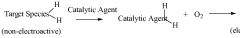Developing Oxaloacetate Sensors for Bioassay Use
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Sensing Technology Background and Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in several biochemical pathways, including the tricarboxylic acid (TCA) cycle, gluconeogenesis, and amino acid metabolism. The development of sensitive and specific sensors for OAA detection has evolved significantly over the past decades, transitioning from traditional colorimetric assays to advanced electrochemical and fluorescence-based detection methods. This technological progression has been driven by increasing demands for real-time metabolic monitoring in both research and clinical settings.
The historical context of OAA sensing begins in the mid-20th century with enzymatic assays utilizing malate dehydrogenase. These methods, while functional, suffered from limited sensitivity and specificity in complex biological matrices. The 1980s and 1990s saw the introduction of high-performance liquid chromatography (HPLC) techniques that improved quantification capabilities but remained laboratory-bound and unsuitable for point-of-care applications.
Recent technological advancements have focused on developing miniaturized, highly sensitive biosensors capable of real-time OAA detection. These innovations leverage developments in nanomaterials, enzyme immobilization techniques, and signal transduction mechanisms to overcome previous limitations. The integration of these technologies has enabled progress toward implantable sensors and lab-on-chip devices that can monitor metabolic fluctuations with unprecedented temporal resolution.
The current technological trajectory points toward multi-modal sensing platforms that can simultaneously detect OAA alongside other metabolites, providing comprehensive metabolic profiles rather than isolated measurements. This holistic approach aligns with the growing emphasis on systems biology and personalized medicine, where understanding metabolic networks is crucial for disease diagnosis and treatment optimization.
The primary objectives for developing next-generation OAA sensors include: enhancing sensitivity to detect physiologically relevant concentrations (typically in the micromolar range); improving specificity to minimize interference from structurally similar metabolites; extending sensor stability for long-term monitoring applications; and developing biocompatible materials suitable for in vivo deployment. Additionally, there is significant interest in creating sensors that function reliably in complex biological environments such as blood, tissue extracts, or cell culture media.
From a broader perspective, advanced OAA sensing technologies aim to bridge fundamental research with clinical applications, potentially enabling early detection of metabolic disorders, monitoring treatment efficacy for conditions like cancer where metabolic reprogramming occurs, and providing insights into cellular energy metabolism under various physiological and pathological conditions. The ultimate goal is to develop cost-effective, user-friendly sensing platforms that can transition from research laboratories to clinical settings, contributing to improved healthcare outcomes through precise metabolic monitoring.
The historical context of OAA sensing begins in the mid-20th century with enzymatic assays utilizing malate dehydrogenase. These methods, while functional, suffered from limited sensitivity and specificity in complex biological matrices. The 1980s and 1990s saw the introduction of high-performance liquid chromatography (HPLC) techniques that improved quantification capabilities but remained laboratory-bound and unsuitable for point-of-care applications.
Recent technological advancements have focused on developing miniaturized, highly sensitive biosensors capable of real-time OAA detection. These innovations leverage developments in nanomaterials, enzyme immobilization techniques, and signal transduction mechanisms to overcome previous limitations. The integration of these technologies has enabled progress toward implantable sensors and lab-on-chip devices that can monitor metabolic fluctuations with unprecedented temporal resolution.
The current technological trajectory points toward multi-modal sensing platforms that can simultaneously detect OAA alongside other metabolites, providing comprehensive metabolic profiles rather than isolated measurements. This holistic approach aligns with the growing emphasis on systems biology and personalized medicine, where understanding metabolic networks is crucial for disease diagnosis and treatment optimization.
The primary objectives for developing next-generation OAA sensors include: enhancing sensitivity to detect physiologically relevant concentrations (typically in the micromolar range); improving specificity to minimize interference from structurally similar metabolites; extending sensor stability for long-term monitoring applications; and developing biocompatible materials suitable for in vivo deployment. Additionally, there is significant interest in creating sensors that function reliably in complex biological environments such as blood, tissue extracts, or cell culture media.
