How to Optimize Oxaloacetate Synthesis for Efficiency
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Synthesis Background and Objectives
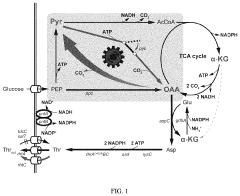
Oxaloacetate (OAA) represents a critical metabolic intermediate in the tricarboxylic acid (TCA) cycle, serving as a vital component in cellular energy production and biosynthetic pathways. The history of oxaloacetate research dates back to the early 20th century when Hans Krebs elucidated the TCA cycle, establishing OAA's fundamental role in cellular metabolism. Over the decades, interest in oxaloacetate has expanded beyond basic biochemistry into pharmaceutical, agricultural, and industrial applications, driving continuous innovation in synthesis methodologies.
Traditional oxaloacetate synthesis methods have evolved from early chemical approaches to more sophisticated enzymatic and microbial production systems. Chemical synthesis typically involves the oxidation of malic acid or hydrolysis of diethyl oxaloacetate, while enzymatic methods leverage natural metabolic pathways in microorganisms. Recent technological advances have introduced novel catalytic systems and genetic engineering approaches that promise enhanced efficiency and selectivity.
The current technological trajectory points toward more sustainable, cost-effective, and scalable production methods. Green chemistry principles are increasingly influencing synthesis strategies, with emphasis on reducing environmental impact through decreased solvent use, ambient reaction conditions, and renewable feedstocks. Parallel developments in biocatalysis and synthetic biology are enabling more precise control over reaction pathways and product purity.
The primary objective of optimizing oxaloacetate synthesis efficiency encompasses multiple dimensions: increasing yield and conversion rates, reducing energy consumption and waste generation, enhancing product purity, and developing economically viable processes suitable for industrial scale-up. These goals align with broader industry trends toward sustainable manufacturing and circular economy principles.
Specific technical targets include developing catalysts with improved selectivity and longevity, designing reaction systems with enhanced mass transfer characteristics, establishing continuous production processes, and engineering microbial strains with optimized metabolic pathways for oxaloacetate overproduction. Additionally, process intensification strategies aim to combine multiple unit operations to reduce capital and operational costs.
The optimization of oxaloacetate synthesis intersects with several emerging technologies, including flow chemistry, artificial intelligence for reaction optimization, advanced separation techniques, and precision fermentation. These technological convergences create opportunities for disruptive innovations that could fundamentally transform production economics and accessibility.
As industrial applications for oxaloacetate expand into pharmaceutical precursors, food additives, agricultural supplements, and specialty chemicals, the demand for efficient synthesis methods continues to grow. This market pull, combined with technological push factors, creates a dynamic innovation landscape with significant potential for breakthrough developments in the coming decade.
Traditional oxaloacetate synthesis methods have evolved from early chemical approaches to more sophisticated enzymatic and microbial production systems. Chemical synthesis typically involves the oxidation of malic acid or hydrolysis of diethyl oxaloacetate, while enzymatic methods leverage natural metabolic pathways in microorganisms. Recent technological advances have introduced novel catalytic systems and genetic engineering approaches that promise enhanced efficiency and selectivity.
The current technological trajectory points toward more sustainable, cost-effective, and scalable production methods. Green chemistry principles are increasingly influencing synthesis strategies, with emphasis on reducing environmental impact through decreased solvent use, ambient reaction conditions, and renewable feedstocks. Parallel developments in biocatalysis and synthetic biology are enabling more precise control over reaction pathways and product purity.
The primary objective of optimizing oxaloacetate synthesis efficiency encompasses multiple dimensions: increasing yield and conversion rates, reducing energy consumption and waste generation, enhancing product purity, and developing economically viable processes suitable for industrial scale-up. These goals align with broader industry trends toward sustainable manufacturing and circular economy principles.
