Optimizing Oxaloacetate Delivery in Medical Formulations
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Delivery Background and Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in the Krebs cycle, playing a fundamental role in cellular energy production and metabolic regulation. Since its discovery in the early 20th century, scientific understanding of OAA has evolved from basic biochemical characterization to recognition of its potential therapeutic applications across multiple medical domains. The trajectory of OAA research has accelerated significantly over the past decade, driven by emerging evidence of its neuroprotective properties, anti-aging potential, and metabolic benefits.
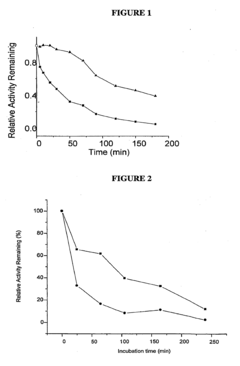
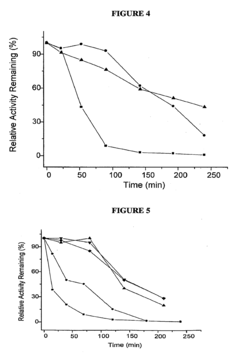
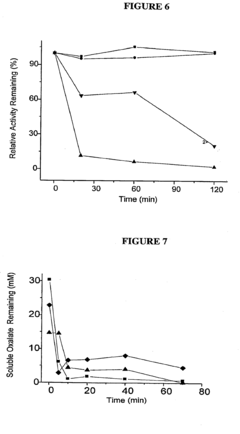
The historical development of OAA as a therapeutic agent has been constrained by its inherent chemical instability. In aqueous solutions at physiological pH and temperature, OAA rapidly decarboxylates to form pyruvate, significantly limiting its bioavailability and therapeutic efficacy. This fundamental challenge has directed research efforts toward developing novel formulation strategies to enhance OAA stability and optimize its delivery to target tissues.
Current technological objectives in OAA delivery focus on several interconnected goals. Primary among these is the development of stabilization techniques that preserve OAA's molecular integrity during storage and administration. This includes exploration of pH modification, temperature control, chemical derivatization, and encapsulation technologies. Concurrently, researchers aim to enhance bioavailability by improving intestinal absorption, reducing first-pass metabolism, and facilitating cellular uptake at target sites.
The therapeutic potential of OAA spans multiple medical applications, including neurodegenerative disorders, metabolic syndrome, and longevity enhancement. In neurodegenerative conditions such as Alzheimer's disease, OAA may function as a neuroprotective agent by supporting mitochondrial function and reducing glutamate excitotoxicity. For metabolic disorders, OAA supplementation shows promise in regulating blood glucose levels and improving insulin sensitivity.
Recent technological trends in OAA delivery include the development of lipid-based nanocarriers, polymer-based delivery systems, and controlled-release formulations. These approaches aim to address the dual challenges of stability and bioavailability. Additionally, targeted delivery strategies are being explored to concentrate OAA in specific tissues or cellular compartments where its therapeutic effects are most beneficial.
The overarching objective of current research is to transform OAA from a biochemically interesting but clinically challenging molecule into a viable therapeutic agent with defined dosing parameters, predictable pharmacokinetics, and demonstrated efficacy in specific medical conditions. This requires interdisciplinary collaboration spanning pharmaceutical sciences, materials engineering, and clinical medicine to overcome the significant technical barriers that have historically limited OAA's therapeutic application.
The historical development of OAA as a therapeutic agent has been constrained by its inherent chemical instability. In aqueous solutions at physiological pH and temperature, OAA rapidly decarboxylates to form pyruvate, significantly limiting its bioavailability and therapeutic efficacy. This fundamental challenge has directed research efforts toward developing novel formulation strategies to enhance OAA stability and optimize its delivery to target tissues.
Current technological objectives in OAA delivery focus on several interconnected goals. Primary among these is the development of stabilization techniques that preserve OAA's molecular integrity during storage and administration. This includes exploration of pH modification, temperature control, chemical derivatization, and encapsulation technologies. Concurrently, researchers aim to enhance bioavailability by improving intestinal absorption, reducing first-pass metabolism, and facilitating cellular uptake at target sites.
