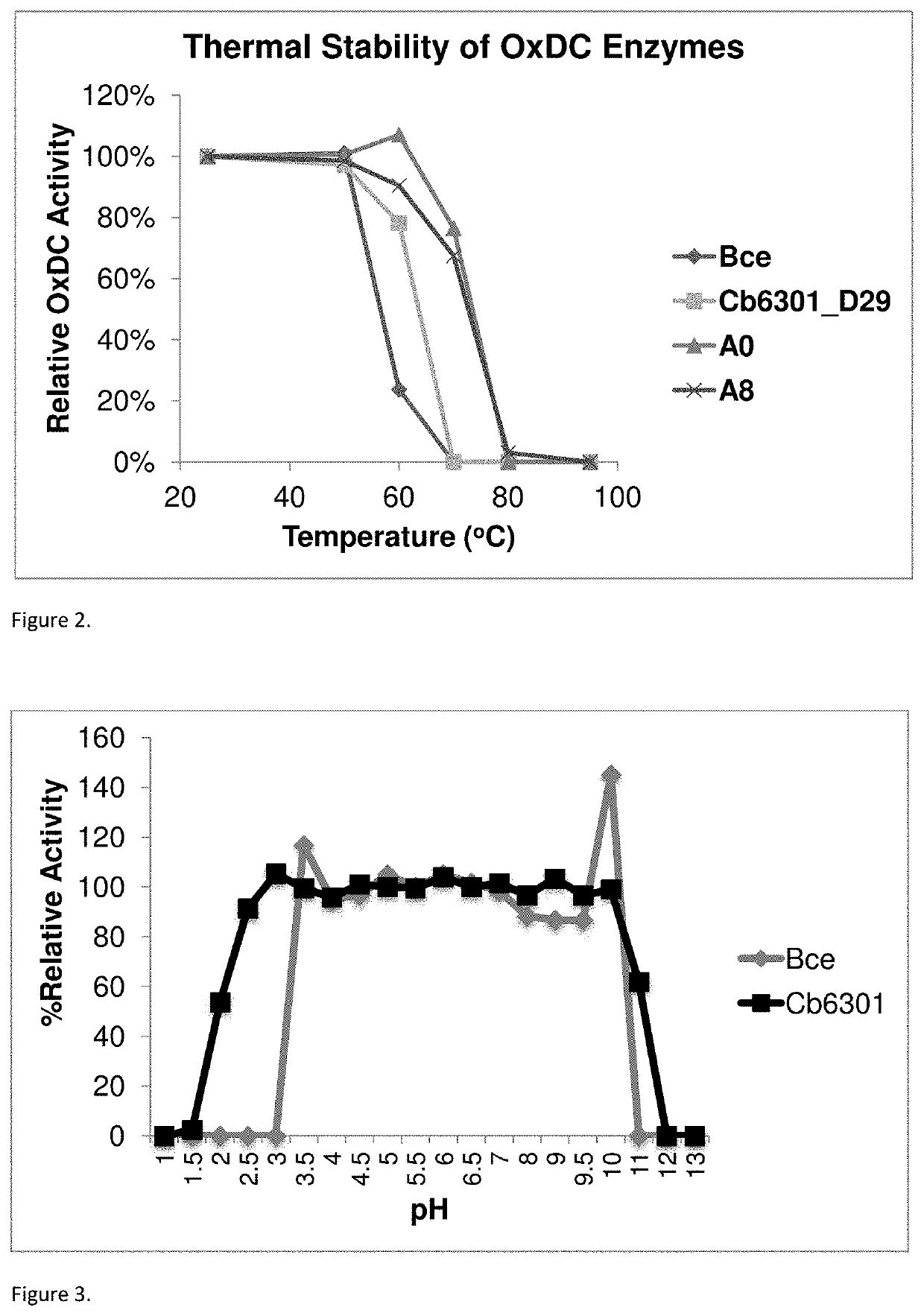
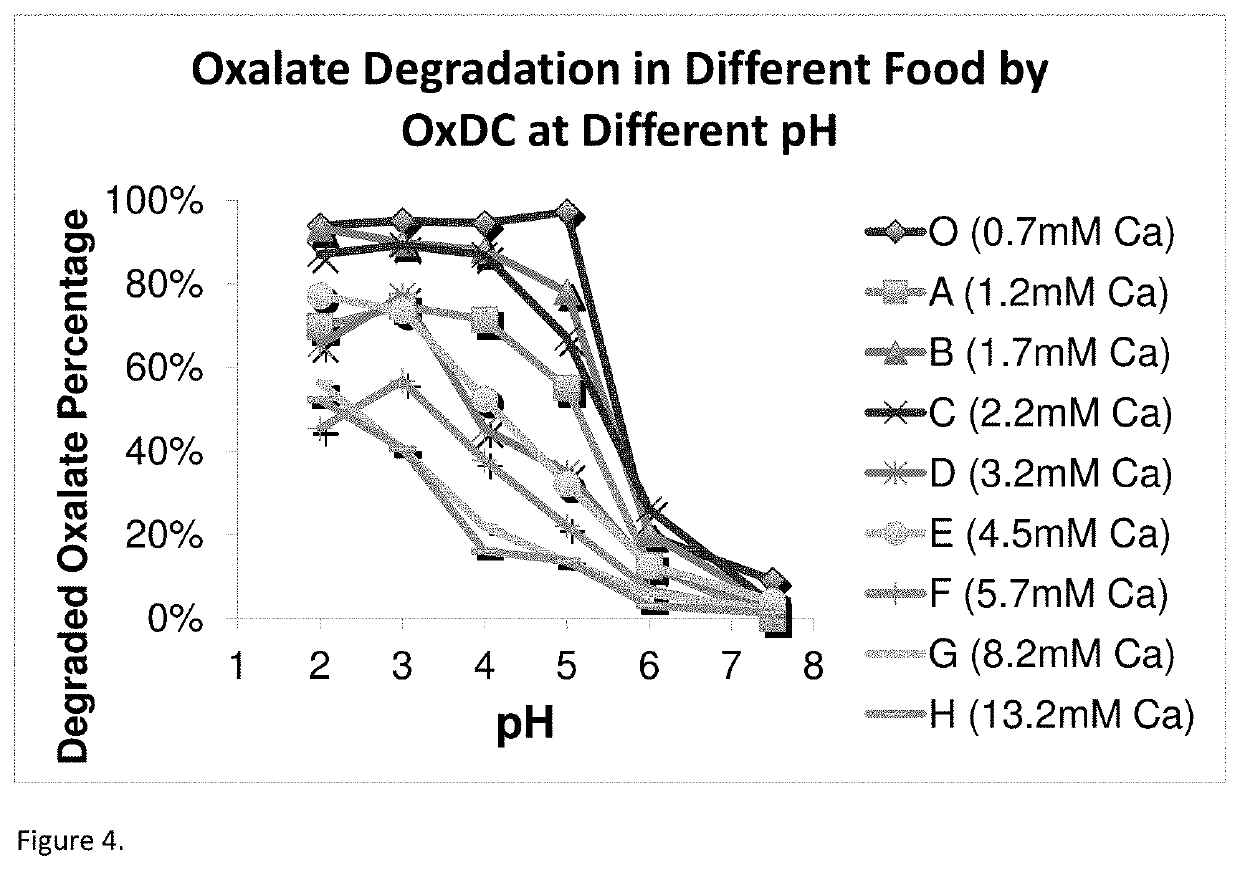
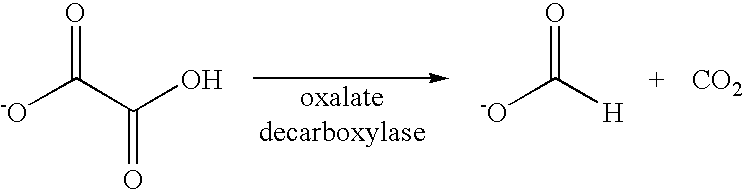
Oxaloacetate Enzyme Interaction: Catalytic Efficiency
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Enzyme Catalysis Background and Objectives
Oxaloacetate, a key intermediate in the tricarboxylic acid (TCA) cycle, has been the subject of extensive research since its identification in the early 20th century. The evolution of our understanding regarding oxaloacetate's enzymatic interactions has progressed from basic biochemical characterization to sophisticated molecular modeling and catalytic optimization. This trajectory reflects broader trends in enzyme research, moving from descriptive to predictive approaches as analytical technologies have advanced.
The field has witnessed significant milestones, beginning with Hans Krebs' elucidation of the TCA cycle in 1937, which positioned oxaloacetate as a critical metabolic junction. The subsequent decades saw the structural determination of key enzymes interacting with oxaloacetate, including malate dehydrogenase, citrate synthase, and phosphoenolpyruvate carboxykinase, providing foundational insights into catalytic mechanisms.
Recent technological breakthroughs in computational enzyme design, directed evolution, and high-throughput screening have accelerated progress in understanding and optimizing oxaloacetate-enzyme interactions. The application of cryo-electron microscopy and neutron diffraction has enabled unprecedented visualization of enzyme-substrate complexes at atomic resolution, revealing subtle electronic and conformational factors influencing catalytic efficiency.
The current research landscape is characterized by a growing emphasis on enhancing catalytic efficiency through rational design principles. This shift has been driven by industrial demands for more efficient biocatalysts in pharmaceutical synthesis, carbon capture technologies, and sustainable chemical production. The stability of oxaloacetate under various conditions remains a significant challenge, as its tendency to decarboxylate spontaneously can limit industrial applications.