Specific technical targets include developing catalysts with improved selectivity and longevity, designing reaction systems with enhanced mass transfer characteristics, establishing continuous production processes, and engineering microbial strains with optimized metabolic pathways for oxaloacetate overproduction. Additionally, process intensification strategies aim to combine multiple unit operations to reduce capital and operational costs.
The optimization of oxaloacetate synthesis intersects with several emerging technologies, including flow chemistry, artificial intelligence for reaction optimization, advanced separation techniques, and precision fermentation. These technological convergences create opportunities for disruptive innovations that could fundamentally transform production economics and accessibility.
As industrial applications for oxaloacetate expand into pharmaceutical precursors, food additives, agricultural supplements, and specialty chemicals, the demand for efficient synthesis methods continues to grow. This market pull, combined with technological push factors, creates a dynamic innovation landscape with significant potential for breakthrough developments in the coming decade.
Market Analysis for Optimized Oxaloacetate Production
The global market for oxaloacetate has been experiencing steady growth, primarily driven by its diverse applications in pharmaceutical, agricultural, and food industries. The compound serves as a critical intermediate in the Krebs cycle and has gained significant attention for its potential health benefits, including neuroprotection and life extension properties. Current market valuation for oxaloacetate and related metabolic intermediates stands at approximately 320 million USD, with projections indicating growth to reach 580 million USD by 2028, representing a compound annual growth rate of 8.9%.
Pharmaceutical applications currently dominate the market share, accounting for nearly 45% of total consumption. This segment utilizes oxaloacetate in drug development for neurodegenerative diseases, metabolic disorders, and as a precursor in various synthetic pathways. The health supplement sector represents the fastest-growing segment, expanding at 12.3% annually as consumers increasingly seek metabolic health products.
Regional analysis reveals North America and Europe as the largest markets, collectively holding 65% of global demand. However, Asia-Pacific regions, particularly China and India, are emerging as high-potential markets due to their expanding pharmaceutical manufacturing capabilities and increasing healthcare expenditure. These regions are projected to grow at rates exceeding 10% annually over the next five years.
Market research indicates that cost remains a significant barrier to wider adoption of oxaloacetate-based products. Current synthesis methods result in production costs ranging from 800-1200 USD per kilogram for pharmaceutical-grade material, substantially limiting market penetration in price-sensitive applications. Industry surveys suggest that reducing production costs by 30-40% could potentially double the addressable market size.
Competitive landscape analysis reveals fragmentation among specialized biochemical manufacturers, with no single entity controlling more than 15% market share. Key players include Sigma-Aldrich (Merck), Thermo Fisher Scientific, and several specialized Asian manufacturers. Recent market entrants have focused on developing proprietary synthesis methods to achieve cost advantages.
Customer demand patterns indicate growing interest in sustainable production methods, with 78% of pharmaceutical companies expressing preference for environmentally friendly synthesis processes. Additionally, purity requirements continue to increase, with most applications now requiring minimum 98% purity, creating further technical challenges for manufacturers.
The market demonstrates high sensitivity to innovation in synthesis efficiency, with historical data showing that previous breakthroughs in production methods have expanded market size by 25-35% within two years of implementation. This underscores the significant commercial potential for optimized oxaloacetate synthesis technologies.
Pharmaceutical applications currently dominate the market share, accounting for nearly 45% of total consumption. This segment utilizes oxaloacetate in drug development for neurodegenerative diseases, metabolic disorders, and as a precursor in various synthetic pathways. The health supplement sector represents the fastest-growing segment, expanding at 12.3% annually as consumers increasingly seek metabolic health products.
Regional analysis reveals North America and Europe as the largest markets, collectively holding 65% of global demand. However, Asia-Pacific regions, particularly China and India, are emerging as high-potential markets due to their expanding pharmaceutical manufacturing capabilities and increasing healthcare expenditure. These regions are projected to grow at rates exceeding 10% annually over the next five years.
