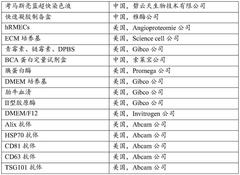
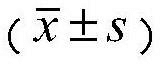Quantify Oxaloacetate Benefits in Aging Research
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate in Aging Research: Background and Objectives
Oxaloacetate (OAA) has emerged as a significant compound in aging research, with its history tracing back to fundamental metabolic studies in the early 20th century. Initially recognized as a key intermediate in the Krebs cycle, oxaloacetate's potential role in longevity and age-related conditions has only gained serious scientific attention in the past two decades. This growing interest coincides with the broader expansion of geroscience, which seeks to understand the biological mechanisms underlying aging and develop interventions to extend healthspan.
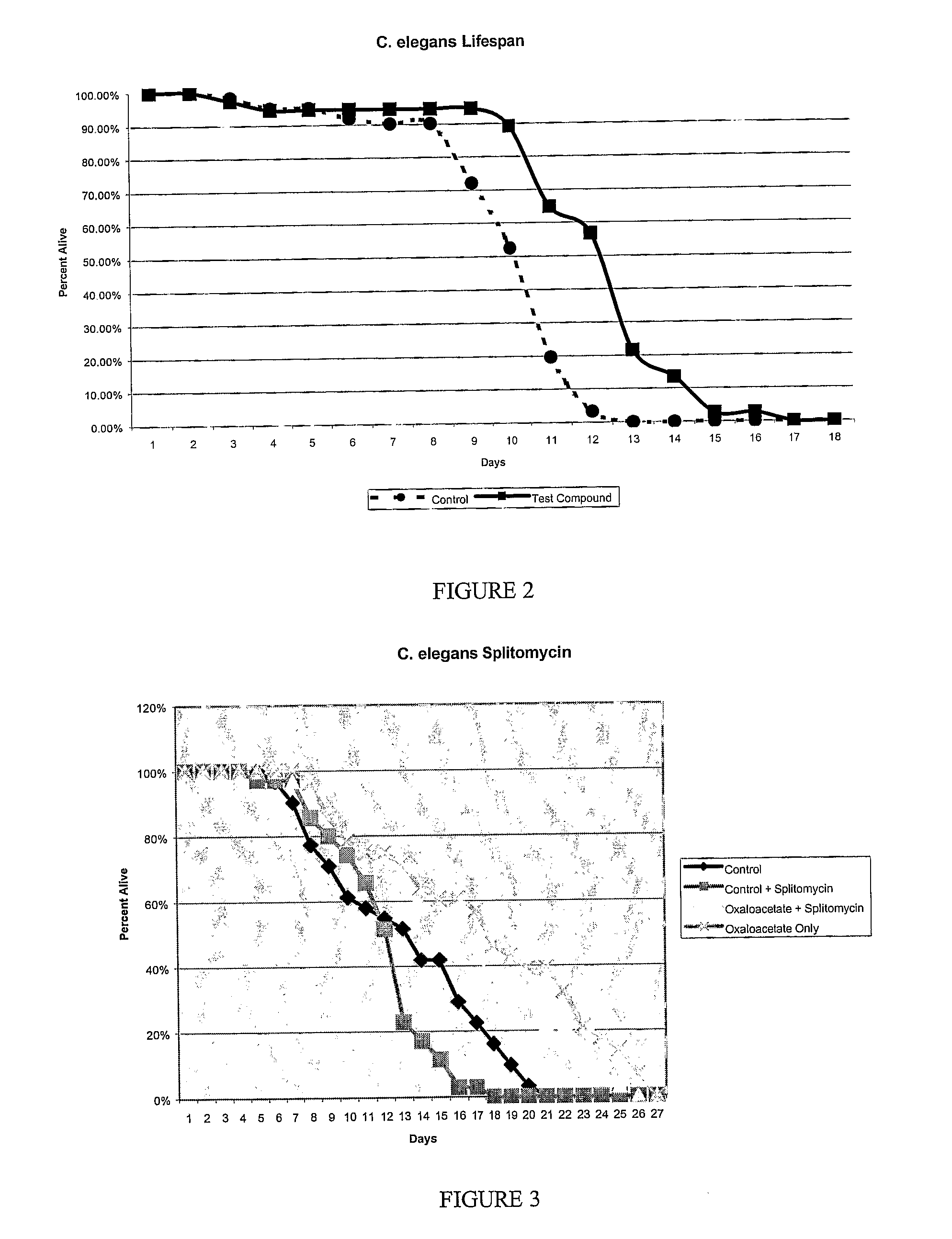
The evolution of oxaloacetate research has progressed from basic biochemical characterization to more sophisticated investigations of its effects on cellular metabolism, mitochondrial function, and systemic aging biomarkers. Early studies in model organisms such as Caenorhabditis elegans demonstrated lifespan extension effects, while subsequent research in rodents has explored its impact on age-related cognitive decline and metabolic parameters.