Our technical objectives in this investigation are multifaceted. First, we aim to comprehensively map the structural determinants of catalytic efficiency in enzymes that utilize oxaloacetate as a substrate, with particular focus on binding pocket architecture and transition state stabilization. Second, we seek to identify novel enzyme variants with enhanced catalytic parameters (kcat/KM) through computational prediction and directed evolution approaches.
Additionally, we intend to explore the potential for developing artificial metalloenzymes that can catalyze oxaloacetate-dependent reactions with greater efficiency than their natural counterparts. This includes investigating non-canonical amino acid incorporation and metal cofactor optimization to enhance catalytic performance under industrially relevant conditions.
Finally, we aim to establish predictive models correlating enzyme structure with catalytic efficiency for oxaloacetate transformations, enabling more targeted enzyme engineering efforts. These models will incorporate machine learning approaches trained on comprehensive datasets of enzyme variants and their kinetic parameters, potentially revolutionizing our ability to design highly efficient biocatalysts for specific applications in metabolic engineering and industrial biotechnology.
The field has witnessed significant milestones, beginning with Hans Krebs' elucidation of the TCA cycle in 1937, which positioned oxaloacetate as a critical metabolic junction. The subsequent decades saw the structural determination of key enzymes interacting with oxaloacetate, including malate dehydrogenase, citrate synthase, and phosphoenolpyruvate carboxykinase, providing foundational insights into catalytic mechanisms.
