How to Enhance Oxaloacetate Concentration in Supplements
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Supplement Development Background and Objectives
Oxaloacetate (OAA) represents a critical metabolite in the Krebs cycle, playing an essential role in cellular energy production and metabolic processes. The exploration of OAA as a dietary supplement has evolved significantly over the past decade, driven by emerging research suggesting its potential benefits in neurological health, longevity, and metabolic regulation. Initially identified primarily as an intermediate metabolite, OAA has gradually gained recognition for its therapeutic potential, particularly in addressing age-related cognitive decline and supporting mitochondrial function.
The supplement industry has witnessed a growing interest in metabolic intermediates as functional ingredients, with OAA emerging as a promising candidate due to its position at the intersection of several key metabolic pathways. Early research in animal models demonstrated OAA's ability to reduce blood glucose levels and potentially extend lifespan, sparking interest in its applications for human health. However, the technical challenges associated with OAA stability have historically limited its commercial viability as a supplement.
The primary technical objective in OAA supplement development centers on enhancing its concentration and stability in supplement formulations. OAA is inherently unstable in aqueous solutions, rapidly decarboxylating to form pyruvate, which significantly reduces its bioavailability and efficacy. This instability presents a fundamental challenge that must be overcome to realize the full potential of OAA as a dietary supplement. Achieving higher concentrations while maintaining stability would enable more effective dosing regimens and potentially enhance therapeutic outcomes.
Current technological approaches have focused on various stabilization methods, including encapsulation technologies, pH modification, and the development of more stable OAA derivatives. These efforts aim to protect the molecule from degradation during storage and enhance its absorption in the gastrointestinal tract. The evolution of these technologies has been gradual but promising, with recent innovations showing potential for significant improvements in OAA stability and bioavailability.
The trajectory of OAA supplement development aligns with broader trends in personalized nutrition and targeted supplementation for specific health outcomes. As research continues to elucidate the mechanisms underlying OAA's biological effects, the potential applications continue to expand, encompassing areas such as neuroprotection, metabolic health, and healthy aging. The technical goals for future development include not only enhancing concentration and stability but also optimizing delivery systems to target specific tissues and cellular compartments.
Achieving these objectives requires interdisciplinary collaboration between biochemists, formulation scientists, and clinical researchers to overcome the technical hurdles while validating the health benefits through rigorous clinical studies. The ultimate goal remains developing a stable, bioavailable OAA supplement that delivers consistent therapeutic effects at commercially viable production costs.
The supplement industry has witnessed a growing interest in metabolic intermediates as functional ingredients, with OAA emerging as a promising candidate due to its position at the intersection of several key metabolic pathways. Early research in animal models demonstrated OAA's ability to reduce blood glucose levels and potentially extend lifespan, sparking interest in its applications for human health. However, the technical challenges associated with OAA stability have historically limited its commercial viability as a supplement.
The primary technical objective in OAA supplement development centers on enhancing its concentration and stability in supplement formulations. OAA is inherently unstable in aqueous solutions, rapidly decarboxylating to form pyruvate, which significantly reduces its bioavailability and efficacy. This instability presents a fundamental challenge that must be overcome to realize the full potential of OAA as a dietary supplement. Achieving higher concentrations while maintaining stability would enable more effective dosing regimens and potentially enhance therapeutic outcomes.
Current technological approaches have focused on various stabilization methods, including encapsulation technologies, pH modification, and the development of more stable OAA derivatives. These efforts aim to protect the molecule from degradation during storage and enhance its absorption in the gastrointestinal tract. The evolution of these technologies has been gradual but promising, with recent innovations showing potential for significant improvements in OAA stability and bioavailability.
The trajectory of OAA supplement development aligns with broader trends in personalized nutrition and targeted supplementation for specific health outcomes. As research continues to elucidate the mechanisms underlying OAA's biological effects, the potential applications continue to expand, encompassing areas such as neuroprotection, metabolic health, and healthy aging. The technical goals for future development include not only enhancing concentration and stability but also optimizing delivery systems to target specific tissues and cellular compartments.
Achieving these objectives requires interdisciplinary collaboration between biochemists, formulation scientists, and clinical researchers to overcome the technical hurdles while validating the health benefits through rigorous clinical studies. The ultimate goal remains developing a stable, bioavailable OAA supplement that delivers consistent therapeutic effects at commercially viable production costs.
