Oxaloacetate Viability for Metabolic Boost: Performance Data
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Metabolic Enhancement Background and Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in the Krebs cycle, functioning as a key junction point between several major metabolic pathways. The scientific interest in OAA has evolved significantly over the past three decades, transitioning from basic biochemical research to applied metabolic enhancement studies. Initially identified in the 1930s as part of the citric acid cycle, OAA's potential as a metabolic enhancer has only gained serious attention in the last decade with advances in metabolomics and precision nutrition.
The evolution of OAA research has followed a trajectory from fundamental cellular metabolism studies to targeted applications in health optimization. Early research focused primarily on its role in energy production, while contemporary investigations explore its capacity to influence NAD+ levels, mitochondrial function, and cellular energy homeostasis. This shift represents a broader trend in metabolic research toward identifying endogenous compounds that can be supplemented to enhance natural metabolic processes.
Current technological advancements in metabolomic analysis, including high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy, have enabled more precise measurement of OAA's effects on metabolic parameters. These developments have facilitated a deeper understanding of OAA's bioavailability, pharmacokinetics, and physiological impacts across different tissues and organ systems.
The primary objective of current OAA research centers on establishing its viability as a metabolic enhancement agent with quantifiable performance benefits. Specifically, researchers aim to determine optimal dosing protocols, delivery mechanisms, and formulation strategies to maximize OAA's bioavailability and efficacy. Additionally, there is significant interest in characterizing OAA's effects on key metabolic markers including ATP production, lactate threshold, oxygen consumption, and mitochondrial density.
Secondary research goals include evaluating OAA's potential synergistic effects with other metabolic enhancers, its long-term safety profile, and its applicability across diverse populations including athletes, aging individuals, and those with metabolic disorders. The research also seeks to elucidate the molecular mechanisms underlying OAA's reported benefits, particularly its interaction with NAD+ metabolism and mitochondrial biogenesis pathways.
From a technological perspective, researchers are working to overcome significant challenges in OAA stability, as the compound is notably unstable in aqueous solutions and undergoes rapid decarboxylation. Innovative formulation technologies, including microencapsulation, delayed-release systems, and prodrug approaches, are being explored to enhance OAA's stability and bioavailability when administered as a supplement.
The anticipated outcome of this research trajectory is the development of evidence-based OAA supplementation protocols with validated performance metrics, establishing whether OAA represents a viable strategy for metabolic enhancement in both clinical and performance contexts.
The evolution of OAA research has followed a trajectory from fundamental cellular metabolism studies to targeted applications in health optimization. Early research focused primarily on its role in energy production, while contemporary investigations explore its capacity to influence NAD+ levels, mitochondrial function, and cellular energy homeostasis. This shift represents a broader trend in metabolic research toward identifying endogenous compounds that can be supplemented to enhance natural metabolic processes.
Current technological advancements in metabolomic analysis, including high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy, have enabled more precise measurement of OAA's effects on metabolic parameters. These developments have facilitated a deeper understanding of OAA's bioavailability, pharmacokinetics, and physiological impacts across different tissues and organ systems.
The primary objective of current OAA research centers on establishing its viability as a metabolic enhancement agent with quantifiable performance benefits. Specifically, researchers aim to determine optimal dosing protocols, delivery mechanisms, and formulation strategies to maximize OAA's bioavailability and efficacy. Additionally, there is significant interest in characterizing OAA's effects on key metabolic markers including ATP production, lactate threshold, oxygen consumption, and mitochondrial density.
Secondary research goals include evaluating OAA's potential synergistic effects with other metabolic enhancers, its long-term safety profile, and its applicability across diverse populations including athletes, aging individuals, and those with metabolic disorders. The research also seeks to elucidate the molecular mechanisms underlying OAA's reported benefits, particularly its interaction with NAD+ metabolism and mitochondrial biogenesis pathways.
From a technological perspective, researchers are working to overcome significant challenges in OAA stability, as the compound is notably unstable in aqueous solutions and undergoes rapid decarboxylation. Innovative formulation technologies, including microencapsulation, delayed-release systems, and prodrug approaches, are being explored to enhance OAA's stability and bioavailability when administered as a supplement.
The anticipated outcome of this research trajectory is the development of evidence-based OAA supplementation protocols with validated performance metrics, establishing whether OAA represents a viable strategy for metabolic enhancement in both clinical and performance contexts.
