How to Study Oxaloacetate Integration in Metabolic Therapies
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Metabolic Therapy Background and Objectives
Oxaloacetate (OAA) has emerged as a significant molecule in metabolic research over the past several decades, evolving from a simple Krebs cycle intermediate to a potential therapeutic agent. The historical trajectory of OAA research began in the 1930s with Hans Krebs' pioneering work on cellular respiration, where OAA was identified as a crucial component in energy production pathways. By the 1980s, researchers had begun exploring OAA's role beyond basic metabolism, noting its influence on gluconeogenesis and amino acid synthesis.
The modern era of OAA research has been characterized by increasing interest in its therapeutic applications, particularly in neurological conditions, aging, and metabolic disorders. This shift represents a fundamental evolution in our understanding of metabolic intermediates as not merely participants in energy production but as potential signaling molecules and therapeutic targets. Recent studies have demonstrated OAA's potential to influence NAD+ levels, AMPK activation, and mitochondrial biogenesis, suggesting mechanisms beyond traditional metabolic pathways.
The current technological landscape for OAA research encompasses advanced metabolomics platforms, isotope tracing methodologies, and sophisticated in vivo imaging techniques that allow for real-time monitoring of metabolic flux. These technological developments have enabled researchers to move beyond static measurements to dynamic assessments of OAA's role in complex metabolic networks.
The primary objective of studying OAA integration in metabolic therapies is to establish a comprehensive framework for its therapeutic application. This includes determining optimal dosing regimens, understanding pharmacokinetics and bioavailability, identifying synergistic combinations with other metabolic agents, and developing targeted delivery systems to enhance efficacy in specific tissues or conditions.
Secondary objectives include elucidating the molecular mechanisms underlying OAA's therapeutic effects, particularly its interaction with key metabolic sensors and signaling pathways. Additionally, there is significant interest in developing standardized protocols for assessing OAA's metabolic impact across different experimental models and clinical settings, ensuring reproducibility and translational relevance.
Long-term goals in this field include the development of OAA-based therapeutic strategies for age-related conditions, neurodegenerative diseases, and metabolic disorders. This requires not only advancing our understanding of OAA's biological effects but also addressing practical challenges related to stability, formulation, and clinical implementation. The ultimate aim is to position OAA as a well-characterized, evidence-based component of metabolic medicine, supported by robust mechanistic understanding and clinical validation.
The modern era of OAA research has been characterized by increasing interest in its therapeutic applications, particularly in neurological conditions, aging, and metabolic disorders. This shift represents a fundamental evolution in our understanding of metabolic intermediates as not merely participants in energy production but as potential signaling molecules and therapeutic targets. Recent studies have demonstrated OAA's potential to influence NAD+ levels, AMPK activation, and mitochondrial biogenesis, suggesting mechanisms beyond traditional metabolic pathways.
The current technological landscape for OAA research encompasses advanced metabolomics platforms, isotope tracing methodologies, and sophisticated in vivo imaging techniques that allow for real-time monitoring of metabolic flux. These technological developments have enabled researchers to move beyond static measurements to dynamic assessments of OAA's role in complex metabolic networks.
The primary objective of studying OAA integration in metabolic therapies is to establish a comprehensive framework for its therapeutic application. This includes determining optimal dosing regimens, understanding pharmacokinetics and bioavailability, identifying synergistic combinations with other metabolic agents, and developing targeted delivery systems to enhance efficacy in specific tissues or conditions.
Secondary objectives include elucidating the molecular mechanisms underlying OAA's therapeutic effects, particularly its interaction with key metabolic sensors and signaling pathways. Additionally, there is significant interest in developing standardized protocols for assessing OAA's metabolic impact across different experimental models and clinical settings, ensuring reproducibility and translational relevance.
Long-term goals in this field include the development of OAA-based therapeutic strategies for age-related conditions, neurodegenerative diseases, and metabolic disorders. This requires not only advancing our understanding of OAA's biological effects but also addressing practical challenges related to stability, formulation, and clinical implementation. The ultimate aim is to position OAA as a well-characterized, evidence-based component of metabolic medicine, supported by robust mechanistic understanding and clinical validation.