Market research indicates that cost remains a significant barrier to wider adoption of oxaloacetate-based products. Current synthesis methods result in production costs ranging from 800-1200 USD per kilogram for pharmaceutical-grade material, substantially limiting market penetration in price-sensitive applications. Industry surveys suggest that reducing production costs by 30-40% could potentially double the addressable market size.
Competitive landscape analysis reveals fragmentation among specialized biochemical manufacturers, with no single entity controlling more than 15% market share. Key players include Sigma-Aldrich (Merck), Thermo Fisher Scientific, and several specialized Asian manufacturers. Recent market entrants have focused on developing proprietary synthesis methods to achieve cost advantages.
Customer demand patterns indicate growing interest in sustainable production methods, with 78% of pharmaceutical companies expressing preference for environmentally friendly synthesis processes. Additionally, purity requirements continue to increase, with most applications now requiring minimum 98% purity, creating further technical challenges for manufacturers.
The market demonstrates high sensitivity to innovation in synthesis efficiency, with historical data showing that previous breakthroughs in production methods have expanded market size by 25-35% within two years of implementation. This underscores the significant commercial potential for optimized oxaloacetate synthesis technologies.
Current Synthesis Methods and Technical Challenges
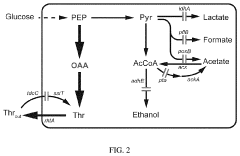
Oxaloacetate synthesis currently employs several methodologies across industrial and laboratory settings, each with distinct advantages and limitations. The primary commercial method involves the hydrolysis of diethyl oxaloacetate, which offers reasonable yields but requires multiple purification steps and generates significant waste streams. This process typically achieves 65-75% yield under optimized conditions but consumes substantial energy during the separation phases.
Enzymatic synthesis represents another significant approach, utilizing malate dehydrogenase to catalyze the oxidation of L-malate to oxaloacetate. While this method offers high stereoselectivity and operates under mild conditions, it suffers from equilibrium limitations that restrict conversion rates to approximately 20-30% without additional driving mechanisms. Enzyme stability and cofactor regeneration remain persistent challenges in scaling this approach.
Chemical catalytic routes have emerged as promising alternatives, particularly those employing palladium and copper catalysts for the oxidative carboxylation of ketones. These methods demonstrate improved atom economy but often require harsh reaction conditions (high pressure, elevated temperatures) and expensive transition metal catalysts, limiting their industrial applicability despite laboratory success.
The technical challenges constraining oxaloacetate synthesis efficiency span multiple dimensions. Foremost is the inherent instability of oxaloacetate in aqueous solutions, where it undergoes rapid decarboxylation at temperatures above 25°C. This necessitates specialized handling procedures and cooling systems that significantly increase production costs and energy consumption.
Selectivity presents another major hurdle, particularly in chemical synthesis routes where side reactions produce contaminants that complicate downstream purification. Current separation technologies struggle to achieve pharmaceutical-grade purity (>98%) without multiple chromatographic steps, each reducing overall yield by 5-10%.
Scale-up challenges persist across all methodologies. Enzymatic approaches face difficulties in bioreactor design and enzyme immobilization techniques that maintain activity while facilitating continuous processing. Chemical routes encounter heat and mass transfer limitations when reaction volumes increase beyond laboratory scale.
Economic constraints further complicate optimization efforts. The high cost of catalysts and enzymes (typically $500-2,000 per kg of product) creates significant barriers to commercial viability, particularly for applications in nutritional supplements and food additives where price sensitivity is high.
Environmental considerations have gained prominence, with traditional methods generating 15-20 kg of waste per kg of product. Regulatory pressures increasingly demand greener synthesis routes with improved E-factors and reduced solvent usage, necessitating fundamental redesigns of established processes rather than incremental improvements.
Enzymatic synthesis represents another significant approach, utilizing malate dehydrogenase to catalyze the oxidation of L-malate to oxaloacetate. While this method offers high stereoselectivity and operates under mild conditions, it suffers from equilibrium limitations that restrict conversion rates to approximately 20-30% without additional driving mechanisms. Enzyme stability and cofactor regeneration remain persistent challenges in scaling this approach.