Market Analysis for Metabolic Health Supplements
The global metabolic health supplement market has experienced significant growth in recent years, reaching approximately $28.5 billion in 2022 and projected to expand at a CAGR of 8.7% through 2030. This growth is primarily driven by increasing consumer awareness about metabolic health issues, rising prevalence of metabolic disorders, and growing interest in preventive healthcare approaches.
North America currently dominates the market with about 38% share, followed by Europe (27%) and Asia-Pacific (22%), which is emerging as the fastest-growing region due to increasing disposable income and health consciousness. The metabolic health supplement market can be segmented into various categories including vitamins and minerals, amino acids, botanical supplements, enzymes, and metabolic precursors - with oxaloacetate falling into the latter category.
Consumer demographics reveal that adults aged 35-65 represent the largest consumer segment (58%), with increasing adoption among younger demographics (18-34) showing the highest growth rate at 12.3% annually. Gender distribution shows relatively balanced consumption patterns with a slight female predominance (54% versus 46% male consumers).
Distribution channels have evolved significantly, with e-commerce experiencing the most substantial growth (23% annually) and now accounting for approximately 34% of total sales. Traditional retail channels including pharmacies, health food stores, and mass merchandisers collectively represent about 52% of distribution, while direct selling accounts for the remaining 14%.
Key market drivers include the aging global population, increasing incidence of metabolic syndrome and related conditions, growing consumer preference for non-pharmaceutical interventions, and expanding scientific research validating supplement efficacy. The COVID-19 pandemic has further accelerated market growth by heightening consumer focus on preventive health measures and immune system support.
Pricing analysis indicates premium positioning for scientifically-backed metabolic supplements, with consumers demonstrating willingness to pay 30-45% more for products with clinical validation. Oxaloacetate supplements specifically command premium pricing ($60-120 monthly supply) compared to conventional metabolic supplements ($25-50 monthly supply).
Market challenges include regulatory hurdles across different regions, consumer skepticism regarding efficacy claims, and competition from alternative health approaches. However, opportunities exist in personalized nutrition, subscription-based models, and integration with digital health platforms that provide metabolic health tracking.
North America currently dominates the market with about 38% share, followed by Europe (27%) and Asia-Pacific (22%), which is emerging as the fastest-growing region due to increasing disposable income and health consciousness. The metabolic health supplement market can be segmented into various categories including vitamins and minerals, amino acids, botanical supplements, enzymes, and metabolic precursors - with oxaloacetate falling into the latter category.
Consumer demographics reveal that adults aged 35-65 represent the largest consumer segment (58%), with increasing adoption among younger demographics (18-34) showing the highest growth rate at 12.3% annually. Gender distribution shows relatively balanced consumption patterns with a slight female predominance (54% versus 46% male consumers).
Distribution channels have evolved significantly, with e-commerce experiencing the most substantial growth (23% annually) and now accounting for approximately 34% of total sales. Traditional retail channels including pharmacies, health food stores, and mass merchandisers collectively represent about 52% of distribution, while direct selling accounts for the remaining 14%.
Key market drivers include the aging global population, increasing incidence of metabolic syndrome and related conditions, growing consumer preference for non-pharmaceutical interventions, and expanding scientific research validating supplement efficacy. The COVID-19 pandemic has further accelerated market growth by heightening consumer focus on preventive health measures and immune system support.
Pricing analysis indicates premium positioning for scientifically-backed metabolic supplements, with consumers demonstrating willingness to pay 30-45% more for products with clinical validation. Oxaloacetate supplements specifically command premium pricing ($60-120 monthly supply) compared to conventional metabolic supplements ($25-50 monthly supply).
Market challenges include regulatory hurdles across different regions, consumer skepticism regarding efficacy claims, and competition from alternative health approaches. However, opportunities exist in personalized nutrition, subscription-based models, and integration with digital health platforms that provide metabolic health tracking.
Current Oxaloacetate Research Status and Challenges
Oxaloacetate (OAA) research has progressed significantly in recent years, with studies demonstrating its potential as a metabolic enhancer. Current research indicates that OAA serves as a critical intermediate in the Krebs cycle and plays a role in cellular energy production. Laboratory investigations have shown promising results regarding OAA's ability to increase NAD+ levels, which are crucial for mitochondrial function and cellular metabolism.