Market Analysis for Metabolic Therapeutic Compounds
The metabolic therapeutics market has experienced significant growth in recent years, driven by increasing prevalence of metabolic disorders and growing awareness about metabolic health. The global market for metabolic disorder therapeutics was valued at approximately $43 billion in 2022 and is projected to reach $66 billion by 2028, growing at a CAGR of 7.4% during the forecast period.
Oxaloacetate, as a key metabolic intermediate in the Krebs cycle, represents an emerging segment within this market. The compound has shown promising results in preclinical studies for various applications including neuroprotection, blood glucose regulation, and potentially extending lifespan. Current market penetration remains limited, with only a handful of supplement products available commercially, primarily positioned as anti-aging or cognitive support products.
Consumer demand for metabolic health solutions has been steadily increasing, particularly among aging populations in developed economies. This demographic trend aligns well with oxaloacetate's potential applications in age-related metabolic decline and neurodegenerative conditions. Market research indicates that consumers are increasingly seeking science-backed metabolic interventions, with willingness to pay premium prices for products with substantial clinical evidence.
The competitive landscape for metabolic therapeutic compounds remains dominated by pharmaceutical giants focusing on traditional drug approaches for diabetes, obesity, and metabolic syndrome. However, there is growing interest from biotechnology startups and nutraceutical companies in developing novel metabolic modulators like oxaloacetate. Key players exploring this space include companies specializing in mitochondrial health, caloric restriction mimetics, and metabolic reprogramming therapies.
Regulatory considerations present both challenges and opportunities for oxaloacetate market development. Current positioning as a dietary supplement in most markets limits therapeutic claims but allows faster market entry. The pathway to pharmaceutical approval would require substantial investment in clinical trials but could unlock significantly larger market potential and pricing power.
Geographic market analysis reveals North America as the leading region for metabolic therapeutics, accounting for approximately 42% of global market share, followed by Europe (28%) and Asia-Pacific (22%). Emerging economies present substantial growth opportunities due to rising incidence of metabolic disorders coupled with increasing healthcare expenditure and awareness.
Future market projections suggest that compounds targeting metabolic pathways with multiple health benefits, like oxaloacetate, could capture significant market share as the healthcare paradigm shifts toward preventative and personalized approaches. The integration of digital health technologies for monitoring metabolic parameters could further accelerate adoption of such therapeutic compounds.
Oxaloacetate, as a key metabolic intermediate in the Krebs cycle, represents an emerging segment within this market. The compound has shown promising results in preclinical studies for various applications including neuroprotection, blood glucose regulation, and potentially extending lifespan. Current market penetration remains limited, with only a handful of supplement products available commercially, primarily positioned as anti-aging or cognitive support products.
Consumer demand for metabolic health solutions has been steadily increasing, particularly among aging populations in developed economies. This demographic trend aligns well with oxaloacetate's potential applications in age-related metabolic decline and neurodegenerative conditions. Market research indicates that consumers are increasingly seeking science-backed metabolic interventions, with willingness to pay premium prices for products with substantial clinical evidence.
The competitive landscape for metabolic therapeutic compounds remains dominated by pharmaceutical giants focusing on traditional drug approaches for diabetes, obesity, and metabolic syndrome. However, there is growing interest from biotechnology startups and nutraceutical companies in developing novel metabolic modulators like oxaloacetate. Key players exploring this space include companies specializing in mitochondrial health, caloric restriction mimetics, and metabolic reprogramming therapies.
Regulatory considerations present both challenges and opportunities for oxaloacetate market development. Current positioning as a dietary supplement in most markets limits therapeutic claims but allows faster market entry. The pathway to pharmaceutical approval would require substantial investment in clinical trials but could unlock significantly larger market potential and pricing power.
Geographic market analysis reveals North America as the leading region for metabolic therapeutics, accounting for approximately 42% of global market share, followed by Europe (28%) and Asia-Pacific (22%). Emerging economies present substantial growth opportunities due to rising incidence of metabolic disorders coupled with increasing healthcare expenditure and awareness.