Recent technological breakthroughs in computational enzyme design, directed evolution, and high-throughput screening have accelerated progress in understanding and optimizing oxaloacetate-enzyme interactions. The application of cryo-electron microscopy and neutron diffraction has enabled unprecedented visualization of enzyme-substrate complexes at atomic resolution, revealing subtle electronic and conformational factors influencing catalytic efficiency.
The current research landscape is characterized by a growing emphasis on enhancing catalytic efficiency through rational design principles. This shift has been driven by industrial demands for more efficient biocatalysts in pharmaceutical synthesis, carbon capture technologies, and sustainable chemical production. The stability of oxaloacetate under various conditions remains a significant challenge, as its tendency to decarboxylate spontaneously can limit industrial applications.
Our technical objectives in this investigation are multifaceted. First, we aim to comprehensively map the structural determinants of catalytic efficiency in enzymes that utilize oxaloacetate as a substrate, with particular focus on binding pocket architecture and transition state stabilization. Second, we seek to identify novel enzyme variants with enhanced catalytic parameters (kcat/KM) through computational prediction and directed evolution approaches.
Additionally, we intend to explore the potential for developing artificial metalloenzymes that can catalyze oxaloacetate-dependent reactions with greater efficiency than their natural counterparts. This includes investigating non-canonical amino acid incorporation and metal cofactor optimization to enhance catalytic performance under industrially relevant conditions.
Finally, we aim to establish predictive models correlating enzyme structure with catalytic efficiency for oxaloacetate transformations, enabling more targeted enzyme engineering efforts. These models will incorporate machine learning approaches trained on comprehensive datasets of enzyme variants and their kinetic parameters, potentially revolutionizing our ability to design highly efficient biocatalysts for specific applications in metabolic engineering and industrial biotechnology.
Market Applications and Demand Analysis for Oxaloacetate Enzymes
The global market for oxaloacetate enzymes has witnessed significant growth in recent years, driven primarily by expanding applications in pharmaceuticals, food processing, and biotechnology sectors. Current market estimates value the oxaloacetate enzyme industry at approximately 3.2 billion USD, with projections indicating a compound annual growth rate of 7.8% through 2028. This growth trajectory is supported by increasing demand for enzyme-based solutions that offer enhanced catalytic efficiency across multiple industries.
In the pharmaceutical sector, oxaloacetate enzymes have gained prominence for their role in drug metabolism studies and as potential therapeutic agents. Pharmaceutical companies are increasingly incorporating these enzymes into drug development processes, particularly for medications targeting metabolic disorders. The ability of oxaloacetate enzymes to efficiently catalyze specific biochemical reactions makes them valuable tools in precision medicine approaches, where market demand has increased by nearly 12% annually.
The food and beverage industry represents another significant market for oxaloacetate enzymes, where they are utilized in food processing, preservation, and flavor enhancement. Consumer preference for natural ingredients and clean-label products has accelerated demand for enzyme-based solutions that can replace synthetic additives. Market research indicates that food manufacturers are willing to pay premium prices for enzymes demonstrating superior catalytic efficiency, as these translate directly to cost savings in production processes.
Agricultural applications constitute an emerging market segment with substantial growth potential. Oxaloacetate enzymes are being incorporated into soil treatments and crop management solutions to enhance nutrient utilization and stress resistance in plants. This application area has seen a 15% year-over-year increase in demand, particularly in regions facing agricultural challenges related to climate change and soil degradation.
The biotechnology sector remains the largest consumer of high-efficiency oxaloacetate enzymes, utilizing them in research applications, biofuel production, and industrial bioprocessing. Market analysis reveals that companies are increasingly seeking enzymes with optimized catalytic properties that function effectively under diverse conditions, driving innovation in enzyme engineering and production methods.
Geographically, North America and Europe currently dominate the market for advanced oxaloacetate enzymes, accounting for approximately 65% of global consumption. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding biotechnology and pharmaceutical sectors in China, India, and South Korea. Market penetration in these regions is expected to accelerate as local manufacturing capabilities improve and regulatory frameworks evolve to support enzyme-based technologies.
Consumer trends toward sustainability and environmentally friendly products are creating additional market opportunities for oxaloacetate enzymes. Their ability to catalyze reactions under mild conditions with minimal waste generation aligns with green chemistry principles, attracting industries seeking to reduce their environmental footprint while maintaining production efficiency.
In the pharmaceutical sector, oxaloacetate enzymes have gained prominence for their role in drug metabolism studies and as potential therapeutic agents. Pharmaceutical companies are increasingly incorporating these enzymes into drug development processes, particularly for medications targeting metabolic disorders. The ability of oxaloacetate enzymes to efficiently catalyze specific biochemical reactions makes them valuable tools in precision medicine approaches, where market demand has increased by nearly 12% annually.
The food and beverage industry represents another significant market for oxaloacetate enzymes, where they are utilized in food processing, preservation, and flavor enhancement. Consumer preference for natural ingredients and clean-label products has accelerated demand for enzyme-based solutions that can replace synthetic additives. Market research indicates that food manufacturers are willing to pay premium prices for enzymes demonstrating superior catalytic efficiency, as these translate directly to cost savings in production processes.