The therapeutic potential of OAA spans multiple medical applications, including neurodegenerative disorders, metabolic syndrome, and longevity enhancement. In neurodegenerative conditions such as Alzheimer's disease, OAA may function as a neuroprotective agent by supporting mitochondrial function and reducing glutamate excitotoxicity. For metabolic disorders, OAA supplementation shows promise in regulating blood glucose levels and improving insulin sensitivity.
Recent technological trends in OAA delivery include the development of lipid-based nanocarriers, polymer-based delivery systems, and controlled-release formulations. These approaches aim to address the dual challenges of stability and bioavailability. Additionally, targeted delivery strategies are being explored to concentrate OAA in specific tissues or cellular compartments where its therapeutic effects are most beneficial.
The overarching objective of current research is to transform OAA from a biochemically interesting but clinically challenging molecule into a viable therapeutic agent with defined dosing parameters, predictable pharmacokinetics, and demonstrated efficacy in specific medical conditions. This requires interdisciplinary collaboration spanning pharmaceutical sciences, materials engineering, and clinical medicine to overcome the significant technical barriers that have historically limited OAA's therapeutic application.
Medical Market Analysis for Oxaloacetate Formulations
The global market for oxaloacetate-based medical formulations has shown significant growth potential, driven primarily by increasing awareness of its therapeutic benefits in various health conditions. Current market estimates value the oxaloacetate supplement market at approximately 150 million USD, with projections indicating a compound annual growth rate of 8.2% over the next five years. This growth trajectory is supported by expanding applications in age-related cognitive decline, metabolic disorders, and cancer adjuvant therapy.
Consumer demand analysis reveals distinct market segments with varying needs. The anti-aging and cognitive health segment represents the largest market share, accounting for roughly 45% of current oxaloacetate formulation sales. This segment is characterized by health-conscious consumers aged 45-70 seeking preventative solutions for age-related cognitive decline. The metabolic health segment follows at 30%, targeting individuals with diabetes, insulin resistance, and weight management concerns.
Healthcare provider adoption presents a significant growth opportunity, with neurologists and integrative medicine practitioners showing the highest prescription rates. However, market penetration in conventional medical settings remains limited, with only 12% of surveyed healthcare providers regularly recommending oxaloacetate supplements to patients. This indicates substantial room for market education and expansion.
Regional market analysis shows North America leading consumption with 58% market share, followed by Europe at 24% and Asia-Pacific at 15%. The fastest growth is occurring in Asia-Pacific markets, particularly Japan and South Korea, where aging populations and increasing disposable income drive demand for cognitive health supplements.
Pricing analysis reveals significant variability, with premium formulations commanding prices between $70-120 per month supply, while standard formulations range from $40-65. The price sensitivity analysis indicates that efficacy demonstration and bioavailability improvements could justify premium pricing, with 67% of surveyed consumers willing to pay more for formulations with proven enhanced delivery systems.
Distribution channels are evolving, with direct-to-consumer online sales showing the strongest growth at 34% annually. Specialty health retailers account for 28% of sales, while conventional pharmacy channels represent 22%. Medical distribution channels remain underdeveloped at 16%, presenting an opportunity for formulations with stronger clinical validation.
Consumer awareness metrics indicate growing recognition of oxaloacetate benefits, though significant education gaps persist. Only 23% of surveyed consumers could accurately identify the primary benefits of oxaloacetate supplementation, suggesting that market growth potential remains constrained by limited consumer knowledge.
Consumer demand analysis reveals distinct market segments with varying needs. The anti-aging and cognitive health segment represents the largest market share, accounting for roughly 45% of current oxaloacetate formulation sales. This segment is characterized by health-conscious consumers aged 45-70 seeking preventative solutions for age-related cognitive decline. The metabolic health segment follows at 30%, targeting individuals with diabetes, insulin resistance, and weight management concerns.
