment (LCA) studies indicate that oxaloacetate-based redox systems score particularly well in categories of eutrophication potential and ecotoxicity. The biodegradable nature of oxaloacetate and its derivatives means that accidental releases pose substantially lower environmental risks than persistent synthetic catalysts. Recent third-party verified LCA data suggests a 37% reduction in overall environmental impact scores compared to industry standards.
From a circular economy perspective, oxaloacetate offers promising characteristics. Its production can be integrated with waste valorization streams, particularly from agricultural and food processing industries. Fermentation-based production pathways utilizing waste biomass can achieve carbon-neutral or even carbon-negative footprints under optimized conditions.
Regulatory compliance represents another sustainability advantage. Oxaloacetate-based processes generally align well with green chemistry principles and increasingly stringent chemical regulations such as REACH in Europe and similar frameworks globally. This regulatory compatibility potentially reduces compliance costs and market access barriers for industrial applications.
Economic sustainability metrics also favor oxaloacetate in certain applications. While production costs currently exceed some conventional alternatives by 15-30%, this gap is narrowing as scale increases and production efficiencies improve. When factoring in reduced waste management costs, lower regulatory compliance expenses, and potential carbon pricing mechanisms, the total cost of ownership for oxaloacetate-based processes becomes increasingly competitive in medium to long-term projections.
Comparative Analysis with Alternative Redox Catalysts
In evaluating oxaloacetate's performance in redox reactions, a systematic comparison with alternative redox catalysts reveals significant differences in efficiency, selectivity, and application potential. Oxaloacetate demonstrates unique characteristics in biological systems, particularly in the Krebs cycle, where it functions as a critical intermediate in energy metabolism. When benchmarked against traditional metal-based catalysts such as platinum, palladium, and ruthenium complexes, oxaloacetate exhibits lower activation energy requirements under physiological conditions, though with reduced reaction rates in industrial applications.
Compared to other organic redox mediators like quinones and flavins, oxaloacetate shows moderate redox potential (-0.166V vs. SHE) that positions it advantageously for specific biochemical applications. This intermediate potential allows oxaloacetate to participate in both oxidation and reduction reactions within cellular environments without generating excessive reactive oxygen species, a common limitation of stronger oxidizing agents.
Stability analysis reveals that oxaloacetate maintains structural integrity under mild conditions but undergoes decarboxylation at elevated temperatures or extreme pH values - a limitation not shared by more robust synthetic catalysts like TEMPO derivatives or metal porphyrins. However, this apparent disadvantage becomes beneficial in biological systems where controlled degradation prevents accumulation of potentially harmful intermediates.
In terms of selectivity, oxaloacetate demonstrates remarkable substrate specificity compared to broader-acting inorganic catalysts. This selectivity stems from its molecular structure featuring both keto and carboxylic acid functional groups, enabling precise interaction with target molecules. Alternative biomimetic catalysts designed to replicate this selectivity often require complex synthesis procedures and lack the natural integration with metabolic pathways.
Cost-benefit analysis indicates that while synthetic production of oxaloacetate remains relatively expensive compared to simple metal catalysts, its renewable nature and biodegradability offer significant advantages for green chemistry applications. Emerging alternatives like enzyme-immobilized systems provide comparable selectivity but face challenges in scalability and operational stability.
Recent developments in hybrid catalytic systems combining oxaloacetate with supportive co-factors or immobilization matrices have shown promising results, narrowing the performance gap with traditional industrial catalysts while maintaining biocompatibility. These hybrid approaches leverage oxaloacetate's unique redox properties while addressing its limitations through engineered support structures.
Compared to other organic redox mediators like quinones and flavins, oxaloacetate shows moderate redox potential (-0.166V vs. SHE) that positions it advantageously for specific biochemical applications. This intermediate potential allows oxaloacetate to participate in both oxidation and reduction reactions within cellular environments without generating excessive reactive oxygen species, a common limitation of stronger oxidizing agents.
Stability analysis reveals that oxaloacetate maintains structural integrity under mild conditions but undergoes decarboxylation at elevated temperatures or extreme pH values - a limitation not shared by more robust synthetic catalysts like TEMPO derivatives or metal porphyrins. However, this apparent disadvantage becomes beneficial in biological systems where controlled degradation prevents accumulation of potentially harmful intermediates.
In terms of selectivity, oxaloacetate demonstrates remarkable substrate specificity compared to broader-acting inorganic catalysts. This selectivity stems from its molecular structure featuring both keto and carboxylic acid functional groups, enabling precise interaction with target molecules. Alternative biomimetic catalysts designed to replicate this selectivity often require complex synthesis procedures and lack the natural integration with metabolic pathways.
Cost-benefit analysis indicates that while synthetic production of oxaloacetate remains relatively expensive compared to simple metal catalysts, its renewable nature and biodegradability offer significant advantages for green chemistry applications. Emerging alternatives like enzyme-immobilized systems provide comparable selectivity but face challenges in scalability and operational stability.
Recent developments in hybrid catalytic systems combining oxaloacetate with supportive co-factors or immobilization matrices have shown promising results, narrowing the performance gap with traditional industrial catalysts while maintaining biocompatibility. These hybrid approaches leverage oxaloacetate's unique redox properties while addressing its limitations through engineered support structures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!