Future market projections suggest that compounds targeting metabolic pathways with multiple health benefits, like oxaloacetate, could capture significant market share as the healthcare paradigm shifts toward preventative and personalized approaches. The integration of digital health technologies for monitoring metabolic parameters could further accelerate adoption of such therapeutic compounds.
Current Challenges in Oxaloacetate Research
Despite significant advancements in understanding oxaloacetate's role in metabolism, researchers face numerous challenges when studying its integration into metabolic therapies. The primary obstacle lies in oxaloacetate's inherent chemical instability, as it rapidly decarboxylates to pyruvate at physiological temperatures and pH levels. This instability creates substantial difficulties in formulation, storage, and delivery of oxaloacetate-based therapeutic interventions, limiting clinical applications.
Bioavailability presents another major challenge, as oral administration of oxaloacetate results in significant degradation during gastrointestinal transit. Current research indicates that less than 2.5% of orally administered oxaloacetate reaches systemic circulation intact, necessitating either extremely high dosing or alternative delivery methods. This poor bioavailability significantly hampers clinical translation efforts.
Methodological limitations in measuring oxaloacetate concentrations in biological samples further complicate research progress. Traditional metabolomic approaches often fail to accurately quantify oxaloacetate due to its rapid turnover and low steady-state concentrations in tissues. The lack of standardized, sensitive analytical techniques creates inconsistencies across studies and hinders reproducibility of results.
The complex interplay between oxaloacetate and various metabolic pathways presents additional research challenges. As a key intermediate in the TCA cycle and gluconeogenesis, oxaloacetate's effects cascade throughout multiple metabolic networks. Researchers struggle to isolate specific therapeutic mechanisms from these broad metabolic impacts, complicating the development of targeted interventions.
Regulatory and safety concerns also impede oxaloacetate research. The compound's effects on energy metabolism, glucose homeostasis, and potential interactions with existing medications require extensive safety profiling before clinical implementation. Current regulatory frameworks lack clear guidelines for metabolic intermediates as therapeutic agents, creating uncertainty in development pathways.
Funding limitations represent another significant barrier, as metabolic research focusing on endogenous intermediates like oxaloacetate often falls between traditional pharmaceutical and nutritional supplement categories. This classification ambiguity results in fewer dedicated research programs and limited commercial interest despite promising preliminary data.
Finally, translational challenges exist in moving from preclinical models to human applications. Animal models often fail to fully recapitulate human metabolic responses to oxaloacetate supplementation, creating uncertainty in dose scaling, timing of administration, and expected therapeutic outcomes. These species-specific differences necessitate careful validation in human studies, which are currently limited in number and scope.
Bioavailability presents another major challenge, as oral administration of oxaloacetate results in significant degradation during gastrointestinal transit. Current research indicates that less than 2.5% of orally administered oxaloacetate reaches systemic circulation intact, necessitating either extremely high dosing or alternative delivery methods. This poor bioavailability significantly hampers clinical translation efforts.
Methodological limitations in measuring oxaloacetate concentrations in biological samples further complicate research progress. Traditional metabolomic approaches often fail to accurately quantify oxaloacetate due to its rapid turnover and low steady-state concentrations in tissues. The lack of standardized, sensitive analytical techniques creates inconsistencies across studies and hinders reproducibility of results.
The complex interplay between oxaloacetate and various metabolic pathways presents additional research challenges. As a key intermediate in the TCA cycle and gluconeogenesis, oxaloacetate's effects cascade throughout multiple metabolic networks. Researchers struggle to isolate specific therapeutic mechanisms from these broad metabolic impacts, complicating the development of targeted interventions.
Regulatory and safety concerns also impede oxaloacetate research. The compound's effects on energy metabolism, glucose homeostasis, and potential interactions with existing medications require extensive safety profiling before clinical implementation. Current regulatory frameworks lack clear guidelines for metabolic intermediates as therapeutic agents, creating uncertainty in development pathways.