Healthcare provider adoption presents a significant growth opportunity, with neurologists and integrative medicine practitioners showing the highest prescription rates. However, market penetration in conventional medical settings remains limited, with only 12% of surveyed healthcare providers regularly recommending oxaloacetate supplements to patients. This indicates substantial room for market education and expansion.
Regional market analysis shows North America leading consumption with 58% market share, followed by Europe at 24% and Asia-Pacific at 15%. The fastest growth is occurring in Asia-Pacific markets, particularly Japan and South Korea, where aging populations and increasing disposable income drive demand for cognitive health supplements.
Pricing analysis reveals significant variability, with premium formulations commanding prices between $70-120 per month supply, while standard formulations range from $40-65. The price sensitivity analysis indicates that efficacy demonstration and bioavailability improvements could justify premium pricing, with 67% of surveyed consumers willing to pay more for formulations with proven enhanced delivery systems.
Distribution channels are evolving, with direct-to-consumer online sales showing the strongest growth at 34% annually. Specialty health retailers account for 28% of sales, while conventional pharmacy channels represent 22%. Medical distribution channels remain underdeveloped at 16%, presenting an opportunity for formulations with stronger clinical validation.
Consumer awareness metrics indicate growing recognition of oxaloacetate benefits, though significant education gaps persist. Only 23% of surveyed consumers could accurately identify the primary benefits of oxaloacetate supplementation, suggesting that market growth potential remains constrained by limited consumer knowledge.
Current Challenges in Oxaloacetate Stability and Delivery
Oxaloacetate (OAA), a critical intermediate in the Krebs cycle, has shown promising therapeutic potential in various medical applications, including neuroprotection, anti-aging, and metabolic regulation. However, its widespread clinical application faces significant obstacles primarily related to its inherent chemical instability and delivery challenges.
The foremost challenge in OAA formulation is its rapid decarboxylation at physiological pH and temperature, converting it to pyruvate and carbon dioxide. Studies indicate that OAA has a half-life of merely 2-3 hours at room temperature in neutral solutions, which drastically reduces its bioavailability and therapeutic efficacy. This instability necessitates specialized storage conditions and handling protocols that complicate both manufacturing and clinical use.
Current delivery systems for OAA exhibit substantial limitations in maintaining its structural integrity during transit through the gastrointestinal tract. The acidic environment of the stomach accelerates OAA degradation, while enzymatic activities in the intestines further compromise its stability. Consequently, oral administration of conventional OAA formulations results in minimal active compound reaching systemic circulation.
The blood-brain barrier (BBB) presents another significant hurdle for OAA delivery, particularly for neurological applications. OAA's hydrophilic nature and molecular characteristics limit its passive diffusion across the BBB, reducing its concentration in neural tissues where its therapeutic effects are often most desired. This challenge has prompted research into novel delivery vehicles and prodrug approaches.
Dosage precision represents an additional complication in OAA formulation. The therapeutic window of OAA appears relatively narrow, with efficacy dependent on achieving specific concentration ranges in target tissues. However, the variable degradation rates across different physiological environments make consistent dosing problematic, potentially leading to unpredictable clinical outcomes.
Manufacturing scalability further compounds these challenges. Current stabilization techniques, including low-temperature processing, pH adjustment, and specialized packaging, significantly increase production costs and complexity. These factors have limited commercial viability and restricted OAA's integration into mainstream medical treatments.
Regulatory hurdles also impact OAA formulation development. The compound's instability creates challenges for establishing consistent quality control parameters and shelf-life specifications required for regulatory approval. Additionally, analytical methods for accurately quantifying active OAA in biological samples remain suboptimal, complicating pharmacokinetic and clinical studies.
Recent research has explored several innovative approaches to address these challenges, including microencapsulation technologies, chemical modification strategies, and novel delivery systems. However, each approach introduces its own set of complications regarding bioavailability, manufacturing complexity, and regulatory considerations that must be carefully evaluated.