From a broader perspective, advanced OAA sensing technologies aim to bridge fundamental research with clinical applications, potentially enabling early detection of metabolic disorders, monitoring treatment efficacy for conditions like cancer where metabolic reprogramming occurs, and providing insights into cellular energy metabolism under various physiological and pathological conditions. The ultimate goal is to develop cost-effective, user-friendly sensing platforms that can transition from research laboratories to clinical settings, contributing to improved healthcare outcomes through precise metabolic monitoring.
Bioassay Market Demand Analysis for Oxaloacetate Detection
The global market for oxaloacetate detection in bioassays has been experiencing significant growth, driven by increasing applications in metabolic research, clinical diagnostics, and pharmaceutical development. Oxaloacetate, a key intermediate in the Krebs cycle, serves as a critical biomarker for various metabolic disorders, mitochondrial function assessment, and cellular energy metabolism studies.
The clinical diagnostics segment represents the largest market share for oxaloacetate detection, with an expanding demand for early disease detection and monitoring of metabolic conditions such as diabetes, obesity, and cardiovascular diseases. Healthcare providers increasingly recognize the value of oxaloacetate measurements in assessing mitochondrial health and metabolic efficiency, creating sustained demand for sensitive and specific detection methods.
Research institutions constitute another major market segment, where oxaloacetate detection is essential for fundamental metabolic research, drug discovery processes, and toxicology studies. The growing focus on personalized medicine and metabolomics has further amplified the need for precise oxaloacetate quantification in biological samples.
Pharmaceutical companies represent a rapidly growing market segment, utilizing oxaloacetate detection in drug development pipelines, particularly for compounds targeting metabolic pathways. The ability to accurately measure oxaloacetate levels provides valuable insights into drug efficacy and potential metabolic side effects during preclinical and clinical trials.
Geographically, North America dominates the market due to advanced healthcare infrastructure and substantial research funding, followed by Europe. However, the Asia-Pacific region is witnessing the fastest growth rate, attributed to increasing healthcare expenditure, expanding biotechnology sectors in China and India, and rising prevalence of metabolic disorders.
Market analysis indicates a strong preference for non-invasive or minimally invasive detection methods that offer real-time monitoring capabilities. End-users consistently emphasize the need for improved sensitivity, specificity, and reliability in oxaloacetate detection, particularly at physiologically relevant concentrations in complex biological matrices.
Cost considerations remain significant market drivers, with healthcare providers and research institutions seeking economically viable solutions that balance performance with affordability. This creates opportunities for innovative sensor technologies that can reduce per-test costs while maintaining analytical quality.
The market trajectory suggests growing demand for point-of-care oxaloacetate detection systems, particularly in clinical settings where rapid results can inform immediate treatment decisions. Additionally, there is increasing interest in multiplexed detection platforms that can simultaneously measure oxaloacetate alongside other metabolic intermediates, providing comprehensive metabolic profiles from single samples.
The clinical diagnostics segment represents the largest market share for oxaloacetate detection, with an expanding demand for early disease detection and monitoring of metabolic conditions such as diabetes, obesity, and cardiovascular diseases. Healthcare providers increasingly recognize the value of oxaloacetate measurements in assessing mitochondrial health and metabolic efficiency, creating sustained demand for sensitive and specific detection methods.
Research institutions constitute another major market segment, where oxaloacetate detection is essential for fundamental metabolic research, drug discovery processes, and toxicology studies. The growing focus on personalized medicine and metabolomics has further amplified the need for precise oxaloacetate quantification in biological samples.
Pharmaceutical companies represent a rapidly growing market segment, utilizing oxaloacetate detection in drug development pipelines, particularly for compounds targeting metabolic pathways. The ability to accurately measure oxaloacetate levels provides valuable insights into drug efficacy and potential metabolic side effects during preclinical and clinical trials.