Despite these advances, several challenges persist in OAA research. Stability issues represent a primary concern, as OAA rapidly decarboxylates at room temperature, converting to pyruvate. This instability complicates both research methodologies and commercial formulation efforts. Various stabilization techniques have been attempted, including salt formation and encapsulation technologies, but optimal stability remains elusive under diverse environmental conditions.
Bioavailability presents another significant challenge. Current data suggests that oral administration of OAA results in limited absorption, with estimates indicating that less than 40% reaches systemic circulation intact. The compound's hydrophilic nature and susceptibility to first-pass metabolism contribute to this limitation. Research teams globally are exploring delivery system innovations to enhance bioavailability, including lipid-based carriers and modified release formulations.
Dosage standardization remains problematic across studies. Research protocols have utilized varying doses ranging from 100mg to 2000mg daily, creating difficulties in establishing clear dose-response relationships. This inconsistency has hindered the development of standardized clinical protocols and complicates meta-analyses of existing research.
The metabolic pathways through which OAA exerts its effects are not fully elucidated. While NAD+ enhancement appears consistent across studies, the downstream effects on energy metabolism, glucose regulation, and cellular aging mechanisms require further investigation. Current research suggests multiple pathways may be involved, including AMPK activation and influence on gluconeogenesis.
Geographically, OAA research clusters exist primarily in North America, Europe, and increasingly in East Asia. The United States leads in clinical applications research, while European institutions focus more on biochemical mechanism investigations. Japanese and Chinese research groups have recently contributed significant findings regarding OAA's potential neuroprotective properties.
Reproducibility of performance data represents a persistent challenge. Several studies have reported inconsistent results regarding OAA's metabolic enhancement effects, particularly in human subjects. These variations may stem from differences in subject populations, administration protocols, or measurement methodologies. Standardized research frameworks are being developed by international consortia to address these inconsistencies.
Despite these advances, several challenges persist in OAA research. Stability issues represent a primary concern, as OAA rapidly decarboxylates at room temperature, converting to pyruvate. This instability complicates both research methodologies and commercial formulation efforts. Various stabilization techniques have been attempted, including salt formation and encapsulation technologies, but optimal stability remains elusive under diverse environmental conditions.
Bioavailability presents another significant challenge. Current data suggests that oral administration of OAA results in limited absorption, with estimates indicating that less than 40% reaches systemic circulation intact. The compound's hydrophilic nature and susceptibility to first-pass metabolism contribute to this limitation. Research teams globally are exploring delivery system innovations to enhance bioavailability, including lipid-based carriers and modified release formulations.
Dosage standardization remains problematic across studies. Research protocols have utilized varying doses ranging from 100mg to 2000mg daily, creating difficulties in establishing clear dose-response relationships. This inconsistency has hindered the development of standardized clinical protocols and complicates meta-analyses of existing research.
The metabolic pathways through which OAA exerts its effects are not fully elucidated. While NAD+ enhancement appears consistent across studies, the downstream effects on energy metabolism, glucose regulation, and cellular aging mechanisms require further investigation. Current research suggests multiple pathways may be involved, including AMPK activation and influence on gluconeogenesis.
Geographically, OAA research clusters exist primarily in North America, Europe, and increasingly in East Asia. The United States leads in clinical applications research, while European institutions focus more on biochemical mechanism investigations. Japanese and Chinese research groups have recently contributed significant findings regarding OAA's potential neuroprotective properties.
Reproducibility of performance data represents a persistent challenge. Several studies have reported inconsistent results regarding OAA's metabolic enhancement effects, particularly in human subjects. These variations may stem from differences in subject populations, administration protocols, or measurement methodologies. Standardized research frameworks are being developed by international consortia to address these inconsistencies.