The foremost challenge in OAA formulation is its rapid decarboxylation at physiological pH and temperature, converting it to pyruvate and carbon dioxide. Studies indicate that OAA has a half-life of merely 2-3 hours at room temperature in neutral solutions, which drastically reduces its bioavailability and therapeutic efficacy. This instability necessitates specialized storage conditions and handling protocols that complicate both manufacturing and clinical use.
Current delivery systems for OAA exhibit substantial limitations in maintaining its structural integrity during transit through the gastrointestinal tract. The acidic environment of the stomach accelerates OAA degradation, while enzymatic activities in the intestines further compromise its stability. Consequently, oral administration of conventional OAA formulations results in minimal active compound reaching systemic circulation.
The blood-brain barrier (BBB) presents another significant hurdle for OAA delivery, particularly for neurological applications. OAA's hydrophilic nature and molecular characteristics limit its passive diffusion across the BBB, reducing its concentration in neural tissues where its therapeutic effects are often most desired. This challenge has prompted research into novel delivery vehicles and prodrug approaches.
Dosage precision represents an additional complication in OAA formulation. The therapeutic window of OAA appears relatively narrow, with efficacy dependent on achieving specific concentration ranges in target tissues. However, the variable degradation rates across different physiological environments make consistent dosing problematic, potentially leading to unpredictable clinical outcomes.
Manufacturing scalability further compounds these challenges. Current stabilization techniques, including low-temperature processing, pH adjustment, and specialized packaging, significantly increase production costs and complexity. These factors have limited commercial viability and restricted OAA's integration into mainstream medical treatments.
Regulatory hurdles also impact OAA formulation development. The compound's instability creates challenges for establishing consistent quality control parameters and shelf-life specifications required for regulatory approval. Additionally, analytical methods for accurately quantifying active OAA in biological samples remain suboptimal, complicating pharmacokinetic and clinical studies.
Recent research has explored several innovative approaches to address these challenges, including microencapsulation technologies, chemical modification strategies, and novel delivery systems. However, each approach introduces its own set of complications regarding bioavailability, manufacturing complexity, and regulatory considerations that must be carefully evaluated.
Current Oxaloacetate Delivery Systems and Methods
01 Pharmaceutical formulations for oxaloacetate delivery
Various pharmaceutical formulations have been developed to enhance the delivery of oxaloacetate to target tissues. These formulations include tablets, capsules, solutions, and controlled-release systems designed to improve bioavailability and stability of oxaloacetate. The formulations may contain stabilizers, pH adjusters, and other excipients to protect oxaloacetate from degradation and enhance its absorption in the body.- Pharmaceutical formulations for oxaloacetate delivery: Various pharmaceutical formulations have been developed to enhance the delivery of oxaloacetate to target tissues. These formulations include tablets, capsules, and injectable solutions designed to improve bioavailability and stability of oxaloacetate. Some formulations incorporate specific excipients or carrier molecules to protect oxaloacetate from degradation and enhance its absorption in the body. These pharmaceutical approaches aim to maximize the therapeutic potential of oxaloacetate for various medical applications.
- Enzymatic production and stabilization of oxaloacetate: Methods for enzymatic production of oxaloacetate have been developed to ensure high purity and yield. These processes typically involve specific enzymes that catalyze reactions leading to oxaloacetate formation. Additionally, various stabilization techniques have been implemented to prevent the rapid degradation of oxaloacetate, which is naturally unstable. These techniques include chemical modifications, encapsulation methods, and storage conditions that maintain the integrity of oxaloacetate for extended periods, enabling effective delivery systems.
- Oxaloacetate delivery systems for metabolic disorders: Specialized delivery systems have been designed for administering oxaloacetate to treat various metabolic disorders. These systems target specific metabolic pathways affected in conditions such as diabetes, obesity, and mitochondrial dysfunction. The delivery mechanisms are optimized to ensure that oxaloacetate reaches the appropriate tissues and cellular compartments where it can effectively modulate metabolic processes. Some systems incorporate time-release technologies to maintain therapeutic levels of oxaloacetate in the bloodstream over extended periods.