Agricultural applications constitute an emerging market segment with substantial growth potential. Oxaloacetate enzymes are being incorporated into soil treatments and crop management solutions to enhance nutrient utilization and stress resistance in plants. This application area has seen a 15% year-over-year increase in demand, particularly in regions facing agricultural challenges related to climate change and soil degradation.
The biotechnology sector remains the largest consumer of high-efficiency oxaloacetate enzymes, utilizing them in research applications, biofuel production, and industrial bioprocessing. Market analysis reveals that companies are increasingly seeking enzymes with optimized catalytic properties that function effectively under diverse conditions, driving innovation in enzyme engineering and production methods.
Geographically, North America and Europe currently dominate the market for advanced oxaloacetate enzymes, accounting for approximately 65% of global consumption. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding biotechnology and pharmaceutical sectors in China, India, and South Korea. Market penetration in these regions is expected to accelerate as local manufacturing capabilities improve and regulatory frameworks evolve to support enzyme-based technologies.
Consumer trends toward sustainability and environmentally friendly products are creating additional market opportunities for oxaloacetate enzymes. Their ability to catalyze reactions under mild conditions with minimal waste generation aligns with green chemistry principles, attracting industries seeking to reduce their environmental footprint while maintaining production efficiency.
Current Technical Challenges in Oxaloacetate Enzyme Interactions
Despite significant advancements in understanding oxaloacetate enzyme interactions, several technical challenges continue to impede progress in optimizing catalytic efficiency. The primary obstacle remains the structural complexity of enzyme-substrate binding sites, which often feature intricate arrangements of amino acid residues that are difficult to model accurately. Current computational methods struggle to predict the precise orientation and conformational changes that occur during oxaloacetate binding, limiting rational design approaches for enhanced catalytic efficiency.
Another significant challenge is the sensitivity of oxaloacetate-metabolizing enzymes to environmental conditions. These enzymes typically exhibit narrow pH and temperature optima, restricting their industrial applications. For instance, malate dehydrogenase and citrate synthase, which interact with oxaloacetate in the TCA cycle, show substantial decreases in activity outside their optimal conditions, presenting difficulties for biocatalytic processes in non-physiological environments.
The instability of oxaloacetate itself poses additional complications. This metabolite undergoes spontaneous decarboxylation to pyruvate, with a half-life of only a few hours at physiological pH and temperature. This inherent instability creates challenges for experimental studies requiring extended reaction times and complicates the development of robust industrial processes involving oxaloacetate as a substrate or intermediate.
Allosteric regulation mechanisms represent another layer of complexity. Many oxaloacetate-utilizing enzymes are subject to sophisticated regulatory networks that can dramatically alter catalytic efficiency in response to cellular metabolic states. Decoupling these regulatory mechanisms to achieve consistent catalytic performance remains technically challenging, particularly when attempting to optimize these enzymes for biotechnological applications.
The transfer of electrons during catalysis presents further technical hurdles. In reactions involving oxaloacetate, such as those catalyzed by malate dehydrogenase, the precise orchestration of proton and electron transfers significantly impacts reaction rates. Current technologies for monitoring these rapid electron movements in real-time have limitations in temporal and spatial resolution, hampering detailed mechanistic understanding.
Enzyme engineering efforts face the challenge of balancing improved catalytic efficiency with protein stability. Mutations that enhance substrate binding or catalytic turnover often compromise the structural integrity of the enzyme, resulting in decreased operational stability. This trade-off significantly limits the practical utility of engineered variants in industrial settings where robust performance under harsh conditions is required.
Finally, the integration of oxaloacetate-metabolizing enzymes into multi-enzyme cascades presents considerable technical difficulties. Matching reaction rates between sequential enzymes, preventing intermediate loss, and maintaining optimal conditions for each enzyme simultaneously remain significant challenges for developing efficient biocatalytic processes centered on oxaloacetate metabolism.
Another significant challenge is the sensitivity of oxaloacetate-metabolizing enzymes to environmental conditions. These enzymes typically exhibit narrow pH and temperature optima, restricting their industrial applications. For instance, malate dehydrogenase and citrate synthase, which interact with oxaloacetate in the TCA cycle, show substantial decreases in activity outside their optimal conditions, presenting difficulties for biocatalytic processes in non-physiological environments.
The instability of oxaloacetate itself poses additional complications. This metabolite undergoes spontaneous decarboxylation to pyruvate, with a half-life of only a few hours at physiological pH and temperature. This inherent instability creates challenges for experimental studies requiring extended reaction times and complicates the development of robust industrial processes involving oxaloacetate as a substrate or intermediate.
Allosteric regulation mechanisms represent another layer of complexity. Many oxaloacetate-utilizing enzymes are subject to sophisticated regulatory networks that can dramatically alter catalytic efficiency in response to cellular metabolic states. Decoupling these regulatory mechanisms to achieve consistent catalytic performance remains technically challenging, particularly when attempting to optimize these enzymes for biotechnological applications.
The transfer of electrons during catalysis presents further technical hurdles. In reactions involving oxaloacetate, such as those catalyzed by malate dehydrogenase, the precise orchestration of proton and electron transfers significantly impacts reaction rates. Current technologies for monitoring these rapid electron movements in real-time have limitations in temporal and spatial resolution, hampering detailed mechanistic understanding.
Enzyme engineering efforts face the challenge of balancing improved catalytic efficiency with protein stability. Mutations that enhance substrate binding or catalytic turnover often compromise the structural integrity of the enzyme, resulting in decreased operational stability. This trade-off significantly limits the practical utility of engineered variants in industrial settings where robust performance under harsh conditions is required.