Geographically, North America dominates the market due to advanced healthcare infrastructure and substantial research funding, followed by Europe. However, the Asia-Pacific region is witnessing the fastest growth rate, attributed to increasing healthcare expenditure, expanding biotechnology sectors in China and India, and rising prevalence of metabolic disorders.
Market analysis indicates a strong preference for non-invasive or minimally invasive detection methods that offer real-time monitoring capabilities. End-users consistently emphasize the need for improved sensitivity, specificity, and reliability in oxaloacetate detection, particularly at physiologically relevant concentrations in complex biological matrices.
Cost considerations remain significant market drivers, with healthcare providers and research institutions seeking economically viable solutions that balance performance with affordability. This creates opportunities for innovative sensor technologies that can reduce per-test costs while maintaining analytical quality.
The market trajectory suggests growing demand for point-of-care oxaloacetate detection systems, particularly in clinical settings where rapid results can inform immediate treatment decisions. Additionally, there is increasing interest in multiplexed detection platforms that can simultaneously measure oxaloacetate alongside other metabolic intermediates, providing comprehensive metabolic profiles from single samples.
Current Challenges in Oxaloacetate Sensing Technologies
Despite significant advancements in biosensing technologies, oxaloacetate (OAA) detection faces several critical challenges that impede widespread application in bioassay systems. The primary obstacle remains sensitivity limitations, as OAA exists at relatively low concentrations in biological samples (typically 1-10 μM in blood), requiring detection methods capable of accurately measuring at submicromolar levels. Current electrochemical and optical sensing approaches often struggle to achieve this sensitivity threshold without complex sample preparation.
Selectivity presents another major hurdle, as biological matrices contain numerous structurally similar metabolites such as pyruvate, malate, and other TCA cycle intermediates that can interfere with OAA detection. This cross-reactivity significantly compromises measurement accuracy, particularly in complex biological samples like blood, tissue extracts, or fermentation media.
Stability issues further complicate OAA sensing, as this metabolite is notably unstable under physiological conditions, readily undergoing decarboxylation to form pyruvate. This inherent instability creates substantial challenges for real-time monitoring applications and necessitates rapid sample processing or specialized stabilization techniques that add complexity to sensing platforms.
Current enzymatic detection methods utilizing malate dehydrogenase (MDH) or citrate synthase face limitations in reaction kinetics and cofactor dependencies. The MDH-based approach requires NADH monitoring as an indirect measure, introducing additional variables and potential sources of error. Meanwhile, the citrate synthase method demands acetyl-CoA as a cofactor, which adds cost and complexity to sensing systems.
Miniaturization and integration challenges persist when developing point-of-care OAA sensors. The complex detection chemistry, often requiring multiple reagents or enzymes, creates significant hurdles for developing compact, user-friendly devices suitable for clinical settings or field applications. Current microfluidic approaches struggle to maintain sensitivity while reducing system complexity.
Calibration and standardization remain problematic across different biological matrices. The varying composition of samples significantly affects sensor response, necessitating matrix-specific calibration protocols that complicate universal application. The lack of standardized reference materials for OAA sensing further exacerbates this challenge.
Cost considerations present additional barriers, particularly for enzymatic sensors that require purified enzymes and cofactors, making widespread deployment economically challenging. Alternative non-enzymatic approaches using nanomaterials or synthetic receptors show promise but currently lack the specificity achieved by enzymatic methods.
Selectivity presents another major hurdle, as biological matrices contain numerous structurally similar metabolites such as pyruvate, malate, and other TCA cycle intermediates that can interfere with OAA detection. This cross-reactivity significantly compromises measurement accuracy, particularly in complex biological samples like blood, tissue extracts, or fermentation media.