Recent technological advances in metabolomics, proteomics, and computational biology have significantly enhanced our ability to quantify and understand oxaloacetate's multifaceted effects on biological systems. These developments have enabled more precise measurements of oxaloacetate's influence on NAD+ levels, glutamate metabolism, and mitochondrial efficiency—all critical factors in the aging process.
The primary technical objectives of current oxaloacetate research center on establishing standardized methodologies for quantifying its benefits across multiple aging parameters. This includes developing reliable biomarkers to assess its impact on cellular senescence, oxidative stress, and inflammatory processes. Additionally, researchers aim to determine optimal dosing regimens, delivery mechanisms, and potential synergistic effects when combined with other geroprotective compounds.
Another crucial goal involves elucidating the molecular pathways through which oxaloacetate exerts its effects. Current evidence suggests it may function through multiple mechanisms, including caloric restriction mimicry, enhancement of mitochondrial biogenesis, and modulation of nutrient-sensing pathways such as AMPK and mTOR. Understanding these mechanisms is essential for developing targeted interventions and predicting potential side effects or contraindications.
From a translational perspective, researchers seek to bridge the gap between promising preclinical findings and human applications. This necessitates the development of robust clinical trial protocols specifically designed to measure aging-related outcomes in response to oxaloacetate supplementation. The field is moving toward more comprehensive assessment frameworks that capture both physiological and functional changes across multiple organ systems.
The ultimate technical objective is to establish whether oxaloacetate represents a viable intervention for extending human healthspan and potentially lifespan. This requires not only demonstrating efficacy but also addressing challenges related to stability, bioavailability, and long-term safety profiles of oxaloacetate supplementation in diverse human populations.
The evolution of oxaloacetate research has progressed from basic biochemical characterization to more sophisticated investigations of its effects on cellular metabolism, mitochondrial function, and systemic aging biomarkers. Early studies in model organisms such as Caenorhabditis elegans demonstrated lifespan extension effects, while subsequent research in rodents has explored its impact on age-related cognitive decline and metabolic parameters.
Recent technological advances in metabolomics, proteomics, and computational biology have significantly enhanced our ability to quantify and understand oxaloacetate's multifaceted effects on biological systems. These developments have enabled more precise measurements of oxaloacetate's influence on NAD+ levels, glutamate metabolism, and mitochondrial efficiency—all critical factors in the aging process.
The primary technical objectives of current oxaloacetate research center on establishing standardized methodologies for quantifying its benefits across multiple aging parameters. This includes developing reliable biomarkers to assess its impact on cellular senescence, oxidative stress, and inflammatory processes. Additionally, researchers aim to determine optimal dosing regimens, delivery mechanisms, and potential synergistic effects when combined with other geroprotective compounds.
Another crucial goal involves elucidating the molecular pathways through which oxaloacetate exerts its effects. Current evidence suggests it may function through multiple mechanisms, including caloric restriction mimicry, enhancement of mitochondrial biogenesis, and modulation of nutrient-sensing pathways such as AMPK and mTOR. Understanding these mechanisms is essential for developing targeted interventions and predicting potential side effects or contraindications.
From a translational perspective, researchers seek to bridge the gap between promising preclinical findings and human applications. This necessitates the development of robust clinical trial protocols specifically designed to measure aging-related outcomes in response to oxaloacetate supplementation. The field is moving toward more comprehensive assessment frameworks that capture both physiological and functional changes across multiple organ systems.
The ultimate technical objective is to establish whether oxaloacetate represents a viable intervention for extending human healthspan and potentially lifespan. This requires not only demonstrating efficacy but also addressing challenges related to stability, bioavailability, and long-term safety profiles of oxaloacetate supplementation in diverse human populations.