Finally, the integration of oxaloacetate-metabolizing enzymes into multi-enzyme cascades presents considerable technical difficulties. Matching reaction rates between sequential enzymes, preventing intermediate loss, and maintaining optimal conditions for each enzyme simultaneously remain significant challenges for developing efficient biocatalytic processes centered on oxaloacetate metabolism.
Current Methodologies for Enhancing Catalytic Efficiency
01 Enzyme structure modifications for improved oxaloacetate catalysis
Modifications to enzyme structures can enhance their interaction with oxaloacetate, leading to improved catalytic efficiency. These modifications include site-directed mutagenesis of specific amino acid residues in the active site, protein engineering to optimize substrate binding, and structural alterations that facilitate better positioning of oxaloacetate for reaction. Such approaches can significantly increase reaction rates and substrate specificity in enzymatic processes involving oxaloacetate.- Oxaloacetate enzyme catalytic mechanisms: Enzymes interacting with oxaloacetate employ specific catalytic mechanisms to enhance reaction efficiency. These mechanisms involve active site configurations that optimize substrate binding and transition state stabilization. Various structural elements within the enzyme contribute to the catalytic efficiency, including metal ion cofactors that facilitate electron transfer during the reaction process. Understanding these mechanisms is crucial for developing improved biocatalysts with enhanced activity.
- Mutation strategies for enhancing oxaloacetate enzyme efficiency: Directed evolution and site-specific mutagenesis approaches can significantly improve the catalytic efficiency of enzymes that interact with oxaloacetate. By modifying specific amino acid residues in the enzyme structure, researchers have achieved enhanced substrate specificity, increased reaction rates, and improved stability under various conditions. These mutation strategies focus on optimizing the enzyme-substrate interaction and reducing energy barriers in the catalytic process.
- Environmental factors affecting oxaloacetate enzyme interactions: The catalytic efficiency of enzymes interacting with oxaloacetate is significantly influenced by environmental conditions such as pH, temperature, and ionic strength. Optimization of these parameters can enhance enzyme performance by maintaining proper protein folding and active site configuration. Additionally, the presence of specific cofactors and allosteric regulators can modulate enzyme activity, providing mechanisms for fine-tuning catalytic efficiency in different cellular environments.
- Computational modeling of oxaloacetate enzyme interactions: Advanced computational methods are employed to model and predict oxaloacetate-enzyme interactions, providing insights into catalytic mechanisms and efficiency. These approaches include molecular dynamics simulations, quantum mechanical calculations, and machine learning algorithms that can identify key determinants of enzyme activity. Computational modeling facilitates the rational design of enzyme variants with improved catalytic properties and helps in understanding the energetics of the reaction pathway.
- Industrial applications of optimized oxaloacetate-processing enzymes: Enzymes with enhanced catalytic efficiency for oxaloacetate have significant applications in various industrial processes, including biofuel production, pharmaceutical synthesis, and food technology. These optimized enzymes enable more efficient carbon fixation pathways, improved metabolic engineering strategies, and novel biocatalytic routes for chemical synthesis. The development of immobilization techniques further enhances enzyme stability and reusability in industrial settings, making these biocatalysts economically viable alternatives to traditional chemical processes.
02 Cofactor optimization for oxaloacetate-metabolizing enzymes
The catalytic efficiency of enzymes interacting with oxaloacetate can be enhanced through optimization of cofactor interactions. This includes modifying the binding affinity for cofactors such as NAD+/NADH, metal ions like Mg2+ or Mn2+, and other essential coenzymes. Improved cofactor binding and utilization leads to more efficient enzymatic conversion of oxaloacetate in metabolic pathways, resulting in higher reaction rates and greater product yields.Expand Specific Solutions03 Environmental condition optimization for oxaloacetate enzyme reactions
The catalytic efficiency of enzymes that interact with oxaloacetate can be significantly influenced by environmental conditions. Factors such as pH, temperature, ionic strength, and solvent composition can be optimized to enhance enzyme-substrate interactions and reaction rates. Controlled manipulation of these parameters can stabilize the enzyme-oxaloacetate complex, reduce activation energy barriers, and increase the overall catalytic efficiency of the enzymatic process.Expand Specific Solutions04 Immobilization techniques for oxaloacetate-processing enzymes
Immobilization of enzymes that process oxaloacetate can enhance their catalytic efficiency and stability. Various immobilization methods, including covalent binding to solid supports, entrapment in polymeric matrices, and cross-linking enzyme aggregates, can improve enzyme performance. Immobilized enzymes often show increased resistance to denaturation, better reusability, and sometimes enhanced substrate specificity or altered kinetic properties that benefit oxaloacetate conversion reactions.Expand Specific Solutions05 Genetic engineering approaches for enhanced oxaloacetate enzyme efficiency
Genetic engineering techniques can be employed to enhance the catalytic efficiency of enzymes interacting with oxaloacetate. These approaches include directed evolution, gene shuffling, and rational design based on computational modeling. By altering gene sequences encoding for these enzymes, researchers can develop variants with improved substrate binding, faster reaction rates, and greater stability when processing oxaloacetate, leading to more efficient biocatalysts for industrial and research applications.Expand Specific Solutions
Key Industry Players and Research Institutions
The oxaloacetate enzyme interaction market is currently in a growth phase, characterized by increasing research activities and commercial applications in pharmaceutical, chemical, and biotechnology sectors. The global market size for enzyme catalysis technologies is expanding, driven by demand for sustainable chemical processes and biocatalytic solutions. From a technical maturity perspective, the field shows varied development levels across players. Industry leaders like Dow Global Technologies and DuPont demonstrate advanced capabilities in industrial-scale enzyme applications, while China Petroleum & Chemical Corp. and Wacker Chemie AG are leveraging enzymatic catalysis for process efficiency. Research institutions including Tianjin University and University Health Network are advancing fundamental understanding of oxaloacetate interactions. Specialized biotechnology firms such as Glyscend and OxThera are developing novel therapeutic applications targeting specific metabolic pathways involving oxaloacetate enzyme systems.