Market Analysis for Stable Oxaloacetate Supplements
The global market for oxaloacetate supplements has shown significant growth in recent years, driven primarily by increasing consumer awareness of its potential health benefits, particularly in the areas of longevity, brain health, and metabolic support. Current market estimates value the brain health supplement sector at approximately $8.5 billion, with metabolic health supplements representing a $5.7 billion segment. Within these categories, stable oxaloacetate supplements, though still occupying a niche position, have demonstrated annual growth rates exceeding 15% since 2019.
Consumer demographics reveal that the primary market for oxaloacetate supplements consists of health-conscious individuals aged 40-65, particularly those concerned with cognitive decline, metabolic health, and anti-aging. This demographic typically possesses higher disposable income and demonstrates willingness to invest in premium supplements with scientific backing. Geographic distribution shows strongest market penetration in North America (particularly the United States), followed by Western Europe, Japan, and increasingly, urban centers in China and South Korea.
Market research indicates several key drivers fueling demand growth. First, the aging global population has intensified interest in supplements targeting age-related cognitive decline and metabolic dysfunction. Second, increasing scientific publications on oxaloacetate's potential benefits have enhanced consumer confidence. Third, the broader trend toward preventative healthcare and wellness has created a receptive environment for supplements promising metabolic optimization.
Pricing analysis reveals significant premium positioning, with stable oxaloacetate supplements commanding 3-5 times the price of conventional supplements. This premium pricing reflects both the technical challenges in stabilization and the perceived value proposition. Current market leaders employ various differentiation strategies, including patented stabilization technologies, clinical trial backing, and specialized delivery systems.
Distribution channels show interesting segmentation, with approximately 60% of sales occurring through online direct-to-consumer channels, 25% through specialty health retailers, and the remainder through healthcare practitioners and conventional retail. This distribution pattern reflects both the specialized nature of the product and the importance of educational marketing in driving consumer adoption.
Market forecasts project continued strong growth, with the stable oxaloacetate supplement segment expected to expand at a compound annual growth rate of 18-22% over the next five years. This growth trajectory is supported by increasing research validation, expanding consumer awareness, and the development of more effective stabilization technologies that may reduce production costs and improve product efficacy.
Consumer demographics reveal that the primary market for oxaloacetate supplements consists of health-conscious individuals aged 40-65, particularly those concerned with cognitive decline, metabolic health, and anti-aging. This demographic typically possesses higher disposable income and demonstrates willingness to invest in premium supplements with scientific backing. Geographic distribution shows strongest market penetration in North America (particularly the United States), followed by Western Europe, Japan, and increasingly, urban centers in China and South Korea.
Market research indicates several key drivers fueling demand growth. First, the aging global population has intensified interest in supplements targeting age-related cognitive decline and metabolic dysfunction. Second, increasing scientific publications on oxaloacetate's potential benefits have enhanced consumer confidence. Third, the broader trend toward preventative healthcare and wellness has created a receptive environment for supplements promising metabolic optimization.
Pricing analysis reveals significant premium positioning, with stable oxaloacetate supplements commanding 3-5 times the price of conventional supplements. This premium pricing reflects both the technical challenges in stabilization and the perceived value proposition. Current market leaders employ various differentiation strategies, including patented stabilization technologies, clinical trial backing, and specialized delivery systems.
Distribution channels show interesting segmentation, with approximately 60% of sales occurring through online direct-to-consumer channels, 25% through specialty health retailers, and the remainder through healthcare practitioners and conventional retail. This distribution pattern reflects both the specialized nature of the product and the importance of educational marketing in driving consumer adoption.
Market forecasts project continued strong growth, with the stable oxaloacetate supplement segment expected to expand at a compound annual growth rate of 18-22% over the next five years. This growth trajectory is supported by increasing research validation, expanding consumer awareness, and the development of more effective stabilization technologies that may reduce production costs and improve product efficacy.
Technical Challenges in Oxaloacetate Stability and Bioavailability
Oxaloacetate (OAA) presents significant challenges in supplement formulation due to its inherent chemical instability. At room temperature and neutral pH, OAA rapidly decarboxylates to form pyruvate, with studies showing degradation rates of up to 50% within 24-48 hours under standard storage conditions. This instability is particularly problematic for commercial supplement production, where shelf-life requirements typically exceed 18-24 months.