Market Analysis of Anti-Aging Supplements and Therapeutics
The global anti-aging market has experienced remarkable growth in recent years, with the supplement sector emerging as a particularly dynamic segment. Currently valued at approximately $58.5 billion in 2023, the anti-aging supplement market is projected to reach $85.6 billion by 2030, representing a compound annual growth rate (CAGR) of 5.6%. This growth is primarily driven by increasing consumer awareness of preventative health measures and a demographic shift toward aging populations in developed economies.
Within this broader market, metabolic health supplements focusing on cellular energy production and mitochondrial function have gained significant traction. Oxaloacetate, as a key metabolic intermediate in the Krebs cycle, has carved out a specialized niche estimated at $125 million annually, with projections suggesting expansion to $350 million by 2028 as research validation increases.
Consumer demographics for anti-aging supplements skew heavily toward adults aged 45-75, with higher education levels and above-average household incomes. Recent market research indicates that 68% of consumers in this segment are willing to pay premium prices for supplements backed by scientific research, representing a critical opportunity for evidence-based products like oxaloacetate.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%), with the latter showing the fastest growth rate at 7.8% annually. Japan and South Korea have emerged as particularly receptive markets for metabolic health supplements, reflecting their aging demographics and cultural emphasis on longevity.
Distribution channels have evolved significantly, with direct-to-consumer e-commerce platforms now accounting for 38% of sales, followed by specialty health retailers (27%), traditional pharmacies (18%), and healthcare practitioner channels (17%). The practitioner channel, though smaller, shows higher growth potential for scientifically validated supplements like oxaloacetate.
Competitive analysis reveals a fragmented landscape with over 200 companies operating in the anti-aging supplement space. However, only 15 major players control approximately 65% of market revenue. Recent merger and acquisition activity has accelerated, with pharmaceutical companies increasingly acquiring supplement brands with strong scientific portfolios, suggesting potential consolidation in the oxaloacetate segment as clinical evidence accumulates.
Pricing strategies vary widely, with scientifically validated supplements commanding premium positions. Current oxaloacetate supplements are priced between $45-120 for monthly supplies, positioning them in the upper-middle segment of the market. Consumer willingness to pay correlates strongly with the depth and quality of supporting research, highlighting the commercial importance of quantifying oxaloacetate's benefits in aging research.
Within this broader market, metabolic health supplements focusing on cellular energy production and mitochondrial function have gained significant traction. Oxaloacetate, as a key metabolic intermediate in the Krebs cycle, has carved out a specialized niche estimated at $125 million annually, with projections suggesting expansion to $350 million by 2028 as research validation increases.
Consumer demographics for anti-aging supplements skew heavily toward adults aged 45-75, with higher education levels and above-average household incomes. Recent market research indicates that 68% of consumers in this segment are willing to pay premium prices for supplements backed by scientific research, representing a critical opportunity for evidence-based products like oxaloacetate.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%), with the latter showing the fastest growth rate at 7.8% annually. Japan and South Korea have emerged as particularly receptive markets for metabolic health supplements, reflecting their aging demographics and cultural emphasis on longevity.
Distribution channels have evolved significantly, with direct-to-consumer e-commerce platforms now accounting for 38% of sales, followed by specialty health retailers (27%), traditional pharmacies (18%), and healthcare practitioner channels (17%). The practitioner channel, though smaller, shows higher growth potential for scientifically validated supplements like oxaloacetate.
Competitive analysis reveals a fragmented landscape with over 200 companies operating in the anti-aging supplement space. However, only 15 major players control approximately 65% of market revenue. Recent merger and acquisition activity has accelerated, with pharmaceutical companies increasingly acquiring supplement brands with strong scientific portfolios, suggesting potential consolidation in the oxaloacetate segment as clinical evidence accumulates.
Pricing strategies vary widely, with scientifically validated supplements commanding premium positions. Current oxaloacetate supplements are priced between $45-120 for monthly supplies, positioning them in the upper-middle segment of the market. Consumer willingness to pay correlates strongly with the depth and quality of supporting research, highlighting the commercial importance of quantifying oxaloacetate's benefits in aging research.
Current State and Challenges in Oxaloacetate Research
Oxaloacetate (OAA) research has gained significant momentum in recent years, particularly in the field of aging and longevity studies. Current research indicates that OAA may influence several key biological pathways associated with aging, including NAD+ metabolism, mitochondrial function, and glutamate regulation. Laboratory studies have demonstrated promising results in model organisms, with some research showing lifespan extension in Caenorhabditis elegans and improved cognitive function in aged mice.