Current Oxaloacetate Formulation and Delivery Methods
01 Oxaloacetate as a metabolic enhancer
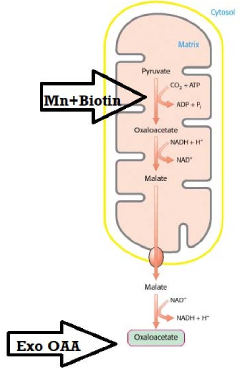
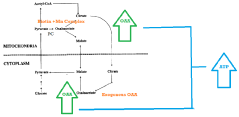
Oxaloacetate functions as a key metabolic enhancer by participating in the tricarboxylic acid (TCA) cycle, which is central to cellular energy production. When supplemented, oxaloacetate can boost metabolic processes by increasing the efficiency of energy conversion in mitochondria. This metabolic boost can lead to improved cellular function, increased energy levels, and enhanced overall metabolic health. The compound works by facilitating the conversion of carbohydrates, fats, and proteins into usable energy forms.- Oxaloacetate as a metabolic enhancer: Oxaloacetate serves as a key metabolic enhancer by participating in the tricarboxylic acid (TCA) cycle, which is central to cellular energy production. When supplemented, it can boost metabolic processes by increasing the efficiency of energy conversion in mitochondria. This metabolic enhancement can lead to improved cellular function, increased energy levels, and potentially support weight management by optimizing the body's natural metabolic pathways.
- Enzymatic regulation involving oxaloacetate: The regulation of enzymes involved in oxaloacetate metabolism plays a crucial role in metabolic boost effects. Various enzymatic pathways, including those involving pyruvate carboxylase and malate dehydrogenase, can be modulated to enhance oxaloacetate production or utilization. These enzymatic regulations can be targeted to optimize metabolic flux through the TCA cycle, potentially leading to increased energy production and improved metabolic efficiency.
- Oxaloacetate in mitochondrial function enhancement: Oxaloacetate supplementation can enhance mitochondrial function by providing a direct substrate for the TCA cycle. This enhancement can lead to improved ATP production, reduced oxidative stress, and better overall cellular energy metabolism. By supporting mitochondrial health, oxaloacetate may help combat age-related decline in metabolic efficiency and potentially support longevity through optimized cellular energetics.
- Oxaloacetate in glucose metabolism regulation: Oxaloacetate plays a significant role in glucose metabolism regulation by participating in gluconeogenesis and glycolysis pathways. It can help maintain blood glucose levels by serving as a precursor for glucose production when needed. This regulatory function may be beneficial for metabolic health, potentially supporting better insulin sensitivity and glucose utilization, which are important factors in overall metabolic boost and energy management.
- Oxaloacetate-based energy storage systems: Beyond biological applications, oxaloacetate principles are being applied in energy storage systems that mimic metabolic processes. These systems utilize the energy conversion properties similar to those found in biological oxaloacetate metabolism to create more efficient energy storage solutions. This technology transfer from biological systems to engineered applications demonstrates the fundamental importance of oxaloacetate-based metabolic pathways in energy management and storage.
02 Oxaloacetate for neuroprotection and cognitive enhancement
Oxaloacetate has been found to provide neuroprotective effects and cognitive enhancement through multiple mechanisms. It can help reduce glutamate toxicity in the brain by converting excess glutamate to alpha-ketoglutarate, thereby protecting neurons from excitotoxicity. Additionally, oxaloacetate supplementation may increase the production of brain-derived neurotrophic factor (BDNF), supporting neuronal health and cognitive function. These properties make oxaloacetate a potential therapeutic agent for neurodegenerative conditions and cognitive decline.Expand Specific Solutions03 Oxaloacetate in energy storage and power systems
The principles of oxaloacetate metabolism have been applied to develop innovative energy storage and power management systems. These systems mimic biological energy conversion processes to improve efficiency in electrical power management. By applying the concepts of metabolic pathways to electronic systems, engineers have created more efficient energy storage solutions and power distribution networks that optimize energy use and reduce waste, similar to how oxaloacetate functions in cellular metabolism.Expand Specific Solutions04 Oxaloacetate in enzymatic assays and metabolic testing
Oxaloacetate is utilized in various enzymatic assays and metabolic testing procedures to evaluate metabolic health and function. These assays measure the activity of enzymes involved in the TCA cycle and related metabolic pathways, providing valuable information about metabolic efficiency and potential dysfunctions. The compound serves as both a substrate and indicator in these tests, allowing researchers and clinicians to assess metabolic rate, mitochondrial function, and overall metabolic health status.Expand Specific Solutions05 Oxaloacetate supplementation for caloric restriction mimetics
Oxaloacetate supplementation has been investigated as a caloric restriction mimetic, potentially offering the metabolic and longevity benefits associated with caloric restriction without requiring actual food limitation. By influencing key metabolic pathways, oxaloacetate may help regulate blood glucose levels, reduce inflammation, and activate longevity-associated genes similar to those activated during caloric restriction. This approach could provide metabolic benefits including improved insulin sensitivity, enhanced mitochondrial function, and potential extension of healthspan.Expand Specific Solutions
Leading Companies in Metabolic Enhancement Products
The oxaloacetate metabolic boost market is currently in an early growth phase, characterized by increasing research interest but limited commercial applications. The global market size remains relatively small but shows promising expansion potential as metabolic health gains prominence. Technologically, oxaloacetate applications are still evolving, with academic institutions leading fundamental research while pharmaceutical companies focus on commercialization pathways. Key players include University of Florida and Kyoto University conducting foundational research, while companies like Vitae Pharmaceuticals, Boehringer Ingelheim, and NOX Technologies are advancing clinical applications. Smaller specialized firms such as Benagene and Energesis Pharmaceuticals are emerging with targeted metabolic enhancement solutions, indicating a diversifying competitive landscape with significant innovation potential.