Stability issues further complicate OAA sensing, as this metabolite is notably unstable under physiological conditions, readily undergoing decarboxylation to form pyruvate. This inherent instability creates substantial challenges for real-time monitoring applications and necessitates rapid sample processing or specialized stabilization techniques that add complexity to sensing platforms.
Current enzymatic detection methods utilizing malate dehydrogenase (MDH) or citrate synthase face limitations in reaction kinetics and cofactor dependencies. The MDH-based approach requires NADH monitoring as an indirect measure, introducing additional variables and potential sources of error. Meanwhile, the citrate synthase method demands acetyl-CoA as a cofactor, which adds cost and complexity to sensing systems.
Miniaturization and integration challenges persist when developing point-of-care OAA sensors. The complex detection chemistry, often requiring multiple reagents or enzymes, creates significant hurdles for developing compact, user-friendly devices suitable for clinical settings or field applications. Current microfluidic approaches struggle to maintain sensitivity while reducing system complexity.
Calibration and standardization remain problematic across different biological matrices. The varying composition of samples significantly affects sensor response, necessitating matrix-specific calibration protocols that complicate universal application. The lack of standardized reference materials for OAA sensing further exacerbates this challenge.
Cost considerations present additional barriers, particularly for enzymatic sensors that require purified enzymes and cofactors, making widespread deployment economically challenging. Alternative non-enzymatic approaches using nanomaterials or synthetic receptors show promise but currently lack the specificity achieved by enzymatic methods.
Current Oxaloacetate Detection Methodologies
01 Enzymatic biosensors for oxaloacetate detection
Enzymatic biosensors utilize specific enzymes to detect oxaloacetate with high sensitivity. These sensors typically incorporate enzymes such as malate dehydrogenase or citrate synthase that interact specifically with oxaloacetate, producing measurable signals. The enzymatic reaction can be coupled with electrochemical, optical, or fluorescent detection methods to enhance sensitivity. These biosensors offer advantages in terms of specificity and can be optimized for various sample matrices.- Enzymatic oxaloacetate detection methods: Enzymatic methods for detecting oxaloacetate involve using specific enzymes that react with oxaloacetate to produce measurable signals. These methods typically employ enzymes such as malate dehydrogenase or citrate synthase that catalyze reactions involving oxaloacetate. The sensitivity of these enzymatic sensors can be enhanced by optimizing reaction conditions, enzyme concentrations, and detection methods. These approaches allow for specific and sensitive detection of oxaloacetate in various biological samples.
- Electrochemical sensor technologies for oxaloacetate: Electrochemical sensors for oxaloacetate detection utilize electrodes modified with specific recognition elements that interact with oxaloacetate. These sensors measure changes in electrical properties such as current, potential, or impedance resulting from oxaloacetate binding or reaction. The sensitivity of electrochemical oxaloacetate sensors can be improved through electrode surface modifications, incorporation of nanomaterials, and signal amplification strategies. These sensors offer advantages of rapid response, miniaturization potential, and direct electronic readout.
- Optical and spectroscopic detection systems: Optical methods for oxaloacetate detection include fluorescence, absorbance, and colorimetric techniques that produce measurable optical signals in response to oxaloacetate presence. These systems often incorporate indicator dyes or fluorescent probes that change their optical properties upon interaction with oxaloacetate. The sensitivity of optical oxaloacetate sensors can be enhanced through signal amplification, improved optical components, and advanced detection algorithms. These methods are valuable for both qualitative and quantitative oxaloacetate analysis.
- Biosensor platforms with enhanced sensitivity: Advanced biosensor platforms for oxaloacetate detection integrate biological recognition elements with transduction mechanisms to achieve high sensitivity. These platforms may incorporate antibodies, aptamers, or molecularly imprinted polymers specific to oxaloacetate. Sensitivity enhancement strategies include signal amplification, noise reduction, and sample pre-concentration techniques. These biosensor platforms enable detection of oxaloacetate at low concentrations in complex biological matrices.