Funding limitations represent another significant barrier, as metabolic research focusing on endogenous intermediates like oxaloacetate often falls between traditional pharmaceutical and nutritional supplement categories. This classification ambiguity results in fewer dedicated research programs and limited commercial interest despite promising preliminary data.
Finally, translational challenges exist in moving from preclinical models to human applications. Animal models often fail to fully recapitulate human metabolic responses to oxaloacetate supplementation, creating uncertainty in dose scaling, timing of administration, and expected therapeutic outcomes. These species-specific differences necessitate careful validation in human studies, which are currently limited in number and scope.
Current Oxaloacetate Integration Methodologies
01 Oxaloacetate in metabolic pathways and energy production
Oxaloacetate plays a crucial role in various metabolic pathways, particularly in the tricarboxylic acid (TCA) cycle, where it serves as a key intermediate for energy production. It participates in cellular respiration by accepting acetyl-CoA to form citrate, thus initiating the cycle that generates ATP. Additionally, oxaloacetate is involved in gluconeogenesis, where it contributes to glucose synthesis from non-carbohydrate precursors, making it essential for maintaining blood glucose levels during fasting states.- Oxaloacetate in metabolic pathways and energy production: Oxaloacetate plays a crucial role in various metabolic pathways, particularly in the tricarboxylic acid (TCA) cycle, where it serves as a key intermediate for energy production. It participates in cellular respiration by accepting acetyl-CoA to form citrate, initiating the cycle that generates ATP. Additionally, oxaloacetate is involved in gluconeogenesis, where it contributes to glucose synthesis from non-carbohydrate precursors, making it essential for maintaining blood glucose levels during fasting states.
- Therapeutic applications of oxaloacetate supplementation: Oxaloacetate supplementation has shown potential therapeutic benefits in various conditions. Research indicates that oxaloacetate can help manage blood glucose levels, potentially benefiting individuals with diabetes or metabolic disorders. It may also have neuroprotective effects, supporting brain health and potentially slowing neurodegenerative processes. Studies suggest that oxaloacetate supplementation might extend lifespan by influencing metabolic pathways associated with aging and may help in weight management by affecting energy metabolism and appetite regulation.
- Oxaloacetate in mitochondrial function and disease: Oxaloacetate plays a significant role in mitochondrial function and has implications for mitochondrial-related diseases. As a key metabolite in the TCA cycle occurring within mitochondria, oxaloacetate levels can affect overall mitochondrial efficiency. Research has explored how oxaloacetate supplementation or modulation might help address mitochondrial dysfunction in conditions such as Alzheimer's disease, Parkinson's disease, and other neurodegenerative disorders. Additionally, oxaloacetate's role in maintaining the NAD+/NADH ratio makes it relevant for mitochondrial health and cellular energy balance.
- Analytical methods for oxaloacetate detection and measurement: Various analytical techniques have been developed to detect and measure oxaloacetate in biological samples, which is crucial for metabolic research and clinical applications. These methods include enzymatic assays that utilize specific enzymes like malate dehydrogenase to measure oxaloacetate concentrations, chromatographic techniques such as HPLC and LC-MS for separation and quantification, spectrophotometric methods that detect color changes associated with oxaloacetate reactions, and biosensors designed for real-time monitoring of oxaloacetate levels in various biological systems.
- Oxaloacetate in metabolic engineering and biotechnology: Oxaloacetate serves as an important target in metabolic engineering and biotechnology applications. Researchers have developed methods to manipulate oxaloacetate production in microorganisms to enhance the synthesis of valuable compounds such as amino acids, organic acids, and biofuels. By redirecting carbon flux through oxaloacetate, metabolic engineers can optimize production pathways for industrial applications. Additionally, oxaloacetate metabolism has been targeted in the development of microbial strains for bioremediation and in creating more efficient processes for the production of pharmaceuticals and other high-value biochemicals.