The molecular structure of OAA contains a β-keto acid group that makes it highly susceptible to spontaneous decarboxylation. This reaction is accelerated by several factors including elevated temperatures, exposure to light, presence of metal ions, and pH fluctuations. Research indicates that even minor temperature increases from 20°C to 30°C can double the degradation rate, creating significant challenges for transportation and storage in various climatic conditions.
Bioavailability represents another major technical hurdle. When ingested, OAA faces several physiological barriers including gastric acid degradation, first-pass metabolism in the liver, and limited cellular uptake mechanisms. Clinical pharmacokinetic studies demonstrate that unprotected OAA has a bioavailability of less than 5% when administered orally, rendering most conventional supplement formulations ineffective at delivering therapeutic concentrations to target tissues.
The blood-brain barrier (BBB) presents an additional challenge for OAA supplements targeting neurological benefits. The molecule's hydrophilic nature and relatively large size limit passive diffusion across the BBB, while specific transporters for OAA have limited capacity. This results in minimal central nervous system penetration, despite promising preclinical data suggesting neuroprotective effects at sufficient concentrations.
Current manufacturing processes also contribute to stability issues. Traditional methods of OAA production and purification often introduce trace contaminants that catalyze degradation reactions. Additionally, common excipients used in supplement formulations may interact unfavorably with OAA, further compromising stability. Production-scale challenges include maintaining precise temperature and humidity control throughout manufacturing and packaging processes.
Analytical challenges further complicate development efforts. Accurate quantification of OAA in complex matrices requires sophisticated analytical techniques such as HPLC-MS/MS with specialized derivatization protocols. The lack of standardized analytical methods across the industry has led to inconsistent potency claims and quality control issues, undermining consumer confidence and regulatory compliance.
Dose-dependent efficacy presents another technical challenge. Preclinical studies suggest that therapeutic effects require sustained plasma concentrations above specific thresholds, which are difficult to achieve with current formulations. This creates a technical paradox where higher doses are needed for efficacy, yet higher concentrations accelerate degradation during storage, necessitating innovative stabilization approaches that can maintain potency while enabling practical dosing regimens.
The molecular structure of OAA contains a β-keto acid group that makes it highly susceptible to spontaneous decarboxylation. This reaction is accelerated by several factors including elevated temperatures, exposure to light, presence of metal ions, and pH fluctuations. Research indicates that even minor temperature increases from 20°C to 30°C can double the degradation rate, creating significant challenges for transportation and storage in various climatic conditions.
Bioavailability represents another major technical hurdle. When ingested, OAA faces several physiological barriers including gastric acid degradation, first-pass metabolism in the liver, and limited cellular uptake mechanisms. Clinical pharmacokinetic studies demonstrate that unprotected OAA has a bioavailability of less than 5% when administered orally, rendering most conventional supplement formulations ineffective at delivering therapeutic concentrations to target tissues.
The blood-brain barrier (BBB) presents an additional challenge for OAA supplements targeting neurological benefits. The molecule's hydrophilic nature and relatively large size limit passive diffusion across the BBB, while specific transporters for OAA have limited capacity. This results in minimal central nervous system penetration, despite promising preclinical data suggesting neuroprotective effects at sufficient concentrations.
Current manufacturing processes also contribute to stability issues. Traditional methods of OAA production and purification often introduce trace contaminants that catalyze degradation reactions. Additionally, common excipients used in supplement formulations may interact unfavorably with OAA, further compromising stability. Production-scale challenges include maintaining precise temperature and humidity control throughout manufacturing and packaging processes.
Analytical challenges further complicate development efforts. Accurate quantification of OAA in complex matrices requires sophisticated analytical techniques such as HPLC-MS/MS with specialized derivatization protocols. The lack of standardized analytical methods across the industry has led to inconsistent potency claims and quality control issues, undermining consumer confidence and regulatory compliance.
Dose-dependent efficacy presents another technical challenge. Preclinical studies suggest that therapeutic effects require sustained plasma concentrations above specific thresholds, which are difficult to achieve with current formulations. This creates a technical paradox where higher doses are needed for efficacy, yet higher concentrations accelerate degradation during storage, necessitating innovative stabilization approaches that can maintain potency while enabling practical dosing regimens.