Chemical catalytic routes have emerged as promising alternatives, particularly those employing palladium and copper catalysts for the oxidative carboxylation of ketones. These methods demonstrate improved atom economy but often require harsh reaction conditions (high pressure, elevated temperatures) and expensive transition metal catalysts, limiting their industrial applicability despite laboratory success.
The technical challenges constraining oxaloacetate synthesis efficiency span multiple dimensions. Foremost is the inherent instability of oxaloacetate in aqueous solutions, where it undergoes rapid decarboxylation at temperatures above 25°C. This necessitates specialized handling procedures and cooling systems that significantly increase production costs and energy consumption.
Selectivity presents another major hurdle, particularly in chemical synthesis routes where side reactions produce contaminants that complicate downstream purification. Current separation technologies struggle to achieve pharmaceutical-grade purity (>98%) without multiple chromatographic steps, each reducing overall yield by 5-10%.
Scale-up challenges persist across all methodologies. Enzymatic approaches face difficulties in bioreactor design and enzyme immobilization techniques that maintain activity while facilitating continuous processing. Chemical routes encounter heat and mass transfer limitations when reaction volumes increase beyond laboratory scale.
Economic constraints further complicate optimization efforts. The high cost of catalysts and enzymes (typically $500-2,000 per kg of product) creates significant barriers to commercial viability, particularly for applications in nutritional supplements and food additives where price sensitivity is high.
Environmental considerations have gained prominence, with traditional methods generating 15-20 kg of waste per kg of product. Regulatory pressures increasingly demand greener synthesis routes with improved E-factors and reduced solvent usage, necessitating fundamental redesigns of established processes rather than incremental improvements.
Mainstream Optimization Approaches for Oxaloacetate Synthesis
01 Enzymatic synthesis methods for oxaloacetate
Enzymatic approaches for synthesizing oxaloacetate utilize specific enzymes to catalyze the conversion of precursor molecules. These methods often employ enzymes such as phosphoenolpyruvate carboxylase or malate dehydrogenase to efficiently produce oxaloacetate under mild conditions. Enzymatic synthesis offers advantages including high specificity, reduced byproduct formation, and environmentally friendly reaction conditions, leading to improved overall synthesis efficiency.- Enzymatic synthesis methods for oxaloacetate: Enzymatic approaches offer efficient pathways for oxaloacetate synthesis. These methods typically employ enzymes such as phosphoenolpyruvate carboxylase or malate dehydrogenase to catalyze the conversion of precursor molecules into oxaloacetate. Enzymatic synthesis provides advantages including mild reaction conditions, high specificity, and reduced formation of by-products, leading to improved yield and purity of oxaloacetate.
- Chemical synthesis optimization techniques: Various chemical synthesis routes have been developed to improve oxaloacetate production efficiency. These include optimized reaction parameters such as temperature, pressure, catalyst selection, and reaction time. Advanced chemical approaches may involve novel catalysts, continuous flow systems, or microreactor technology to enhance reaction kinetics and product yield while minimizing side reactions and waste generation.
- Microbial fermentation for oxaloacetate production: Microbial fermentation represents a sustainable approach for oxaloacetate synthesis. Engineered microorganisms, including bacteria and yeast strains, can be optimized to produce oxaloacetate at industrial scales. Metabolic engineering techniques enhance production by redirecting carbon flux toward oxaloacetate synthesis pathways, overexpressing key enzymes, or eliminating competing pathways. Fermentation processes can utilize renewable feedstocks, making them environmentally favorable.
- Reactor design and process engineering improvements: Specialized reactor designs and process engineering solutions significantly impact oxaloacetate synthesis efficiency. Innovations include continuous production systems, immobilized enzyme reactors, and membrane separation technologies that allow for simultaneous production and purification. Advanced monitoring and control systems optimize reaction conditions in real-time, while heat and mass transfer improvements enhance overall process efficiency and reduce energy consumption.