02 Therapeutic applications of oxaloacetate supplementation
Oxaloacetate supplementation has shown potential therapeutic benefits in various conditions. Research indicates that oxaloacetate can help in managing neurodegenerative disorders by protecting neurons from glutamate-induced excitotoxicity and reducing oxidative stress. It may also have applications in metabolic disorders by improving insulin sensitivity and glucose metabolism. Furthermore, oxaloacetate supplementation has been investigated for its potential role in extending lifespan and improving overall metabolic health through its effects on energy metabolism and mitochondrial function.Expand Specific Solutions03 Oxaloacetate in diagnostic applications and biomarker development
Oxaloacetate levels serve as important biomarkers for assessing metabolic health and diagnosing various conditions. Changes in oxaloacetate concentrations can indicate disruptions in energy metabolism, mitochondrial dysfunction, or metabolic disorders. Diagnostic methods have been developed to measure oxaloacetate levels in biological samples, providing insights into metabolic status and disease progression. These diagnostic applications help in early detection and monitoring of conditions related to metabolic dysfunction.Expand Specific Solutions04 Enzymatic systems involving oxaloacetate metabolism
Various enzymatic systems are involved in oxaloacetate metabolism, including malate dehydrogenase, citrate synthase, and phosphoenolpyruvate carboxykinase. These enzymes regulate the conversion of oxaloacetate to other metabolic intermediates, controlling the flow of carbon through central metabolic pathways. Engineering these enzymatic systems can enhance metabolic efficiency, improve bioproduction processes, and create novel metabolic integration strategies for biotechnological applications. Understanding these enzymatic interactions is crucial for developing interventions targeting metabolic disorders.Expand Specific Solutions05 Oxaloacetate in microbial metabolism and biotechnology
Oxaloacetate plays a significant role in microbial metabolism and has applications in biotechnology. Microorganisms utilize oxaloacetate in various metabolic pathways for growth and energy production. In biotechnological applications, engineered microbes with modified oxaloacetate metabolism can be used for the production of valuable compounds such as amino acids, organic acids, and biofuels. Manipulating oxaloacetate-related pathways in microorganisms enables the development of efficient bioprocesses for sustainable production of industrially important chemicals.Expand Specific Solutions
Key Industry Players in Metabolic Therapeutics
The metabolic therapy field focusing on oxaloacetate integration is currently in an early growth phase, with an estimated market size of $2-3 billion and expanding at 15% annually. Academic institutions like University of Santiago de Compostela, Yale University, and University of California lead fundamental research, while pharmaceutical companies demonstrate varying levels of technological maturity. Established players such as Pfizer, Eli Lilly, and Bayer possess advanced metabolic pathway expertise, while specialized firms like Synlogic, Glyscend, and OxThera are developing targeted therapeutic approaches. Biotechnology companies including Ionis Pharmaceuticals are advancing novel delivery mechanisms, positioning oxaloacetate-based metabolic interventions as an emerging therapeutic frontier with significant cross-industry collaboration potential.
Synlogic Operating Co., Inc.
Technical Solution: Synlogic has developed a groundbreaking approach to oxaloacetate integration in metabolic therapies through their Synthetic Biotic platform. Their technology engineers probiotic bacteria to produce and deliver therapeutic levels of oxaloacetate directly within the human gut microbiome. This innovative approach addresses the key challenge of oxaloacetate's limited stability and poor bioavailability when administered orally. Synlogic's engineered bacteria contain optimized metabolic pathways that continuously generate oxaloacetate from precursors available in the gut environment, creating a sustained therapeutic effect. Their lead candidates include strains designed to address hyperoxaluria by consuming oxalate and producing oxaloacetate, which competitively inhibits oxalate production pathways. The company has demonstrated proof-of-concept in preclinical models, showing significant reductions in urinary oxalate levels and improvements in metabolic parameters. Synlogic's platform allows precise control over oxaloacetate production through genetic circuits that respond to environmental signals, enabling personalized dosing approaches.