- Data processing and calibration methods: Advanced data processing and calibration methods are crucial for improving the sensitivity of oxaloacetate sensors. These approaches include signal filtering algorithms, machine learning techniques for pattern recognition, and statistical methods for noise reduction. Proper calibration procedures using standard solutions of known oxaloacetate concentrations help establish accurate response curves. These computational methods enhance the limit of detection, dynamic range, and reliability of oxaloacetate measurements across different sensor platforms.
02 Electrochemical sensing methods for oxaloacetate
Electrochemical sensors for oxaloacetate detection utilize redox reactions to generate electrical signals proportional to oxaloacetate concentration. These sensors often incorporate modified electrodes with catalytic materials or mediators that facilitate electron transfer during oxaloacetate oxidation or reduction. Techniques such as amperometry, potentiometry, and impedance spectroscopy are employed to measure the resulting signals. Electrochemical methods offer advantages including rapid response times, miniaturization potential, and direct electronic readout.Expand Specific Solutions03 Optical and fluorescence-based oxaloacetate sensors
Optical sensing techniques for oxaloacetate detection utilize changes in light absorption, fluorescence, or luminescence properties. These sensors may incorporate indicator dyes or fluorescent molecules that interact with oxaloacetate directly or through coupled reactions. The resulting optical changes are measured using spectrophotometry, fluorimetry, or specialized imaging techniques. These methods offer high sensitivity and can be adapted for real-time monitoring applications, with potential for non-invasive measurements in certain contexts.Expand Specific Solutions04 Microfluidic and lab-on-chip platforms for oxaloacetate sensing
Microfluidic systems integrate oxaloacetate sensing components into miniaturized platforms, enabling precise sample handling and analysis. These systems typically incorporate microchannels, reaction chambers, and integrated detection elements to perform automated oxaloacetate measurements. The miniaturized format allows for reduced sample volumes, faster analysis times, and potential for multiplexed detection. Lab-on-chip devices can combine sample preparation, reaction, and detection steps in a single integrated platform, enhancing sensitivity through optimized reaction conditions.Expand Specific Solutions05 Signal processing and sensitivity enhancement techniques
Various signal processing and enhancement techniques are employed to improve the sensitivity of oxaloacetate sensors. These include noise reduction algorithms, signal amplification methods, and data analysis approaches that extract meaningful information from complex sensor responses. Advanced mathematical models and machine learning algorithms can be applied to sensor data to improve detection limits and quantification accuracy. Additionally, physical and chemical modifications to sensor components, such as surface treatments or nanomaterial incorporation, can significantly enhance sensitivity by improving signal-to-noise ratios.Expand Specific Solutions
Key Industry Players in Biosensor Development
The oxaloacetate sensor bioassay market is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size remains relatively modest, estimated below $100 million, but shows promising expansion potential within the broader $30+ billion biosensor industry. Technologically, oxaloacetate sensors are advancing from laboratory proof-of-concept toward practical applications, with varying degrees of maturity across competitors. Leading players include established medical device companies like Medtronic MiniMed, Abbott Diabetes Care, and Roche Diagnostics, who leverage their extensive biosensor expertise. Academic institutions (University of Florida, Colorado State University) are driving fundamental research innovations, while specialized firms like Nova Biomedical, OptiEnz Sensors, and AquAffirm are developing targeted solutions. The competitive landscape reflects a blend of commercial development and academic research, with increasing cross-sector collaborations accelerating technology maturation.
Nova Biomedical Corp.
Technical Solution: Nova Biomedical has pioneered optical biosensor technology for oxaloacetate detection using fluorescence resonance energy transfer (FRET) principles. Their platform employs genetically engineered protein sensors with binding domains specific to oxaloacetate, triggering conformational changes that alter fluorescence intensity proportional to analyte concentration. The company's StatSensor technology incorporates these protein-based sensors into microfluidic cartridges with integrated optics for portable analysis. Nova's approach achieves detection sensitivity in the 5-50 μM range with minimal sample preparation requirements. Their sensors utilize a proprietary stabilization matrix that extends shelf-life to over 12 months at room temperature, addressing a key limitation in enzyme-based alternatives. The technology has been validated across various sample types including blood, urine, and cell culture media, with coefficient of variation below 8% across the physiological range.