Current Formulation Approaches for Oxaloacetate Supplements
01 Effective concentration ranges for oxaloacetate supplements
Research indicates specific concentration ranges for oxaloacetate supplements to achieve therapeutic effects. These concentrations typically range from 100mg to 1000mg per day for adults, with some studies suggesting optimal efficacy at 250-500mg daily dosages. The concentration may be adjusted based on individual factors such as body weight, age, and specific health conditions being targeted. Higher concentrations may be used for specific neurological applications, while lower doses are often recommended for general health maintenance.- Effective concentration ranges for oxaloacetate supplements: Research indicates specific concentration ranges for oxaloacetate supplements to achieve therapeutic effects. These concentrations typically range from 100mg to 1000mg per day for human consumption, with some formulations suggesting divided doses for optimal absorption. The concentration is critical for maintaining stability of the compound while providing sufficient active ingredient to produce the desired metabolic and neuroprotective effects.
- Stabilization methods for oxaloacetate in supplement formulations: Oxaloacetate is inherently unstable in solution, requiring specific stabilization methods for supplement formulations. These include encapsulation technologies, pH adjustment, addition of specific buffer systems, and combination with stabilizing agents. Some formulations use enteric coating to protect oxaloacetate from stomach acid degradation, while others employ freeze-drying or spray-drying techniques to maintain potency during storage.
- Bioavailability enhancement techniques for oxaloacetate: Various techniques are employed to enhance the bioavailability of oxaloacetate supplements. These include formulating with specific carriers, using nanoparticle delivery systems, combining with absorption enhancers, and developing time-release mechanisms. Some formulations incorporate lipid-based delivery systems to improve cellular uptake, while others use specific excipients to protect the compound during digestion and facilitate transport across intestinal membranes.
- Combination with other active ingredients for synergistic effects: Oxaloacetate supplements are often formulated in combination with other active ingredients to achieve synergistic effects. Common combinations include vitamins (particularly B vitamins), minerals like magnesium and zinc, antioxidants, and other metabolic intermediates such as alpha-ketoglutarate. These combinations are designed to enhance mitochondrial function, support the Krebs cycle, improve energy production, and provide neuroprotective benefits beyond what oxaloacetate alone can deliver.
- Testing methods for determining oxaloacetate concentration and purity: Various analytical methods are employed to determine the concentration and purity of oxaloacetate in supplement formulations. These include high-performance liquid chromatography (HPLC), mass spectrometry, enzymatic assays, and spectrophotometric techniques. Quality control protocols typically involve stability testing under different storage conditions, assessment of degradation products, and verification of bioactivity to ensure that the stated concentration of active oxaloacetate is maintained throughout the product's shelf life.
02 Formulation techniques for oxaloacetate stability
Oxaloacetate is inherently unstable in solution, requiring specific formulation techniques to maintain potency. Methods include microencapsulation, use of stabilizing agents, pH control systems, and specialized coating technologies. Some formulations employ enteric coatings to protect oxaloacetate from stomach acid degradation, while others use time-release mechanisms to optimize absorption. Freeze-drying and anhydrous formulations have also been developed to extend shelf life and preserve the active compound's efficacy.Expand Specific Solutions03 Bioavailability enhancement methods for oxaloacetate
Various methods have been developed to enhance the bioavailability of oxaloacetate supplements. These include combining oxaloacetate with specific carriers like lipid nanoparticles, cyclodextrins, or protein complexes. Some formulations incorporate enzyme inhibitors to prevent premature breakdown of oxaloacetate in the digestive system. Other approaches utilize co-factors such as vitamin B1 (thiamine) to enhance cellular uptake and utilization of oxaloacetate, resulting in improved therapeutic outcomes at lower concentrations.Expand Specific Solutions04 Oxaloacetate concentration for specific health applications
Different concentrations of oxaloacetate supplements are recommended for various health applications. For neuroprotection and cognitive enhancement, higher concentrations (500-1000mg) have been studied. Lower concentrations (100-250mg) are often used for general metabolic support and anti-aging applications. For blood glucose management, moderate concentrations with specific timing protocols have shown efficacy. Some formulations combine oxaloacetate with other active ingredients at precise ratios to target specific health conditions or metabolic pathways.Expand Specific Solutions05 Testing and measurement methods for oxaloacetate concentration