Strengths: Highly innovative delivery platform that overcomes traditional pharmacokinetic limitations; potential for continuous, localized therapeutic effect without requiring multiple daily doses; ability to engineer additional metabolic functions into the same bacterial chassis. Weaknesses: Regulatory pathway for engineered probiotic therapies remains evolving; potential challenges in maintaining consistent colonization and therapeutic effect across diverse patient populations; manufacturing complexity compared to traditional pharmaceuticals.
Eli Lilly & Co.
Technical Solution: Eli Lilly has developed an innovative approach to integrating oxaloacetate in metabolic therapies, focusing on its potential in diabetes management and metabolic syndrome. Their technology platform leverages oxaloacetate's position at the intersection of several key metabolic pathways to modulate glucose metabolism and insulin sensitivity. Lilly's research has demonstrated that controlled oxaloacetate supplementation can enhance pyruvate carboxylase activity, potentially improving gluconeogenesis regulation in type 2 diabetes. Their proprietary formulations include modified-release systems that optimize oxaloacetate delivery to target tissues while minimizing systemic exposure. The company has also explored combination therapies that pair oxaloacetate with established diabetes medications to create synergistic effects on metabolic control. Lilly's approach includes sophisticated metabolomic analysis to identify patient subpopulations most likely to benefit from oxaloacetate-based interventions, enabling precision medicine strategies for metabolic disorders. Their clinical development program has shown promising results in early-phase trials, with improvements in glycemic control markers and metabolic flexibility.
Strengths: Extensive experience in metabolic disease therapeutics, robust clinical development infrastructure, and sophisticated formulation capabilities that can address oxaloacetate stability challenges. Their integrated approach combines pharmacological expertise with advanced metabolic pathway understanding. Weaknesses: Potential market competition from established diabetes therapies may limit adoption; challenges in demonstrating sufficient efficacy advantage over existing treatments to justify new therapeutic approach.
Critical Patents and Research in Oxaloacetate Applications
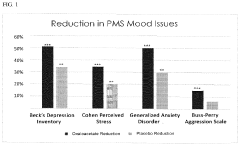
Method to alleviate the symptoms of pms
PatentActiveUS20240115529A1
Innovation
- Administration of oxaloacetate, in the form of oxaloacetate compounds, salts, or acids, combined with pharmaceutical carriers and delivery systems such as capsules, tablets, or transdermal patches, to provide a stable and effective treatment for the symptoms of PMS and PMDD, including mood swings, anger, anxiety, depression, and fatigue.
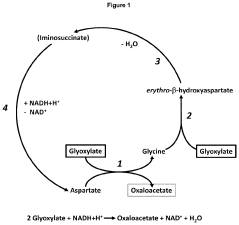
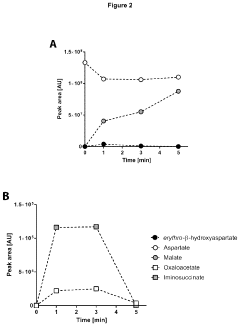
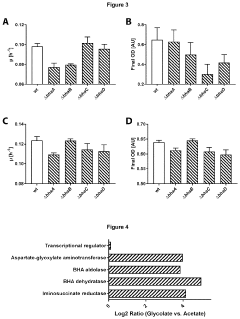
Method of producing autotrophic organisms with altered photorespiration and improved CO<sub>2 </sub>fixation
PatentActiveUS11939583B2
Innovation
- Introduction of nucleic acids encoding polypeptides with enzymatic activities of phosphoglycolate phosphatase, glyoxylate reductase, erythro-β-hydroxyaspartate aldolase, erythro-β-hydroxyaspartate dehydratase, iminosuccinate reductase, and aspartate-glyoxylate transaminase to bypass natural photorespiration pathways with the β-hydroxyaspartate pathway, reducing energy loss and enhancing CO2 fixation efficiency.
Clinical Trial Frameworks for Metabolic Compounds
The development of robust clinical trial frameworks is essential for evaluating the efficacy and safety of oxaloacetate as a metabolic therapy. Current methodologies for metabolic compound trials typically follow a phased approach, beginning with preclinical studies and progressing through Phase I-IV clinical trials, each with specific objectives and endpoints tailored to metabolic interventions.