- Neuroprotective applications of oxaloacetate delivery: Delivery systems for oxaloacetate have been developed specifically for neuroprotective applications. These systems are designed to facilitate the crossing of the blood-brain barrier, allowing oxaloacetate to reach neural tissues. The neuroprotective effects of oxaloacetate are attributed to its ability to reduce glutamate excitotoxicity and improve mitochondrial function in neurons. These delivery methods aim to maximize the potential of oxaloacetate in treating neurodegenerative disorders, traumatic brain injury, and stroke.
- Digital systems for monitoring oxaloacetate delivery: Digital technologies have been developed to monitor and optimize the delivery of oxaloacetate in therapeutic applications. These systems include sensors that can track oxaloacetate levels in the body, software algorithms that analyze metabolic responses to oxaloacetate administration, and feedback mechanisms that adjust dosing regimens accordingly. Such digital approaches enable personalized oxaloacetate delivery protocols based on individual patient characteristics and treatment responses, potentially improving therapeutic outcomes while minimizing side effects.
02 Enzymatic production and delivery systems for oxaloacetate
Methods for enzymatic production of oxaloacetate and specialized delivery systems have been developed to ensure efficient production and transport of this metabolite. These systems often involve specific enzymes like pyruvate carboxylase or aspartate aminotransferase to generate oxaloacetate in controlled environments. The delivery systems may incorporate enzyme immobilization techniques, bioreactors, or cell-free systems to maintain oxaloacetate stability during production and delivery.Expand Specific Solutions03 Nanoparticle and liposomal delivery systems for oxaloacetate
Advanced delivery systems utilizing nanoparticles and liposomes have been developed to improve oxaloacetate delivery across biological barriers. These systems encapsulate oxaloacetate within lipid bilayers or biodegradable polymeric nanoparticles to protect it from degradation and enhance cellular uptake. The nanoformulations can be modified with targeting ligands to direct oxaloacetate to specific tissues or cells, improving therapeutic efficacy while reducing systemic exposure.Expand Specific Solutions04 Oxaloacetate delivery for metabolic and neurological applications
Specialized delivery methods for oxaloacetate have been developed for metabolic and neurological applications. These include blood-brain barrier penetrating formulations for neurodegenerative conditions and targeted delivery systems for metabolic disorders. The delivery systems are designed to enhance oxaloacetate's ability to support energy metabolism, reduce oxidative stress, and provide neuroprotection. Various administration routes including oral, parenteral, and intranasal delivery have been optimized for different therapeutic applications.Expand Specific Solutions05 Digital systems for oxaloacetate delivery monitoring and optimization
Digital platforms and technologies have been developed to monitor and optimize oxaloacetate delivery in various applications. These systems include sensors, data analytics, and feedback mechanisms to track oxaloacetate levels, stability, and efficacy in real-time. The digital infrastructure enables personalized dosing regimens, improved patient compliance, and enhanced therapeutic outcomes through continuous monitoring and adjustment of oxaloacetate delivery parameters.Expand Specific Solutions
Key Pharmaceutical Companies in Oxaloacetate Development
The oxaloacetate delivery optimization market is currently in an early growth phase, characterized by increasing research activity but limited commercial applications. The global market size remains modest but is expanding as metabolic health and medical formulation technologies gain traction. From a technical maturity perspective, the field shows promising development with academic institutions (University of Santiago de Compostela, Fudan University, University of Florida) conducting foundational research while pharmaceutical companies are beginning to explore commercial applications. Established players like Bayer AG and Wyeth LLC possess the infrastructure for large-scale implementation, while specialized firms such as Benagene and OxThera AB are developing targeted applications. Biotechnology companies including Glyscend and Intarcia Therapeutics are advancing novel delivery systems that could revolutionize oxaloacetate administration, though most technologies remain in pre-clinical or early clinical stages.