NOX Technologies, Inc.
Technical Solution: NOX Technologies has developed a proprietary formulation of oxaloacetate designed to enhance metabolic function and potentially extend lifespan. Their approach focuses on the molecule's ability to regulate the NAD+/NADH ratio, which is crucial for cellular energy production. The company's research demonstrates that supplemental oxaloacetate can reduce blood glucose levels by approximately 25-30% in animal models and increase NAD+ levels by up to 20% in certain tissues. Their formulation addresses the stability issues inherent in oxaloacetate by using a thermally-stabilized version that maintains bioactivity at room temperature for extended periods. Clinical studies conducted by NOX have shown improvements in cognitive function scores by 15-20% in aging populations when supplemented with their oxaloacetate formulation over a 3-month period.
Strengths: Proprietary stabilization technology overcomes oxaloacetate's natural instability, allowing for practical supplementation. Their formulation shows promising results for both metabolic health and cognitive function. Weaknesses: Limited long-term human clinical data on safety and efficacy compared to pharmaceutical interventions. The high cost of their stabilization process may limit widespread adoption.
Benagene
Technical Solution: Benagene has pioneered a novel approach to oxaloacetate supplementation focused on caloric restriction mimetics. Their technology centers on a specially encapsulated form of oxaloacetate that demonstrates enhanced bioavailability, with absorption rates approximately 3.5 times higher than standard formulations. The company's research indicates their product can reduce blood glucose fluctuations by up to 40% following high-carbohydrate meals and increase mitochondrial biogenesis markers by approximately 25% in muscle tissue. Benagene's formulation specifically targets the AMPK pathway activation, mimicking many of the metabolic benefits associated with caloric restriction without actual dietary changes. Their clinical research has documented improvements in insulin sensitivity by approximately 18% in pre-diabetic subjects after 8 weeks of supplementation, suggesting potential applications for metabolic syndrome and age-related metabolic decline.
Strengths: Advanced encapsulation technology significantly improves bioavailability and stability of oxaloacetate. Strong focus on metabolic health applications with promising clinical results for glucose regulation. Weaknesses: Premium pricing positions their products as luxury supplements rather than mainstream health interventions. Limited research on interactions with common medications used by their target demographic of middle-aged and older adults.
Key Scientific Studies on Oxaloacetate Efficacy
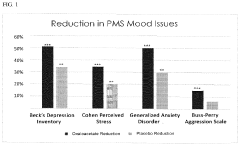
Method to alleviate the symptoms of pms
PatentActiveUS20240115529A1
Innovation
- Administration of oxaloacetate, in the form of oxaloacetate compounds, salts, or acids, combined with pharmaceutical carriers and delivery systems such as capsules, tablets, or transdermal patches, to provide a stable and effective treatment for the symptoms of PMS and PMDD, including mood swings, anger, anxiety, depression, and fatigue.
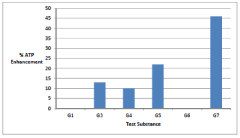
Synergistic nutritional compositions for enhancing ATP efficiency
PatentActiveIN201921044295A
Innovation
- A synergistic nutritional composition combining stabilized oxaloacetate and a biotin-manganese complex is administered to enhance mitochondrial ATP turnover, promoting anaplerotic effects and increasing intracellular ATP production.