Strengths: Room-temperature stable sensors with extended shelf-life; portable format suitable for field deployment; minimal sample preparation requirements; broad sample compatibility. Weaknesses: Lower sensitivity compared to some electrochemical methods; optical components increase device complexity and cost; potential for photobleaching affecting long-term measurements.
ARKRAY, Inc.
Technical Solution: ARKRAY has developed a novel colorimetric biosensor platform for oxaloacetate detection based on nanoparticle-enhanced enzymatic reactions. Their approach utilizes gold nanoparticles functionalized with malate dehydrogenase and NADH, creating a visible color change proportional to oxaloacetate concentration. The company's proprietary stabilization technology preserves enzyme activity through controlled dehydration processes, allowing ambient storage for up to 18 months. ARKRAY's sensors are integrated into lateral flow test strips with smartphone-compatible readout capabilities, enabling quantitative analysis through their dedicated mobile application. The technology achieves detection limits of approximately 20 μM with a dynamic range spanning physiological concentrations (50-500 μM). Their manufacturing process employs roll-to-roll printing techniques that significantly reduce production costs compared to traditional biosensor fabrication methods. The system has been validated in clinical settings for monitoring metabolic disorders and has shown promising applications in sports medicine for tracking metabolic efficiency.
Strengths: Cost-effective manufacturing process; smartphone integration for accessible analytics; excellent ambient stability reducing cold-chain requirements; simple user interface suitable for consumer applications. Weaknesses: Moderate sensitivity limits applications requiring trace detection; potential for color interpretation variability in different lighting conditions; limited multiplexing capabilities.
Critical Patents and Literature in Oxaloacetate Sensing
Biosensing systems and methods for assessing analyte concentrations
PatentInactiveUS20150232913A1
Innovation
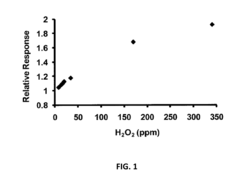
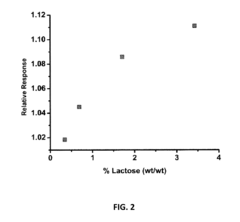
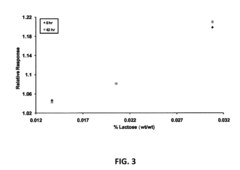
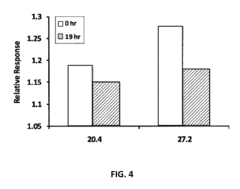
- A biosensing system comprising an optical fiber with a luminescent transducer layer and a biocomponent layer, optimized with specific enzymes and diffusion coefficients, allowing for continuous, real-time measurement of analytes like lactose and hydrogen peroxide without the need for sample dilution, using enzymes such as beta-galactosidase and glucose oxidase, and catalase, with a porous membrane for effective analyte detection.
Monitoring target endogenous species
PatentWO2009053370A1
Innovation
- An electrode with a conducting substrate, a catalytic agent for converting non-electroactive molecules to electroactive species, and an oxygen reservoir within a polymer matrix to maintain a steady oxygen supply, ensuring accurate monitoring even in oxygen-depleted environments and rejecting interfering species.
Regulatory Framework for Clinical Biosensor Applications
The regulatory landscape for oxaloacetate biosensors in clinical applications presents a complex framework that developers must navigate carefully. In the United States, the Food and Drug Administration (FDA) classifies biosensors based on their intended use and risk profile, with most diagnostic biosensors falling under Class II medical devices requiring 510(k) clearance. For oxaloacetate sensors specifically, demonstration of analytical validity, clinical validity, and clinical utility becomes paramount in the regulatory submission process.