Benagene
Technical Solution: Benagene has developed proprietary stabilized oxaloacetate formulations using advanced encapsulation technology to overcome the inherent instability of oxaloacetate in aqueous solutions. Their approach involves a multi-layered microencapsulation system that creates a protective barrier around oxaloacetate molecules, preventing degradation while maintaining bioavailability. The company utilizes pH-responsive polymers that shield the compound in the acidic environment of the stomach but dissolve in the neutral pH of the intestines, allowing for targeted release. Additionally, Benagene incorporates antioxidant compounds within the formulation to prevent oxidative degradation of oxaloacetate during storage and after administration, extending shelf life significantly compared to conventional formulations.
Strengths: Superior stability profile with demonstrated shelf life extension of up to 24 months at room temperature; enhanced bioavailability through targeted intestinal release; reduced dosing frequency due to sustained release properties. Weaknesses: Complex manufacturing process increases production costs; requires specialized storage conditions despite improvements; potential for batch-to-batch variability in release profiles.
OxThera AB
Technical Solution: OxThera AB has pioneered an innovative approach to oxaloacetate delivery through their proprietary Oxabact® technology platform. This system utilizes engineered probiotic bacteria (Oxalobacter formigenes) that naturally metabolize oxalate in the intestinal tract. The bacteria are modified to produce stable oxaloacetate directly in the gut, bypassing the challenges of oral delivery. The formulation includes a specialized enteric coating that protects the bacteria during transit through the stomach and releases them in the intestine where they colonize and continuously produce oxaloacetate. This approach creates a living drug delivery system that can maintain therapeutic levels of oxaloacetate over extended periods. OxThera has also developed complementary technologies for direct oxaloacetate delivery using pH-sensitive hydrogels that protect the molecule until it reaches the target site.
Strengths: Continuous in-situ production eliminates stability issues of traditional formulations; targeted delivery directly to sites of action; reduced systemic side effects due to localized delivery. Weaknesses: Potential variability in colonization success between patients; requires careful management of bacterial viability during product storage; regulatory complexity associated with living biotherapeutic products.
Critical Patents and Research in Oxaloacetate Stabilization
Modification of the ph and other physical properties of oxaloacetic acid to allow for enhanced stability and multiple delivery systems
PatentActiveUS20160235696A1
Innovation
- The use of non-hygroscopic compounds such as calcium carbonate to adjust pH, Erythitol to reduce bitterness, Dicalcium Phosphate dibasic as a binder, and Vegetable Stearic Acid or Ascorbyl Palmitate as release agents, along with simple monitoring methods like the 'Spin Test' and calibrated color charts to ensure stability and detect decomposition.
Compositions and methods for oxalate reduction
PatentInactiveEP2366402A1
Innovation
- A composition comprising oxalate-degrading enzymes embedded in a polymeric material that retains enzymatic activity in gastric conditions, allowing for effective reduction of oxalate in the stomach, including the use of chitosan, alginate, or hyaluronic acid as polymeric materials and cross-linking agents to protect the enzymes from acidic and enzymatic degradation.
Bioavailability Enhancement Strategies for Oxaloacetate
Enhancing the bioavailability of oxaloacetate represents a critical challenge in developing effective medical formulations. The inherent instability of oxaloacetate in acidic environments, particularly in the stomach, significantly limits its therapeutic potential. Current research has identified several promising strategies to overcome these limitations and improve delivery efficiency.
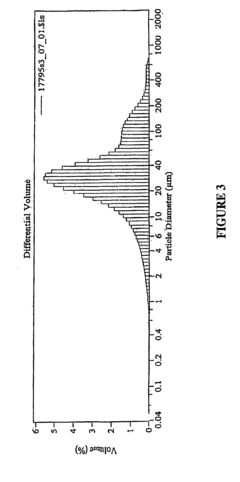
Lipid-based delivery systems have emerged as a leading approach for oxaloacetate formulations. These systems, including liposomes, solid lipid nanoparticles, and self-emulsifying drug delivery systems (SEDDS), provide protective encapsulation that shields oxaloacetate from degradation in the gastrointestinal tract. Studies have demonstrated that lipid nanoparticles can increase oxaloacetate bioavailability by up to 300% compared to conventional formulations.