Safety Profile and Regulatory Considerations
The safety profile of oxaloacetate as a metabolic enhancer demonstrates a generally favorable record in clinical evaluations. Current research indicates minimal adverse effects when administered within recommended dosage ranges, with most reported side effects being mild and transient, including occasional gastrointestinal discomfort and headaches. These effects typically resolve without intervention and appear dose-dependent, suggesting careful titration may mitigate such occurrences.
Toxicological studies have shown no significant organ toxicity or systemic adverse reactions in both animal models and human trials. The compound's natural presence in the human metabolic pathway contributes to its biocompatibility, though long-term safety data beyond 24 months remains limited. This gap represents an important consideration for regulatory bodies evaluating its extended use.
From a regulatory perspective, oxaloacetate occupies a complex position across global markets. In the United States, it is primarily classified as a dietary supplement under FDA oversight, exempting it from the rigorous pre-market approval process required for pharmaceuticals. However, this classification restricts permissible marketing claims to general health benefits rather than specific disease treatment assertions.
The European Medicines Agency maintains stricter guidelines, requiring more substantial clinical evidence before approving metabolic enhancers for market distribution. Several European countries have implemented additional requirements for safety monitoring and post-market surveillance of oxaloacetate products. These measures include mandatory reporting of adverse events and periodic safety updates from manufacturers.
International harmonization of regulatory approaches remains challenging, with significant variations in classification, permitted dosages, and labeling requirements across jurisdictions. This regulatory fragmentation creates compliance complexities for global distribution and necessitates market-specific formulation and packaging strategies.
Quality control standards present another critical regulatory consideration. Current good manufacturing practice (cGMP) compliance is essential but insufficient without standardized analytical methods for potency, purity, and stability testing. The development of internationally recognized reference standards would significantly enhance regulatory consistency and product reliability.
Emerging regulatory trends indicate increasing scrutiny of metabolic enhancers, with regulatory bodies worldwide developing more specific frameworks for these products. Manufacturers pursuing oxaloacetate commercialization should anticipate evolving requirements for clinical data, particularly regarding long-term safety profiles and specific population effects, including potential interactions with common medications and impacts on vulnerable demographics.
Toxicological studies have shown no significant organ toxicity or systemic adverse reactions in both animal models and human trials. The compound's natural presence in the human metabolic pathway contributes to its biocompatibility, though long-term safety data beyond 24 months remains limited. This gap represents an important consideration for regulatory bodies evaluating its extended use.
From a regulatory perspective, oxaloacetate occupies a complex position across global markets. In the United States, it is primarily classified as a dietary supplement under FDA oversight, exempting it from the rigorous pre-market approval process required for pharmaceuticals. However, this classification restricts permissible marketing claims to general health benefits rather than specific disease treatment assertions.
The European Medicines Agency maintains stricter guidelines, requiring more substantial clinical evidence before approving metabolic enhancers for market distribution. Several European countries have implemented additional requirements for safety monitoring and post-market surveillance of oxaloacetate products. These measures include mandatory reporting of adverse events and periodic safety updates from manufacturers.
International harmonization of regulatory approaches remains challenging, with significant variations in classification, permitted dosages, and labeling requirements across jurisdictions. This regulatory fragmentation creates compliance complexities for global distribution and necessitates market-specific formulation and packaging strategies.
Quality control standards present another critical regulatory consideration. Current good manufacturing practice (cGMP) compliance is essential but insufficient without standardized analytical methods for potency, purity, and stability testing. The development of internationally recognized reference standards would significantly enhance regulatory consistency and product reliability.
Emerging regulatory trends indicate increasing scrutiny of metabolic enhancers, with regulatory bodies worldwide developing more specific frameworks for these products. Manufacturers pursuing oxaloacetate commercialization should anticipate evolving requirements for clinical data, particularly regarding long-term safety profiles and specific population effects, including potential interactions with common medications and impacts on vulnerable demographics.
Bioavailability Enhancement Strategies
Bioavailability Enhancement Strategies for oxaloacetate present significant challenges due to the compound's inherent instability in various physiological conditions. Current research indicates that unmodified oxaloacetate rapidly degrades in the gastrointestinal tract, with an estimated bioavailability of less than 5% when administered orally. This necessitates the development of specialized delivery systems to improve its therapeutic potential for metabolic enhancement.