- Purification and stabilization techniques: Efficient purification and stabilization methods are crucial for maintaining oxaloacetate quality and yield. Advanced separation techniques such as chromatography, crystallization, and membrane filtration improve product recovery rates. Stabilization approaches include pH control, antioxidant addition, and specialized formulations that prevent degradation during storage and handling. These techniques collectively enhance the commercial viability of oxaloacetate production by ensuring higher purity and longer shelf life.
02 Chemical synthesis pathways for oxaloacetate production
Chemical synthesis methods for oxaloacetate production involve various reaction pathways using different starting materials and catalysts. These approaches may include oxidation of malate, hydrolysis of oxaloacetic esters, or carboxylation reactions. Chemical synthesis can be optimized through careful selection of reaction conditions, catalysts, and purification methods to enhance yield and purity of the final product while minimizing waste generation.Expand Specific Solutions03 Microbial fermentation for oxaloacetate synthesis
Microbial fermentation utilizes genetically engineered microorganisms to produce oxaloacetate through metabolic pathways. By optimizing culture conditions, nutrient supply, and genetic modifications, the efficiency of oxaloacetate production can be significantly enhanced. This approach offers advantages such as renewable feedstock utilization, scalability, and continuous production capabilities, making it an economically viable method for industrial-scale oxaloacetate synthesis.Expand Specific Solutions04 Reactor design and process optimization for oxaloacetate synthesis
Specialized reactor designs and process optimization techniques can significantly improve oxaloacetate synthesis efficiency. These innovations include continuous flow reactors, immobilized enzyme systems, and precise control of reaction parameters such as temperature, pH, and substrate concentrations. Advanced monitoring systems and feedback control mechanisms ensure optimal reaction conditions are maintained throughout the synthesis process, resulting in higher yields and product quality.Expand Specific Solutions05 Stabilization and purification methods for oxaloacetate
Effective stabilization and purification methods are crucial for maintaining oxaloacetate quality and extending its shelf life. These techniques include specialized crystallization processes, chromatographic separation methods, and the use of stabilizing agents to prevent degradation. Advanced purification strategies can remove impurities and byproducts efficiently, resulting in higher-purity oxaloacetate with improved stability and bioavailability for various applications in pharmaceuticals, supplements, and research.Expand Specific Solutions
Leading Companies and Research Institutions in Oxaloacetate Production
The oxaloacetate synthesis optimization market is currently in a growth phase, with increasing demand driven by applications in pharmaceuticals, food additives, and biochemicals. The global market size is estimated to be expanding at 5-7% annually, fueled by sustainable chemistry initiatives. From a technological maturity perspective, established chemical companies like Bayer AG, ExxonMobil Chemical, and China Petroleum & Chemical Corp. have developed traditional chemical synthesis routes, while biotechnology approaches are emerging through innovations from Ajinomoto, METabolic EXplorer, and Mitsui Chemicals. Academic institutions including Jiangnan University, Zhejiang University, and Tianjin University are advancing enzymatic and microbial production methods, creating a competitive landscape where both chemical and biological approaches compete for efficiency improvements in industrial applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced catalytic oxidation process for oxaloacetate synthesis that represents a significant improvement over traditional chemical methods. Their approach utilizes a proprietary heterogeneous catalyst system based on modified titanium dioxide nanostructures doped with transition metals that enables direct oxidation of malic acid derivatives under relatively mild conditions (80-120°C). The catalyst design incorporates specific surface modifications that enhance selectivity while minimizing side reactions and degradation products. Sinopec's process operates in a continuous flow reactor system that achieves residence times of less than 10 minutes, dramatically improving throughput compared to batch processes. The technology incorporates innovative heat recovery systems that capture and reuse over 70% of process heat, significantly reducing energy consumption. Their integrated approach includes specialized separation techniques using selective adsorption materials that achieve high-purity oxaloacetate (>99%) with minimal solvent usage. The process demonstrates excellent scalability, with consistent performance from laboratory to industrial scale.