The European Union's regulatory approach differs through the In Vitro Diagnostic Regulation (IVDR 2017/746), which has replaced the previous IVDD directive with more stringent requirements. Under this framework, oxaloacetate biosensors would likely be classified as Class B or C devices, necessitating conformity assessment involving notified bodies and comprehensive technical documentation including clinical evidence and post-market surveillance plans.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established common principles for biosensor evaluation, though regional differences persist. The IMDRF's risk-based classification system and guidance on clinical evidence requirements provide valuable reference points for global market access strategies for oxaloacetate sensor developers.
Quality management systems compliant with ISO 13485:2016 represent a fundamental regulatory requirement across jurisdictions. For oxaloacetate sensors, specific technical standards such as ISO 15197 for in vitro glucose monitoring systems may provide analogous frameworks until specific standards emerge for metabolite sensing technologies.
Data privacy considerations introduce additional regulatory complexity, particularly when biosensors connect to digital platforms. The General Data Protection Regulation (GDPR) in Europe and HIPAA in the United States impose strict requirements on patient data handling, necessitating privacy-by-design approaches in oxaloacetate sensor development.
Reimbursement pathways constitute another critical regulatory consideration. Novel biosensors like those for oxaloacetate measurement must demonstrate not only safety and efficacy but also cost-effectiveness to secure coverage decisions from public and private payers. This often requires specialized clinical studies beyond those needed for regulatory approval.
Emerging regulatory frameworks for software as a medical device (SaMD) and artificial intelligence/machine learning components are increasingly relevant as oxaloacetate sensors incorporate advanced algorithms for data interpretation. The FDA's Digital Health Innovation Action Plan and similar initiatives globally are shaping how these technologies will be regulated in bioassay applications.
The European Union's regulatory approach differs through the In Vitro Diagnostic Regulation (IVDR 2017/746), which has replaced the previous IVDD directive with more stringent requirements. Under this framework, oxaloacetate biosensors would likely be classified as Class B or C devices, necessitating conformity assessment involving notified bodies and comprehensive technical documentation including clinical evidence and post-market surveillance plans.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established common principles for biosensor evaluation, though regional differences persist. The IMDRF's risk-based classification system and guidance on clinical evidence requirements provide valuable reference points for global market access strategies for oxaloacetate sensor developers.
Quality management systems compliant with ISO 13485:2016 represent a fundamental regulatory requirement across jurisdictions. For oxaloacetate sensors, specific technical standards such as ISO 15197 for in vitro glucose monitoring systems may provide analogous frameworks until specific standards emerge for metabolite sensing technologies.
Data privacy considerations introduce additional regulatory complexity, particularly when biosensors connect to digital platforms. The General Data Protection Regulation (GDPR) in Europe and HIPAA in the United States impose strict requirements on patient data handling, necessitating privacy-by-design approaches in oxaloacetate sensor development.
Reimbursement pathways constitute another critical regulatory consideration. Novel biosensors like those for oxaloacetate measurement must demonstrate not only safety and efficacy but also cost-effectiveness to secure coverage decisions from public and private payers. This often requires specialized clinical studies beyond those needed for regulatory approval.
Emerging regulatory frameworks for software as a medical device (SaMD) and artificial intelligence/machine learning components are increasingly relevant as oxaloacetate sensors incorporate advanced algorithms for data interpretation. The FDA's Digital Health Innovation Action Plan and similar initiatives globally are shaping how these technologies will be regulated in bioassay applications.
Scalability and Manufacturing Considerations
The scalability of oxaloacetate sensor manufacturing represents a critical consideration for commercial viability in bioassay applications. Current laboratory-scale production methods typically involve small-batch synthesis of sensing elements, which presents significant challenges when transitioning to industrial-scale manufacturing. The primary bottleneck exists in maintaining consistent quality and performance characteristics across large production volumes, particularly for enzyme-based sensors where protein stability and activity must be preserved throughout the manufacturing process.