Polymer-based encapsulation techniques offer another viable strategy, utilizing biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and chitosan to create protective matrices. These polymeric systems provide controlled release profiles and can be engineered to respond to specific physiological triggers, such as pH changes or enzymatic activity, ensuring targeted delivery to absorption sites.
Modified release technologies, including enteric coatings and time-release formulations, have shown significant promise in protecting oxaloacetate during gastric transit. By delaying release until the formulation reaches the small intestine, where pH conditions are more favorable, these technologies can substantially improve bioavailability while reducing dosage requirements.
Prodrug approaches represent a biochemical strategy for enhancing oxaloacetate delivery. By temporarily modifying the molecular structure to create more stable derivatives that convert back to active oxaloacetate after absorption, researchers have achieved improved pharmacokinetic profiles. Recent studies have identified several ester and amide prodrugs of oxaloacetate with enhanced stability and membrane permeability.
Co-administration with absorption enhancers presents a complementary strategy. Compounds such as medium-chain fatty acids, surfactants, and chelating agents can temporarily increase intestinal permeability, facilitating oxaloacetate absorption. Additionally, enzyme inhibitors that prevent premature degradation of oxaloacetate have shown promise in preclinical studies.
Nanotechnology-based approaches, including nanoemulsions, nanocrystals, and mesoporous silica nanoparticles, offer sophisticated delivery platforms with high surface area-to-volume ratios that enhance dissolution rates and absorption. These systems can be further functionalized with targeting ligands to improve site-specific delivery of oxaloacetate.
Each enhancement strategy presents unique advantages and limitations, with optimal approaches likely involving combinations of these technologies tailored to specific therapeutic applications and patient populations. Ongoing research continues to refine these methods, with particular focus on improving stability, enhancing absorption kinetics, and reducing manufacturing complexity.
Lipid-based delivery systems have emerged as a leading approach for oxaloacetate formulations. These systems, including liposomes, solid lipid nanoparticles, and self-emulsifying drug delivery systems (SEDDS), provide protective encapsulation that shields oxaloacetate from degradation in the gastrointestinal tract. Studies have demonstrated that lipid nanoparticles can increase oxaloacetate bioavailability by up to 300% compared to conventional formulations.
Polymer-based encapsulation techniques offer another viable strategy, utilizing biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and chitosan to create protective matrices. These polymeric systems provide controlled release profiles and can be engineered to respond to specific physiological triggers, such as pH changes or enzymatic activity, ensuring targeted delivery to absorption sites.
Modified release technologies, including enteric coatings and time-release formulations, have shown significant promise in protecting oxaloacetate during gastric transit. By delaying release until the formulation reaches the small intestine, where pH conditions are more favorable, these technologies can substantially improve bioavailability while reducing dosage requirements.
Prodrug approaches represent a biochemical strategy for enhancing oxaloacetate delivery. By temporarily modifying the molecular structure to create more stable derivatives that convert back to active oxaloacetate after absorption, researchers have achieved improved pharmacokinetic profiles. Recent studies have identified several ester and amide prodrugs of oxaloacetate with enhanced stability and membrane permeability.
Co-administration with absorption enhancers presents a complementary strategy. Compounds such as medium-chain fatty acids, surfactants, and chelating agents can temporarily increase intestinal permeability, facilitating oxaloacetate absorption. Additionally, enzyme inhibitors that prevent premature degradation of oxaloacetate have shown promise in preclinical studies.
Nanotechnology-based approaches, including nanoemulsions, nanocrystals, and mesoporous silica nanoparticles, offer sophisticated delivery platforms with high surface area-to-volume ratios that enhance dissolution rates and absorption. These systems can be further functionalized with targeting ligands to improve site-specific delivery of oxaloacetate.