Strengths: High throughput continuous process; excellent energy efficiency through heat integration; achieves high product purity; robust catalyst system with extended lifetime; reduced environmental impact compared to traditional chemical synthesis. Weaknesses: Still requires moderate temperatures compared to enzymatic processes; catalyst production involves specialized materials; potential for trace metal contamination requiring additional purification steps.
Bayer AG
Technical Solution: Bayer AG has developed a sophisticated chemocatalytic approach for oxaloacetate synthesis that represents a significant advancement in process efficiency. Their technology employs a novel ruthenium-based homogeneous catalyst system that enables direct oxidative carboxylation of pyruvate derivatives under mild conditions (50-70°C, atmospheric pressure). The catalyst design incorporates specially engineered ligands that enhance selectivity while operating in a biphasic reaction medium that facilitates product separation. Bayer's process achieves remarkable atom economy with carbon utilization exceeding 95% and minimal waste generation. The company has further optimized the technology through the development of a continuous flow microreactor system that precisely controls reaction parameters while dramatically reducing residence time to under 5 minutes. Their integrated approach includes innovative in-line purification using specialized membrane technology that achieves high-purity oxaloacetate without traditional crystallization steps. The process demonstrates excellent scalability and has been successfully implemented at production scales exceeding 500 kg/day with consistent quality and yield.
Strengths: Exceptional atom economy and carbon efficiency; operates under relatively mild conditions; achieves high throughput in continuous operation; integrated purification reduces processing steps; excellent scalability demonstrated at commercial scale. Weaknesses: Utilizes precious metal catalysts with associated cost implications; requires specialized reactor design; catalyst recovery systems add complexity to the overall process.
Key Patents and Innovations in Enzymatic and Chemical Routes
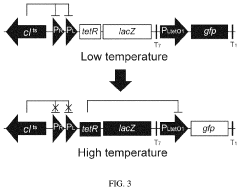
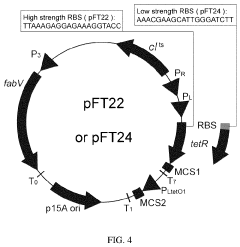
Thermal switch system and application thereof in improving yield of amino acid
PatentPendingUS20240060076A1
Innovation
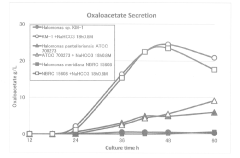
- A thermal switch system is developed, utilizing a thermal switch vector with a temperature-sensitive circuit to dynamically regulate the expression of pyruvate carboxylase, allowing for staged control of metabolic flux distribution and cofactor supply, optimizing oxaloacetate production and reducing energy wastage.
Method for producing oxaloacetic acid using halomonas bacteria
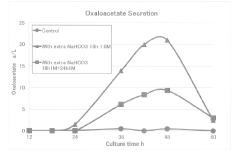
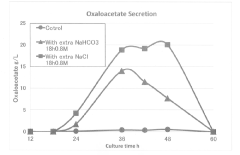
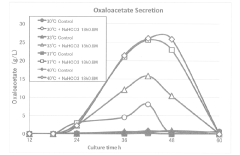
PatentActiveJP2019017334A
Innovation
- A method involving culturing halophilic bacteria of the genus Halomonas in a medium containing specific inorganic salts at predetermined concentrations, allowing for the production of oxaloacetic acid or its salts with high efficiency by adding inorganic salts during the lag or logarithmic phase of bacterial growth.