Various analytical techniques have been developed to accurately measure oxaloacetate concentration in supplements and biological samples. These include high-performance liquid chromatography (HPLC), mass spectrometry, enzymatic assays, and spectrophotometric methods. Quality control protocols often employ multiple testing methodologies to ensure consistent concentration and stability throughout the product lifecycle. Some patents describe novel biosensor technologies for real-time monitoring of oxaloacetate levels in biological systems or during manufacturing processes.Expand Specific Solutions
Key Industry Players in Metabolic Supplement Production
The oxaloacetate supplement market is currently in a growth phase, characterized by increasing research interest and emerging commercial applications. The global market size for metabolic health supplements is expanding, with oxaloacetate gaining attention for its potential benefits in neurological health and metabolic regulation. From a technological maturity perspective, the field shows varied development stages across key players. Established pharmaceutical companies like Ionis Pharmaceuticals and BASF Corp are leveraging their R&D capabilities to advance formulation technologies, while specialized biotechnology firms such as Glyscend, Synlogic, and Codexis are applying enzymatic and synthetic biology approaches to enhance oxaloacetate stability and bioavailability. Academic institutions including the University of Florida and University of Santiago de Compostela are contributing fundamental research, creating a competitive landscape balanced between established corporations and innovative startups.
Benagene
Technical Solution: Benagene has developed a proprietary stabilization technology for oxaloacetate supplements that addresses the compound's inherent instability. Their approach involves microencapsulation techniques that protect oxaloacetate from degradation in both storage and gastrointestinal environments. The company utilizes a specialized freeze-drying process combined with pH-controlled environments to maintain the molecular integrity of oxaloacetate. Their formulation includes specific buffering agents that prevent decarboxylation, the primary degradation pathway for oxaloacetate. Additionally, Benagene has incorporated bioavailability enhancers such as modified cyclodextrins that form inclusion complexes with oxaloacetate molecules, further protecting them while improving cellular uptake. Their supplements undergo rigorous stability testing under various temperature and humidity conditions to ensure consistent potency throughout shelf life.
Strengths: Superior stabilization technology providing longer shelf life than competitors; enhanced bioavailability through proprietary delivery systems; comprehensive quality control protocols. Weaknesses: Higher production costs leading to premium pricing; complex manufacturing process requiring specialized equipment; potential limitations in scaling production to meet increasing market demand.
Synlogic Operating Co., Inc.
Technical Solution: Synlogic has pioneered an innovative approach to enhancing oxaloacetate concentration through engineered probiotic bacteria. Their Synthetic Biotic™ platform utilizes genetically modified probiotics that can produce oxaloacetate directly in the gut microbiome. These engineered microbes contain optimized metabolic pathways with upregulated expression of key enzymes like phosphoenolpyruvate carboxylase and malate dehydrogenase, which are critical for oxaloacetate synthesis. The bacteria are designed with genetic circuits that respond to specific gut environmental cues, triggering oxaloacetate production when and where needed. This approach circumvents the stability challenges of traditional supplements by generating fresh oxaloacetate in situ. Synlogic's technology includes controlled release mechanisms that regulate the rate of oxaloacetate production and specialized transport systems that facilitate absorption across intestinal epithelia. Their formulations also incorporate prebiotics that selectively nourish the engineered probiotics, creating a synergistic effect for sustained oxaloacetate delivery.
Strengths: Continuous in situ production eliminates stability concerns; targeted delivery directly to absorption sites; potential for personalized dosing based on individual microbiome profiles. Weaknesses: Regulatory hurdles associated with genetically modified organisms; variable efficacy depending on individual gut microbiome composition; potential immunological responses to engineered bacteria in some individuals.
Critical Patents and Research in Oxaloacetate Enhancement
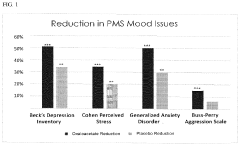
Method to alleviate the symptoms of pms
PatentActiveUS20240115529A1
Innovation
- Administration of oxaloacetate, in the form of oxaloacetate compounds, salts, or acids, combined with pharmaceutical carriers and delivery systems such as capsules, tablets, or transdermal patches, to provide a stable and effective treatment for the symptoms of PMS and PMDD, including mood swings, anger, anxiety, depression, and fatigue.