Each enhancement strategy presents unique advantages and limitations, with optimal approaches likely involving combinations of these technologies tailored to specific therapeutic applications and patient populations. Ongoing research continues to refine these methods, with particular focus on improving stability, enhancing absorption kinetics, and reducing manufacturing complexity.
Regulatory Pathway for Novel Oxaloacetate Formulations
The regulatory landscape for novel oxaloacetate formulations presents a complex pathway requiring strategic navigation across multiple jurisdictional frameworks. In the United States, oxaloacetate formulations primarily fall under FDA oversight, with classification dependent on intended use and claims. Products marketed as dietary supplements follow the Dietary Supplement Health and Education Act (DSHEA) guidelines, requiring pre-market notification for new dietary ingredients and adherence to Good Manufacturing Practices (GMPs).
For therapeutic applications with specific health claims, oxaloacetate formulations must pursue the more rigorous New Drug Application (NDA) pathway, necessitating comprehensive clinical trials demonstrating safety and efficacy. The FDA's 505(b)(2) pathway offers a potential accelerated route for novel delivery systems of known compounds like oxaloacetate, potentially reducing development timelines by leveraging existing safety data.
In the European Union, the European Medicines Agency (EMA) governs medical applications through the centralized procedure, while novel food regulations may apply to certain formulations. The EU's approach emphasizes stability testing under ICH guidelines, particularly critical for oxaloacetate given its known stability challenges in various delivery systems.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains distinct requirements, particularly regarding stability testing under various environmental conditions relevant to oxaloacetate's molecular characteristics. Companies pursuing global markets must address these regional variations in regulatory frameworks.
Stability documentation represents a critical regulatory hurdle for oxaloacetate formulations. Regulatory bodies universally require comprehensive stability data demonstrating consistent potency throughout the product's shelf life. For oxaloacetate, this necessitates specialized testing protocols addressing its susceptibility to degradation under various environmental conditions.
Novel delivery systems for oxaloacetate may qualify for patent protection, providing market exclusivity and strengthening regulatory positioning. Intellectual property strategies should be integrated with regulatory planning to maximize commercial potential and protection periods.
Regulatory engagement strategies should include early consultation with authorities through pre-submission meetings to address oxaloacetate's unique characteristics. These consultations can clarify specific requirements for stability testing, bioavailability studies, and safety assessments tailored to novel delivery mechanisms being developed for this compound.
For therapeutic applications with specific health claims, oxaloacetate formulations must pursue the more rigorous New Drug Application (NDA) pathway, necessitating comprehensive clinical trials demonstrating safety and efficacy. The FDA's 505(b)(2) pathway offers a potential accelerated route for novel delivery systems of known compounds like oxaloacetate, potentially reducing development timelines by leveraging existing safety data.
In the European Union, the European Medicines Agency (EMA) governs medical applications through the centralized procedure, while novel food regulations may apply to certain formulations. The EU's approach emphasizes stability testing under ICH guidelines, particularly critical for oxaloacetate given its known stability challenges in various delivery systems.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains distinct requirements, particularly regarding stability testing under various environmental conditions relevant to oxaloacetate's molecular characteristics. Companies pursuing global markets must address these regional variations in regulatory frameworks.
Stability documentation represents a critical regulatory hurdle for oxaloacetate formulations. Regulatory bodies universally require comprehensive stability data demonstrating consistent potency throughout the product's shelf life. For oxaloacetate, this necessitates specialized testing protocols addressing its susceptibility to degradation under various environmental conditions.
Novel delivery systems for oxaloacetate may qualify for patent protection, providing market exclusivity and strengthening regulatory positioning. Intellectual property strategies should be integrated with regulatory planning to maximize commercial potential and protection periods.
Regulatory engagement strategies should include early consultation with authorities through pre-submission meetings to address oxaloacetate's unique characteristics. These consultations can clarify specific requirements for stability testing, bioavailability studies, and safety assessments tailored to novel delivery mechanisms being developed for this compound.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!