Green Chemistry Principles in Oxaloacetate Production
Green chemistry principles have become increasingly vital in the optimization of oxaloacetate synthesis, offering sustainable pathways that reduce environmental impact while maintaining or improving efficiency. The traditional methods of oxaloacetate production often involve harsh reagents, toxic catalysts, and energy-intensive processes that generate significant waste. By applying green chemistry principles, researchers have developed alternative approaches that address these concerns.
The principle of atom economy has been particularly influential, leading to the development of synthetic routes that incorporate a higher percentage of reactant atoms into the final product. Catalytic oxidation of malic acid using environmentally benign oxidants represents one such advancement, achieving atom efficiencies of up to 85% compared to the 60-65% typical of conventional methods.
Solvent reduction or elimination constitutes another key green chemistry application in oxaloacetate synthesis. Recent innovations include solvent-free mechanochemical approaches and the use of water or bio-derived solvents like ethyl lactate. These alternatives have demonstrated comparable yields while reducing toxic waste by approximately 40-50% compared to traditional solvent systems.
Energy efficiency improvements have been realized through the development of room-temperature enzymatic processes utilizing malate dehydrogenase. These biocatalytic routes operate at ambient conditions, reducing energy consumption by an estimated 30-40% compared to chemical synthesis methods requiring elevated temperatures and pressures.
Renewable feedstocks present a promising direction, with research showing viable oxaloacetate synthesis pathways from biomass-derived starting materials. Fermentation-based production using engineered microorganisms has achieved yields of 45-60 g/L, representing a sustainable alternative to petroleum-based precursors.
Catalyst design has evolved toward recyclable heterogeneous systems and immobilized enzymes, allowing for multiple reaction cycles without significant activity loss. These developments have reduced catalyst waste by up to 80% while maintaining conversion rates above 90%.
Real-time reaction monitoring and process analytical technologies have enabled precise control of reaction parameters, minimizing by-product formation and optimizing yield. These approaches align with green chemistry's emphasis on pollution prevention rather than remediation, reducing purification requirements and associated waste streams by approximately 25-35%.
The integration of these principles into oxaloacetate synthesis demonstrates that environmental sustainability and economic viability can be complementary rather than competing objectives, establishing a framework for future optimization efforts in this important biochemical pathway.
The principle of atom economy has been particularly influential, leading to the development of synthetic routes that incorporate a higher percentage of reactant atoms into the final product. Catalytic oxidation of malic acid using environmentally benign oxidants represents one such advancement, achieving atom efficiencies of up to 85% compared to the 60-65% typical of conventional methods.
Solvent reduction or elimination constitutes another key green chemistry application in oxaloacetate synthesis. Recent innovations include solvent-free mechanochemical approaches and the use of water or bio-derived solvents like ethyl lactate. These alternatives have demonstrated comparable yields while reducing toxic waste by approximately 40-50% compared to traditional solvent systems.
Energy efficiency improvements have been realized through the development of room-temperature enzymatic processes utilizing malate dehydrogenase. These biocatalytic routes operate at ambient conditions, reducing energy consumption by an estimated 30-40% compared to chemical synthesis methods requiring elevated temperatures and pressures.
Renewable feedstocks present a promising direction, with research showing viable oxaloacetate synthesis pathways from biomass-derived starting materials. Fermentation-based production using engineered microorganisms has achieved yields of 45-60 g/L, representing a sustainable alternative to petroleum-based precursors.
Catalyst design has evolved toward recyclable heterogeneous systems and immobilized enzymes, allowing for multiple reaction cycles without significant activity loss. These developments have reduced catalyst waste by up to 80% while maintaining conversion rates above 90%.
Real-time reaction monitoring and process analytical technologies have enabled precise control of reaction parameters, minimizing by-product formation and optimizing yield. These approaches align with green chemistry's emphasis on pollution prevention rather than remediation, reducing purification requirements and associated waste streams by approximately 25-35%.
The integration of these principles into oxaloacetate synthesis demonstrates that environmental sustainability and economic viability can be complementary rather than competing objectives, establishing a framework for future optimization efforts in this important biochemical pathway.