Material sourcing emerges as a fundamental consideration, with high-purity reagents and specialized enzymes requiring reliable supply chains capable of delivering consistent quality at increased volumes. The cost structure of these materials significantly impacts the economic feasibility of large-scale production, with enzyme purification processes often representing a substantial portion of manufacturing expenses. Implementation of recombinant enzyme production technologies offers promising cost reduction potential through improved yields and simplified purification protocols.
Production equipment and facilities for oxaloacetate sensor manufacturing must accommodate specialized requirements for temperature control, sterility, and precision handling. The capital investment for establishing such manufacturing infrastructure varies considerably based on production volume targets and sensor complexity. Modular manufacturing approaches have demonstrated particular promise, allowing for incremental scaling of production capacity while minimizing initial capital expenditure.
Quality control systems represent another critical element in scaling production, with automated inspection technologies increasingly essential for maintaining consistent performance across large manufacturing batches. Implementation of in-line testing protocols enables real-time monitoring of critical quality attributes, reducing waste and improving manufacturing efficiency. Statistical process control methodologies provide frameworks for continuous process improvement while ensuring regulatory compliance.
Packaging and storage considerations significantly impact sensor shelf-life and performance stability. Moisture-resistant packaging with controlled atmosphere conditions has demonstrated effectiveness in extending viable storage periods for enzyme-based sensors. The development of stabilizing formulations incorporating trehalose and other protective agents shows promise for enhancing long-term stability under various environmental conditions.
Regulatory compliance frameworks present varying requirements across global markets, necessitating adaptable manufacturing protocols capable of meeting diverse standards. The implementation of Quality by Design principles in manufacturing process development facilitates regulatory approval while simultaneously improving production efficiency and product consistency. Early engagement with regulatory authorities during manufacturing scale-up can identify potential compliance challenges before significant capital investment occurs.
Material sourcing emerges as a fundamental consideration, with high-purity reagents and specialized enzymes requiring reliable supply chains capable of delivering consistent quality at increased volumes. The cost structure of these materials significantly impacts the economic feasibility of large-scale production, with enzyme purification processes often representing a substantial portion of manufacturing expenses. Implementation of recombinant enzyme production technologies offers promising cost reduction potential through improved yields and simplified purification protocols.
Production equipment and facilities for oxaloacetate sensor manufacturing must accommodate specialized requirements for temperature control, sterility, and precision handling. The capital investment for establishing such manufacturing infrastructure varies considerably based on production volume targets and sensor complexity. Modular manufacturing approaches have demonstrated particular promise, allowing for incremental scaling of production capacity while minimizing initial capital expenditure.
Quality control systems represent another critical element in scaling production, with automated inspection technologies increasingly essential for maintaining consistent performance across large manufacturing batches. Implementation of in-line testing protocols enables real-time monitoring of critical quality attributes, reducing waste and improving manufacturing efficiency. Statistical process control methodologies provide frameworks for continuous process improvement while ensuring regulatory compliance.
Packaging and storage considerations significantly impact sensor shelf-life and performance stability. Moisture-resistant packaging with controlled atmosphere conditions has demonstrated effectiveness in extending viable storage periods for enzyme-based sensors. The development of stabilizing formulations incorporating trehalose and other protective agents shows promise for enhancing long-term stability under various environmental conditions.
Regulatory compliance frameworks present varying requirements across global markets, necessitating adaptable manufacturing protocols capable of meeting diverse standards. The implementation of Quality by Design principles in manufacturing process development facilitates regulatory approval while simultaneously improving production efficiency and product consistency. Early engagement with regulatory authorities during manufacturing scale-up can identify potential compliance challenges before significant capital investment occurs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!