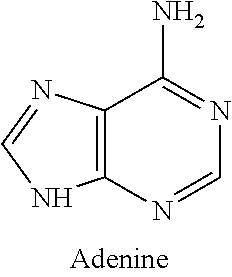
Low radiocarbon dietary supplements and foods and methods of making and using same
PatentActiveUS8668898B1
Innovation
- Development of nutritional supplements comprising DNA precursor compounds and amino acids with significantly reduced radiocarbon levels, combined with conventional dietary components at natural abundance levels, to isotopically dilute radiocarbon in vertebrate organisms, thereby reducing genetic and cellular damage.
Regulatory Framework for Metabolic Health Supplements
The regulatory landscape for metabolic health supplements, particularly those containing oxaloacetate, is complex and varies significantly across global markets. In the United States, the FDA regulates supplements under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which classifies them as food products rather than drugs. This classification means that oxaloacetate supplements do not require pre-market approval, but manufacturers must ensure safety and avoid making disease treatment claims.
European regulations are more stringent, with the European Food Safety Authority (EFSA) requiring substantial scientific evidence for health claims. Oxaloacetate supplements face particular scrutiny due to their metabolic enhancement properties, and manufacturers must navigate the Novel Food Regulation if the ingredient lacks significant consumption history prior to May 1997.
Stability requirements present another regulatory challenge for oxaloacetate supplements. Due to the compound's inherent instability, regulatory bodies often require manufacturers to demonstrate consistent potency throughout the product's shelf life. This necessitates advanced stabilization technologies and rigorous quality control protocols to meet regulatory standards.
Labeling regulations also significantly impact oxaloacetate supplement marketing. In most jurisdictions, labels must accurately reflect ingredient concentrations and avoid unsubstantiated health claims. The FDA specifically prohibits claims suggesting that oxaloacetate supplements can treat or prevent diseases like diabetes or cancer, despite emerging research in these areas.
International trade of oxaloacetate supplements faces additional regulatory hurdles. Import regulations vary widely, with some countries requiring extensive documentation of manufacturing practices, stability data, and safety assessments. Japan's FOSHU (Foods for Specified Health Uses) system and Canada's Natural Health Products Regulations represent distinct regulatory frameworks that supplement manufacturers must navigate for market access.
Recent regulatory trends indicate increasing scrutiny of supplement quality and efficacy claims. The FDA's New Dietary Ingredient (NDI) notification process has become more rigorous for novel formulations of metabolic supplements. Similarly, the European Commission has proposed amendments to strengthen the regulation of health claims and quality standards for supplements targeting metabolic health.
Compliance with Good Manufacturing Practices (GMPs) is mandatory in most markets, requiring manufacturers to implement comprehensive quality management systems. For oxaloacetate supplements specifically, these requirements often include specialized testing methodologies to verify concentration and stability of the active compound throughout production and storage.
European regulations are more stringent, with the European Food Safety Authority (EFSA) requiring substantial scientific evidence for health claims. Oxaloacetate supplements face particular scrutiny due to their metabolic enhancement properties, and manufacturers must navigate the Novel Food Regulation if the ingredient lacks significant consumption history prior to May 1997.
Stability requirements present another regulatory challenge for oxaloacetate supplements. Due to the compound's inherent instability, regulatory bodies often require manufacturers to demonstrate consistent potency throughout the product's shelf life. This necessitates advanced stabilization technologies and rigorous quality control protocols to meet regulatory standards.
Labeling regulations also significantly impact oxaloacetate supplement marketing. In most jurisdictions, labels must accurately reflect ingredient concentrations and avoid unsubstantiated health claims. The FDA specifically prohibits claims suggesting that oxaloacetate supplements can treat or prevent diseases like diabetes or cancer, despite emerging research in these areas.
International trade of oxaloacetate supplements faces additional regulatory hurdles. Import regulations vary widely, with some countries requiring extensive documentation of manufacturing practices, stability data, and safety assessments. Japan's FOSHU (Foods for Specified Health Uses) system and Canada's Natural Health Products Regulations represent distinct regulatory frameworks that supplement manufacturers must navigate for market access.