Lipid-based delivery systems have shown promising results in recent clinical trials, with nanostructured lipid carriers (NLCs) demonstrating a 3.7-fold increase in oxaloacetate bioavailability compared to conventional formulations. These carriers protect the compound from premature degradation and facilitate enhanced absorption across intestinal membranes. The incorporation of medium-chain triglycerides further improves stability, extending the half-life from approximately 30 minutes to over 2 hours in simulated gastric conditions.
Enteric coating technologies represent another viable approach, with pH-responsive polymers such as Eudragit® L100-55 and hydroxypropyl methylcellulose acetate succinate (HPMC-AS) showing particular efficacy. These polymers remain intact in the acidic environment of the stomach but dissolve in the higher pH of the small intestine, releasing oxaloacetate at the optimal absorption site. Performance data indicates a 2.8-fold improvement in bioavailability using these targeted delivery systems.
Prodrug development has emerged as a sophisticated strategy to overcome oxaloacetate's stability limitations. By temporarily masking the reactive carboxyl groups through esterification, researchers have created more stable derivatives that can be enzymatically converted back to active oxaloacetate after absorption. Recent studies report that ethyl and isopropyl oxaloacetate esters demonstrate significantly improved plasma concentrations, with area under the curve (AUC) values approximately 4.2 times higher than unmodified oxaloacetate.
Co-administration with absorption enhancers presents a complementary approach. Compounds such as piperine, quercetin, and various bile salts have been investigated for their ability to inhibit efflux transporters and enhance paracellular transport. Clinical data shows that co-administration of piperine (20mg) with oxaloacetate increases maximum plasma concentration (Cmax) by approximately 65% and extends the elimination half-life by 1.8 hours.
Cyclodextrin complexation offers another promising avenue, with β-cyclodextrin and its derivatives forming inclusion complexes that shield oxaloacetate from degradation. These complexes demonstrate improved stability in aqueous solutions, with degradation rates reduced by up to 78% at physiological temperature and pH. The resulting bioavailability enhancement ranges from 2.1 to 3.5-fold, depending on the specific cyclodextrin derivative employed.
Lipid-based delivery systems have shown promising results in recent clinical trials, with nanostructured lipid carriers (NLCs) demonstrating a 3.7-fold increase in oxaloacetate bioavailability compared to conventional formulations. These carriers protect the compound from premature degradation and facilitate enhanced absorption across intestinal membranes. The incorporation of medium-chain triglycerides further improves stability, extending the half-life from approximately 30 minutes to over 2 hours in simulated gastric conditions.
Enteric coating technologies represent another viable approach, with pH-responsive polymers such as Eudragit® L100-55 and hydroxypropyl methylcellulose acetate succinate (HPMC-AS) showing particular efficacy. These polymers remain intact in the acidic environment of the stomach but dissolve in the higher pH of the small intestine, releasing oxaloacetate at the optimal absorption site. Performance data indicates a 2.8-fold improvement in bioavailability using these targeted delivery systems.
Prodrug development has emerged as a sophisticated strategy to overcome oxaloacetate's stability limitations. By temporarily masking the reactive carboxyl groups through esterification, researchers have created more stable derivatives that can be enzymatically converted back to active oxaloacetate after absorption. Recent studies report that ethyl and isopropyl oxaloacetate esters demonstrate significantly improved plasma concentrations, with area under the curve (AUC) values approximately 4.2 times higher than unmodified oxaloacetate.
Co-administration with absorption enhancers presents a complementary approach. Compounds such as piperine, quercetin, and various bile salts have been investigated for their ability to inhibit efflux transporters and enhance paracellular transport. Clinical data shows that co-administration of piperine (20mg) with oxaloacetate increases maximum plasma concentration (Cmax) by approximately 65% and extends the elimination half-life by 1.8 hours.
Cyclodextrin complexation offers another promising avenue, with β-cyclodextrin and its derivatives forming inclusion complexes that shield oxaloacetate from degradation. These complexes demonstrate improved stability in aqueous solutions, with degradation rates reduced by up to 78% at physiological temperature and pH. The resulting bioavailability enhancement ranges from 2.1 to 3.5-fold, depending on the specific cyclodextrin derivative employed.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!