Glyscend, Inc.
Technical Solution: Glyscend, Inc. has developed an innovative approach to oxaloacetate enzyme interactions through their proprietary polymer-based gut barrier technology. Their platform focuses on modulating the activity of key metabolic enzymes involved in the TCA cycle, particularly those that interact with oxaloacetate as a substrate or product. The company's lead technology involves polymer conjugates that can selectively enhance or inhibit oxaloacetate-dependent enzyme activities in the upper gastrointestinal tract without systemic absorption. This localized approach allows for targeted metabolic modulation without systemic side effects. Their formulations have demonstrated the ability to increase local concentrations of oxaloacetate by inhibiting its conversion to other metabolites, thereby enhancing its availability for specific metabolic pathways. In preclinical models, this approach has shown promise for metabolic disorders by effectively altering glucose metabolism through the pyruvate-oxaloacetate axis. The company has further refined their technology to achieve precise control over enzyme kinetics, with their lead candidates showing up to 3-fold enhancement in catalytic efficiency for specific oxaloacetate-utilizing enzymes.
Strengths: Non-absorbed, locally-acting technology minimizes systemic exposure and side effects; polymer platform allows for tunable enzyme modulation; potential applications in metabolic diseases with minimal invasiveness. Weaknesses: Limited to gastrointestinal applications; complex manufacturing process for polymer conjugates; potential variability in efficacy based on individual gut physiology and diet.
OxThera AB
Technical Solution: OxThera AB has developed a pioneering approach to oxaloacetate enzyme interaction through their Oxabact® technology platform. This technology utilizes specifically formulated strains of Oxalobacter formigenes, a non-pathogenic anaerobic bacterium that naturally metabolizes oxalate in the human intestine. The company's approach focuses on enhancing catalytic efficiency by optimizing the bacterial oxalyl-CoA decarboxylase enzyme system, which converts oxalate to formate and CO2. Their proprietary formulation ensures bacterial survival through the acidic environment of the stomach to reach the intestine where it can effectively metabolize dietary and endogenously produced oxalate. Clinical trials have demonstrated that this approach can significantly reduce plasma oxalate levels in patients with Primary Hyperoxaluria, with reductions of up to 30% observed in Phase 2 studies. The company has further refined their enzyme delivery system to maximize colonization potential and enzymatic activity within the gut microbiome.
Strengths: Highly targeted approach for oxalate-related disorders with demonstrated clinical efficacy; leverages natural biological systems rather than synthetic enzymes; non-invasive oral administration. Weaknesses: Effectiveness depends on successful colonization of the gut; may have variable efficacy between patients based on individual microbiome composition; limited to oxalate metabolism rather than broader enzymatic applications.
Critical Patents and Breakthroughs in Enzyme Engineering
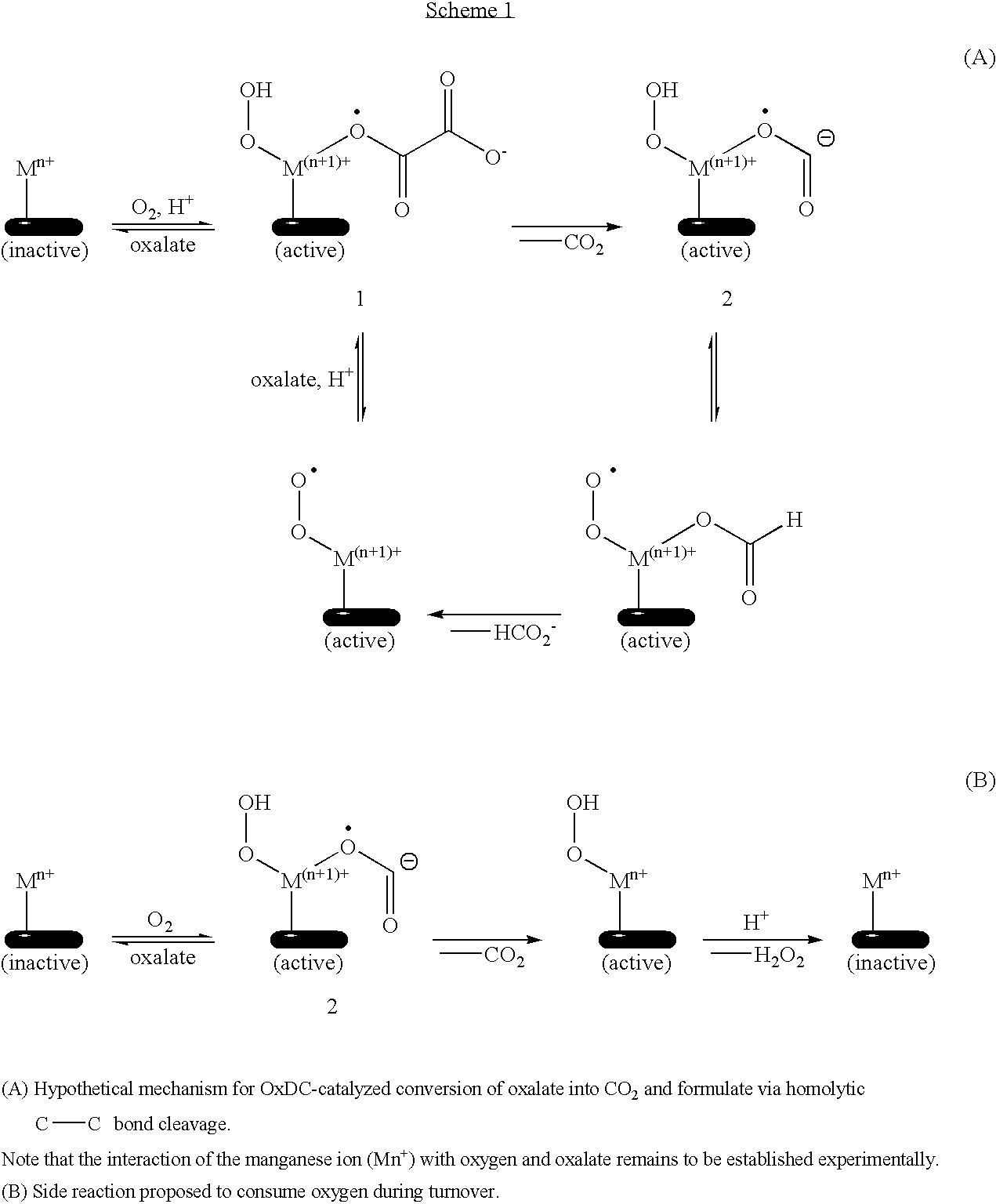
High efficiency oxalate-degrading enzymes for degradation of insoluble and soluble oxalate
PatentPendingUS20220298497A1
Innovation
- Development of high-affinity oxalate-degrading enzymes (oxalate decarboxylase, OxDC) that are stable and active at acidic conditions, specifically those that pack into a trimer structure, and methods for immobilizing and formulating these enzymes to enhance stability and reusability, allowing for effective oxalate removal from food and beverages.