Standard clinical trial designs for metabolic compounds include randomized controlled trials (RCTs), crossover studies, and adaptive designs. RCTs remain the gold standard, allowing for direct comparison between oxaloacetate intervention and placebo or standard-of-care treatments. Crossover designs are particularly valuable for metabolic studies as they allow subjects to serve as their own controls, reducing inter-individual variability in metabolic responses.
Patient selection criteria for oxaloacetate trials must consider metabolic phenotypes, existing comorbidities, and concurrent medications that might influence metabolic pathways. Stratification based on baseline metabolic parameters such as insulin sensitivity, mitochondrial function, or NAD+/NADH ratios may enhance the ability to detect treatment effects in specific subpopulations.
Biomarker selection represents a critical component of metabolic compound trial frameworks. For oxaloacetate studies, relevant biomarkers include direct measurements of TCA cycle intermediates, glucose homeostasis parameters, inflammatory markers, and mitochondrial function indicators. Novel metabolomic approaches allow for comprehensive profiling of metabolic changes induced by oxaloacetate supplementation.
Duration considerations for metabolic therapy trials must balance the need for sufficient intervention time to observe metabolic adaptations against practical constraints. Short-term studies (4-12 weeks) may capture immediate effects on metabolic parameters, while longer interventions (6-12 months) are necessary to evaluate sustained benefits and safety profiles.
Regulatory considerations for metabolic compound trials include adherence to FDA or EMA guidelines for investigational new drugs or, in some cases, dietary supplement regulations depending on the intended use and claims. The regulatory pathway chosen significantly impacts trial design requirements, endpoint selection, and the overall development timeline.
Ethical frameworks must address the unique considerations of metabolic intervention trials, including appropriate risk-benefit assessments for preventive applications in otherwise healthy individuals versus therapeutic applications in those with established metabolic disorders. Informed consent processes should clearly communicate the experimental nature of metabolic interventions and potential long-term implications.
Standard clinical trial designs for metabolic compounds include randomized controlled trials (RCTs), crossover studies, and adaptive designs. RCTs remain the gold standard, allowing for direct comparison between oxaloacetate intervention and placebo or standard-of-care treatments. Crossover designs are particularly valuable for metabolic studies as they allow subjects to serve as their own controls, reducing inter-individual variability in metabolic responses.
Patient selection criteria for oxaloacetate trials must consider metabolic phenotypes, existing comorbidities, and concurrent medications that might influence metabolic pathways. Stratification based on baseline metabolic parameters such as insulin sensitivity, mitochondrial function, or NAD+/NADH ratios may enhance the ability to detect treatment effects in specific subpopulations.
Biomarker selection represents a critical component of metabolic compound trial frameworks. For oxaloacetate studies, relevant biomarkers include direct measurements of TCA cycle intermediates, glucose homeostasis parameters, inflammatory markers, and mitochondrial function indicators. Novel metabolomic approaches allow for comprehensive profiling of metabolic changes induced by oxaloacetate supplementation.
Duration considerations for metabolic therapy trials must balance the need for sufficient intervention time to observe metabolic adaptations against practical constraints. Short-term studies (4-12 weeks) may capture immediate effects on metabolic parameters, while longer interventions (6-12 months) are necessary to evaluate sustained benefits and safety profiles.
Regulatory considerations for metabolic compound trials include adherence to FDA or EMA guidelines for investigational new drugs or, in some cases, dietary supplement regulations depending on the intended use and claims. The regulatory pathway chosen significantly impacts trial design requirements, endpoint selection, and the overall development timeline.
Ethical frameworks must address the unique considerations of metabolic intervention trials, including appropriate risk-benefit assessments for preventive applications in otherwise healthy individuals versus therapeutic applications in those with established metabolic disorders. Informed consent processes should clearly communicate the experimental nature of metabolic interventions and potential long-term implications.