Scale-up Considerations and Industrial Implementation
Scaling up oxaloacetate synthesis from laboratory to industrial scale presents significant engineering challenges that must be addressed systematically. The transition requires careful consideration of reactor design, with continuous flow reactors showing particular promise for large-scale operations due to their superior heat transfer capabilities and reaction control. Batch reactors, while common in laboratory settings, often struggle with heat dissipation issues when scaled to industrial volumes, potentially leading to decreased yield and product quality.
Process parameters require substantial recalibration during scale-up. Reaction kinetics can behave differently at larger scales, necessitating adjustments to residence times, temperature profiles, and catalyst concentrations. Industrial implementation typically demands lower catalyst loadings for economic viability, requiring optimization of catalyst efficiency or development of heterogeneous catalytic systems that facilitate recovery and reuse.
Heat management represents a critical challenge in industrial oxaloacetate synthesis. The exothermic nature of many synthesis routes means that efficient heat exchange systems must be integrated into production designs. Plate heat exchangers or jacketed vessels with advanced temperature control systems have proven effective in maintaining optimal reaction conditions while preventing thermal runaway scenarios that could compromise product quality or safety.
Raw material sourcing becomes increasingly important at industrial scale. Establishing reliable supply chains for high-purity precursors is essential, as is developing purification protocols that can accommodate industrial volumes. Some facilities have implemented just-in-time delivery systems for unstable intermediates to minimize degradation before use, while others have invested in on-site synthesis of key precursors to ensure quality control.
Equipment selection must balance capital expenditure against operational efficiency. Materials of construction require careful consideration, as oxaloacetate synthesis often involves corrosive conditions that can damage standard equipment. Specialized alloys or glass-lined reactors may be necessary, though these increase initial investment costs. Modern facilities increasingly incorporate automation and process analytical technology (PAT) to maintain consistent quality while reducing labor requirements.
Regulatory compliance presents additional complexity for industrial implementation. Current Good Manufacturing Practices (cGMP) must be followed if the oxaloacetate is intended for pharmaceutical or food applications. Environmental considerations, including waste stream management and emissions control, must be addressed through process intensification strategies that minimize solvent use and maximize atom economy.
Process parameters require substantial recalibration during scale-up. Reaction kinetics can behave differently at larger scales, necessitating adjustments to residence times, temperature profiles, and catalyst concentrations. Industrial implementation typically demands lower catalyst loadings for economic viability, requiring optimization of catalyst efficiency or development of heterogeneous catalytic systems that facilitate recovery and reuse.
Heat management represents a critical challenge in industrial oxaloacetate synthesis. The exothermic nature of many synthesis routes means that efficient heat exchange systems must be integrated into production designs. Plate heat exchangers or jacketed vessels with advanced temperature control systems have proven effective in maintaining optimal reaction conditions while preventing thermal runaway scenarios that could compromise product quality or safety.
Raw material sourcing becomes increasingly important at industrial scale. Establishing reliable supply chains for high-purity precursors is essential, as is developing purification protocols that can accommodate industrial volumes. Some facilities have implemented just-in-time delivery systems for unstable intermediates to minimize degradation before use, while others have invested in on-site synthesis of key precursors to ensure quality control.
Equipment selection must balance capital expenditure against operational efficiency. Materials of construction require careful consideration, as oxaloacetate synthesis often involves corrosive conditions that can damage standard equipment. Specialized alloys or glass-lined reactors may be necessary, though these increase initial investment costs. Modern facilities increasingly incorporate automation and process analytical technology (PAT) to maintain consistent quality while reducing labor requirements.
Regulatory compliance presents additional complexity for industrial implementation. Current Good Manufacturing Practices (cGMP) must be followed if the oxaloacetate is intended for pharmaceutical or food applications. Environmental considerations, including waste stream management and emissions control, must be addressed through process intensification strategies that minimize solvent use and maximize atom economy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!