Recent regulatory trends indicate increasing scrutiny of supplement quality and efficacy claims. The FDA's New Dietary Ingredient (NDI) notification process has become more rigorous for novel formulations of metabolic supplements. Similarly, the European Commission has proposed amendments to strengthen the regulation of health claims and quality standards for supplements targeting metabolic health.
Compliance with Good Manufacturing Practices (GMPs) is mandatory in most markets, requiring manufacturers to implement comprehensive quality management systems. For oxaloacetate supplements specifically, these requirements often include specialized testing methodologies to verify concentration and stability of the active compound throughout production and storage.
Quality Control Methods for Oxaloacetate Concentration Verification
Ensuring accurate and consistent oxaloacetate concentration in supplements requires robust quality control methodologies. High-performance liquid chromatography (HPLC) stands as the gold standard for oxaloacetate quantification, offering precise measurement capabilities with detection limits as low as 0.01 mg/mL. This technique effectively separates oxaloacetate from other compounds in complex supplement matrices, providing reliable concentration verification.
Mass spectrometry represents another advanced analytical approach, particularly when coupled with liquid chromatography (LC-MS). This combination delivers exceptional sensitivity and specificity for oxaloacetate detection, enabling accurate identification and quantification even in complex formulations. The method proves especially valuable for detecting potential degradation products that might form during supplement manufacturing or storage.
Enzymatic assays offer a more accessible alternative for routine quality control procedures. These assays utilize specific enzymes like malate dehydrogenase that react with oxaloacetate, producing measurable signals proportional to concentration. While less expensive than chromatographic methods, enzymatic approaches require careful calibration and validation to ensure accuracy across different supplement formulations.
Spectrophotometric methods provide rapid screening capabilities for oxaloacetate concentration. These techniques typically involve chemical reactions that produce colored compounds, with absorbance measurements correlating to oxaloacetate levels. Though less precise than HPLC or mass spectrometry, spectrophotometric methods serve well for high-throughput preliminary screening in manufacturing environments.
Stability testing constitutes a critical component of quality control protocols. Accelerated aging studies under various temperature and humidity conditions help predict oxaloacetate stability over time. Such testing enables manufacturers to establish appropriate shelf-life parameters and storage recommendations for maintaining effective concentrations throughout the product lifecycle.
Reference standards and certified reference materials play an essential role in calibrating analytical instruments and validating test methods. These standards ensure measurement accuracy and facilitate inter-laboratory comparisons, contributing to consistent quality across different production batches and manufacturing facilities.
Statistical process control techniques should be implemented to monitor oxaloacetate concentration trends during production. Control charts and capability analyses help identify process variations that might affect concentration consistency, allowing for timely corrective actions before products fall outside specification limits.
Mass spectrometry represents another advanced analytical approach, particularly when coupled with liquid chromatography (LC-MS). This combination delivers exceptional sensitivity and specificity for oxaloacetate detection, enabling accurate identification and quantification even in complex formulations. The method proves especially valuable for detecting potential degradation products that might form during supplement manufacturing or storage.
Enzymatic assays offer a more accessible alternative for routine quality control procedures. These assays utilize specific enzymes like malate dehydrogenase that react with oxaloacetate, producing measurable signals proportional to concentration. While less expensive than chromatographic methods, enzymatic approaches require careful calibration and validation to ensure accuracy across different supplement formulations.
Spectrophotometric methods provide rapid screening capabilities for oxaloacetate concentration. These techniques typically involve chemical reactions that produce colored compounds, with absorbance measurements correlating to oxaloacetate levels. Though less precise than HPLC or mass spectrometry, spectrophotometric methods serve well for high-throughput preliminary screening in manufacturing environments.
Stability testing constitutes a critical component of quality control protocols. Accelerated aging studies under various temperature and humidity conditions help predict oxaloacetate stability over time. Such testing enables manufacturers to establish appropriate shelf-life parameters and storage recommendations for maintaining effective concentrations throughout the product lifecycle.
Reference standards and certified reference materials play an essential role in calibrating analytical instruments and validating test methods. These standards ensure measurement accuracy and facilitate inter-laboratory comparisons, contributing to consistent quality across different production batches and manufacturing facilities.
Statistical process control techniques should be implemented to monitor oxaloacetate concentration trends during production. Control charts and capability analyses help identify process variations that might affect concentration consistency, allowing for timely corrective actions before products fall outside specification limits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!