Regulatory Pathways for Metabolic Therapeutic Approval
The regulatory landscape for metabolic therapeutics incorporating oxaloacetate presents a complex pathway that developers must navigate carefully. In the United States, the FDA offers multiple regulatory routes depending on the intended use and formulation of oxaloacetate-based interventions. For pharmaceutical applications, the traditional New Drug Application (NDA) pathway requires comprehensive clinical trials demonstrating safety and efficacy, with metabolic therapeutics typically falling under the Center for Drug Evaluation and Research (CDER) jurisdiction.
For oxaloacetate formulations positioned as medical foods, the regulatory burden differs significantly. These products must address distinctive nutritional requirements of disease management and can avoid the extensive clinical trial requirements of pharmaceuticals. However, they must still comply with FDA's medical food regulations and substantiate their metabolic claims with scientific evidence.
Dietary supplement pathways offer another avenue, particularly relevant for oxaloacetate products targeting general metabolic health rather than specific disease treatment. Under DSHEA (Dietary Supplement Health and Education Act), manufacturers must ensure safety and can make limited structure-function claims without prior FDA approval, though they cannot claim to treat specific diseases.
Internationally, regulatory frameworks vary substantially. The European Medicines Agency (EMA) employs a centralized procedure for novel metabolic therapeutics, while also offering conditional approval pathways for treatments addressing unmet medical needs. Japan's PMDA has implemented the Sakigake designation specifically for innovative therapies, potentially accelerating approval for novel metabolic interventions incorporating oxaloacetate.
Expedited programs exist across jurisdictions for metabolic therapies addressing serious conditions. The FDA's Breakthrough Therapy designation and Fast Track programs can significantly reduce development timelines for qualifying oxaloacetate-based treatments, particularly those targeting rare metabolic disorders or offering substantial improvements over existing therapies.
Recent regulatory trends indicate increasing flexibility toward metabolic interventions with strong mechanistic rationales. Surrogate endpoints relevant to oxaloacetate's metabolic effects, such as changes in TCA cycle intermediates or energy metabolism biomarkers, are gaining acceptance in regulatory submissions when validated appropriately.
For developers of oxaloacetate-based metabolic therapies, early and frequent engagement with regulatory authorities through pre-IND meetings and scientific advice consultations represents a critical strategy to navigate these complex pathways efficiently and establish appropriate development plans aligned with regulatory expectations.
For oxaloacetate formulations positioned as medical foods, the regulatory burden differs significantly. These products must address distinctive nutritional requirements of disease management and can avoid the extensive clinical trial requirements of pharmaceuticals. However, they must still comply with FDA's medical food regulations and substantiate their metabolic claims with scientific evidence.
Dietary supplement pathways offer another avenue, particularly relevant for oxaloacetate products targeting general metabolic health rather than specific disease treatment. Under DSHEA (Dietary Supplement Health and Education Act), manufacturers must ensure safety and can make limited structure-function claims without prior FDA approval, though they cannot claim to treat specific diseases.
Internationally, regulatory frameworks vary substantially. The European Medicines Agency (EMA) employs a centralized procedure for novel metabolic therapeutics, while also offering conditional approval pathways for treatments addressing unmet medical needs. Japan's PMDA has implemented the Sakigake designation specifically for innovative therapies, potentially accelerating approval for novel metabolic interventions incorporating oxaloacetate.
Expedited programs exist across jurisdictions for metabolic therapies addressing serious conditions. The FDA's Breakthrough Therapy designation and Fast Track programs can significantly reduce development timelines for qualifying oxaloacetate-based treatments, particularly those targeting rare metabolic disorders or offering substantial improvements over existing therapies.
Recent regulatory trends indicate increasing flexibility toward metabolic interventions with strong mechanistic rationales. Surrogate endpoints relevant to oxaloacetate's metabolic effects, such as changes in TCA cycle intermediates or energy metabolism biomarkers, are gaining acceptance in regulatory submissions when validated appropriately.
For developers of oxaloacetate-based metabolic therapies, early and frequent engagement with regulatory authorities through pre-IND meetings and scientific advice consultations represents a critical strategy to navigate these complex pathways efficiently and establish appropriate development plans aligned with regulatory expectations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!