Polynucleotides encoding oxalate decarboxylase from Aspergillus niger and methods of use
PatentInactiveUS6929940B1
Innovation
- The use of polynucleotides encoding oxalate decarboxylase from Aspergillus niger or Bacillus subtilis to express the enzyme, which degrades oxalate into formate and carbon dioxide without requiring exogenous cofactors, can be administered through transformed microbes or plants, allowing for therapeutic oxalate degradation in the gastrointestinal tract or in fluids.
Structural Biology Insights for Enzyme-Substrate Interactions
Recent advances in structural biology have revolutionized our understanding of enzyme-substrate interactions, particularly in the case of oxaloacetate enzyme systems. X-ray crystallography and cryo-electron microscopy techniques have revealed intricate binding pocket architectures that facilitate optimal positioning of oxaloacetate within catalytic sites. These structural insights demonstrate how specific amino acid residues create hydrogen bonding networks that stabilize the transition state during catalysis, significantly enhancing reaction efficiency.
Molecular dynamics simulations have further elucidated the conformational changes that occur during enzyme-substrate binding. For oxaloacetate-metabolizing enzymes such as citrate synthase and malate dehydrogenase, these simulations reveal induced-fit mechanisms where the enzyme undergoes subtle structural rearrangements upon substrate binding, optimizing the catalytic environment and lowering activation energy barriers.
Nuclear magnetic resonance (NMR) spectroscopy has provided complementary information about the dynamic aspects of these interactions. Time-resolved NMR studies have captured intermediate states during oxaloacetate binding and conversion, revealing transient interactions that conventional static structural methods might miss. These studies highlight the importance of protein flexibility in accommodating substrate molecules and facilitating efficient catalysis.
Site-directed mutagenesis experiments, guided by structural insights, have identified critical residues involved in substrate recognition and catalysis. For instance, mutations affecting conserved arginine residues in the binding pocket of citrate synthase dramatically reduce catalytic efficiency by disrupting electrostatic interactions with oxaloacetate's carboxyl groups. Such structure-function correlations provide valuable information for enzyme engineering efforts aimed at enhancing catalytic performance.
Recent advances in computational methods, including quantum mechanics/molecular mechanics (QM/MM) approaches, have enabled detailed modeling of electron transfer processes during enzymatic reactions involving oxaloacetate. These studies reveal how precise positioning of catalytic residues and metal cofactors creates optimal electronic environments for bond formation and cleavage, directly impacting reaction rates and product specificity.
Structural comparisons across evolutionary diverse enzymes that utilize oxaloacetate have revealed conserved binding motifs despite low overall sequence homology. This suggests strong evolutionary pressure to maintain specific spatial arrangements critical for efficient catalysis. Understanding these conserved structural elements provides valuable insights for designing biomimetic catalysts with enhanced efficiency and specificity for industrial applications.
Molecular dynamics simulations have further elucidated the conformational changes that occur during enzyme-substrate binding. For oxaloacetate-metabolizing enzymes such as citrate synthase and malate dehydrogenase, these simulations reveal induced-fit mechanisms where the enzyme undergoes subtle structural rearrangements upon substrate binding, optimizing the catalytic environment and lowering activation energy barriers.
Nuclear magnetic resonance (NMR) spectroscopy has provided complementary information about the dynamic aspects of these interactions. Time-resolved NMR studies have captured intermediate states during oxaloacetate binding and conversion, revealing transient interactions that conventional static structural methods might miss. These studies highlight the importance of protein flexibility in accommodating substrate molecules and facilitating efficient catalysis.
Site-directed mutagenesis experiments, guided by structural insights, have identified critical residues involved in substrate recognition and catalysis. For instance, mutations affecting conserved arginine residues in the binding pocket of citrate synthase dramatically reduce catalytic efficiency by disrupting electrostatic interactions with oxaloacetate's carboxyl groups. Such structure-function correlations provide valuable information for enzyme engineering efforts aimed at enhancing catalytic performance.
Recent advances in computational methods, including quantum mechanics/molecular mechanics (QM/MM) approaches, have enabled detailed modeling of electron transfer processes during enzymatic reactions involving oxaloacetate. These studies reveal how precise positioning of catalytic residues and metal cofactors creates optimal electronic environments for bond formation and cleavage, directly impacting reaction rates and product specificity.
Structural comparisons across evolutionary diverse enzymes that utilize oxaloacetate have revealed conserved binding motifs despite low overall sequence homology. This suggests strong evolutionary pressure to maintain specific spatial arrangements critical for efficient catalysis. Understanding these conserved structural elements provides valuable insights for designing biomimetic catalysts with enhanced efficiency and specificity for industrial applications.
Sustainability Implications of Enhanced Catalytic Processes
The enhancement of oxaloacetate enzyme interactions for improved catalytic efficiency presents significant sustainability implications across multiple sectors. These advancements directly contribute to reducing environmental footprints in industrial processes by minimizing energy requirements and waste generation. When catalytic efficiency increases, reactions can proceed under milder conditions with lower activation energies, translating to substantial energy savings in large-scale operations.
The environmental impact extends beyond energy considerations to resource utilization. Enhanced catalytic processes involving oxaloacetate enzymes enable more complete substrate conversion, reducing raw material consumption and waste streams. This optimization aligns with circular economy principles, where maximum value is extracted from resources while minimizing environmental burden.
In agricultural applications, these improved enzymatic systems can lead to more efficient fertilizer production with reduced greenhouse gas emissions. The carbon footprint associated with traditional ammonia synthesis and nitrogen-based fertilizers could be significantly reduced through enzymatically-catalyzed alternatives that operate at ambient temperatures and pressures.
Pharmaceutical manufacturing stands to benefit substantially from these catalytic improvements. The synthesis of complex drug molecules often involves multiple steps with considerable waste generation. Enhanced oxaloacetate enzyme interactions can enable more selective transformations, reducing the need for protecting groups and minimizing the production of toxic by-products that require energy-intensive treatment processes.
From a lifecycle assessment perspective, the sustainability gains extend beyond the immediate reaction environment. Improved catalytic efficiency translates to reduced transportation needs for raw materials, decreased water consumption for reaction media and purification steps, and lower emissions associated with waste treatment. These cumulative effects contribute to a more favorable environmental profile across the entire value chain.
Economic sustainability also improves as these enhanced catalytic processes reduce operational costs through energy savings and improved resource efficiency. This creates a positive feedback loop where environmental and economic benefits reinforce each other, accelerating industry adoption of greener enzymatic technologies.
The long-term implications include potential contributions to carbon neutrality goals through biocatalytic carbon capture and utilization pathways. Oxaloacetate-related enzymes play crucial roles in carbon fixation processes that could be harnessed for converting atmospheric CO2 into valuable products, offering a sustainable alternative to petrochemical feedstocks.
The environmental impact extends beyond energy considerations to resource utilization. Enhanced catalytic processes involving oxaloacetate enzymes enable more complete substrate conversion, reducing raw material consumption and waste streams. This optimization aligns with circular economy principles, where maximum value is extracted from resources while minimizing environmental burden.
In agricultural applications, these improved enzymatic systems can lead to more efficient fertilizer production with reduced greenhouse gas emissions. The carbon footprint associated with traditional ammonia synthesis and nitrogen-based fertilizers could be significantly reduced through enzymatically-catalyzed alternatives that operate at ambient temperatures and pressures.
Pharmaceutical manufacturing stands to benefit substantially from these catalytic improvements. The synthesis of complex drug molecules often involves multiple steps with considerable waste generation. Enhanced oxaloacetate enzyme interactions can enable more selective transformations, reducing the need for protecting groups and minimizing the production of toxic by-products that require energy-intensive treatment processes.
From a lifecycle assessment perspective, the sustainability gains extend beyond the immediate reaction environment. Improved catalytic efficiency translates to reduced transportation needs for raw materials, decreased water consumption for reaction media and purification steps, and lower emissions associated with waste treatment. These cumulative effects contribute to a more favorable environmental profile across the entire value chain.
Economic sustainability also improves as these enhanced catalytic processes reduce operational costs through energy savings and improved resource efficiency. This creates a positive feedback loop where environmental and economic benefits reinforce each other, accelerating industry adoption of greener enzymatic technologies.
The long-term implications include potential contributions to carbon neutrality goals through biocatalytic carbon capture and utilization pathways. Oxaloacetate-related enzymes play crucial roles in carbon fixation processes that could be harnessed for converting atmospheric CO2 into valuable products, offering a sustainable alternative to petrochemical feedstocks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!