Despite these encouraging findings, the field faces substantial challenges in translating these results to human applications. One primary obstacle is the inherent instability of oxaloacetate in its natural form, which rapidly decarboxylates at room temperature. This chemical instability presents significant hurdles for formulation, storage, and delivery of effective OAA supplements or therapeutic agents.
Methodological inconsistencies across studies represent another major challenge. Variations in dosing protocols, delivery methods, and measurement techniques have led to conflicting results, making it difficult to establish standardized approaches for quantifying OAA benefits. The lack of large-scale, long-term human clinical trials further compounds this issue, as most evidence remains limited to animal models or small-scale human studies.
Bioavailability presents a significant technical barrier, with current research indicating that oral administration results in relatively poor absorption and rapid metabolism. This necessitates the development of novel delivery systems or chemical modifications to enhance stability and bioavailability while maintaining biological activity.
The complexity of aging mechanisms also complicates research efforts. Aging involves numerous interconnected biological pathways, making it challenging to isolate and quantify the specific contributions of OAA to longevity and healthspan. Researchers struggle to determine whether observed benefits stem directly from OAA or from its metabolites and downstream effects.
Regulatory hurdles further impede progress in this field. The classification of OAA as a supplement rather than a pharmaceutical in many jurisdictions has limited funding for rigorous clinical research and created uncertainty regarding appropriate quality standards and efficacy claims.
Technological limitations in measuring relevant biomarkers consistently and accurately across different studies have hampered efforts to establish definitive correlations between OAA supplementation and aging outcomes. Current analytical methods vary widely in sensitivity and specificity, particularly for in vivo measurements of metabolic parameters affected by OAA.
These challenges collectively highlight the need for interdisciplinary approaches combining expertise in biochemistry, pharmacology, and gerontology to advance the quantification of oxaloacetate benefits in aging research.
Despite these encouraging findings, the field faces substantial challenges in translating these results to human applications. One primary obstacle is the inherent instability of oxaloacetate in its natural form, which rapidly decarboxylates at room temperature. This chemical instability presents significant hurdles for formulation, storage, and delivery of effective OAA supplements or therapeutic agents.
Methodological inconsistencies across studies represent another major challenge. Variations in dosing protocols, delivery methods, and measurement techniques have led to conflicting results, making it difficult to establish standardized approaches for quantifying OAA benefits. The lack of large-scale, long-term human clinical trials further compounds this issue, as most evidence remains limited to animal models or small-scale human studies.
Bioavailability presents a significant technical barrier, with current research indicating that oral administration results in relatively poor absorption and rapid metabolism. This necessitates the development of novel delivery systems or chemical modifications to enhance stability and bioavailability while maintaining biological activity.
The complexity of aging mechanisms also complicates research efforts. Aging involves numerous interconnected biological pathways, making it challenging to isolate and quantify the specific contributions of OAA to longevity and healthspan. Researchers struggle to determine whether observed benefits stem directly from OAA or from its metabolites and downstream effects.
Regulatory hurdles further impede progress in this field. The classification of OAA as a supplement rather than a pharmaceutical in many jurisdictions has limited funding for rigorous clinical research and created uncertainty regarding appropriate quality standards and efficacy claims.
Technological limitations in measuring relevant biomarkers consistently and accurately across different studies have hampered efforts to establish definitive correlations between OAA supplementation and aging outcomes. Current analytical methods vary widely in sensitivity and specificity, particularly for in vivo measurements of metabolic parameters affected by OAA.
These challenges collectively highlight the need for interdisciplinary approaches combining expertise in biochemistry, pharmacology, and gerontology to advance the quantification of oxaloacetate benefits in aging research.
Current Methodologies for Quantifying Oxaloacetate Effects
01 Neuroprotective effects of oxaloacetate
Oxaloacetate has been shown to have neuroprotective properties by reducing glutamate levels in the brain, which can help protect neurons from excitotoxicity. This mechanism may be beneficial in treating or preventing neurodegenerative conditions such as Alzheimer's disease, Parkinson's disease, and traumatic brain injury. The compound works by converting excess glutamate to alpha-ketoglutarate, thereby reducing neurotoxicity and promoting brain health.- Neuroprotective effects of oxaloacetate: Oxaloacetate has been found to have neuroprotective properties that can help protect brain cells from damage and degeneration. It works by reducing glutamate levels in the brain, which can be toxic in high concentrations. This neuroprotective effect may be beneficial for conditions such as traumatic brain injury, stroke, and neurodegenerative diseases. Research suggests that oxaloacetate supplementation could help maintain cognitive function and protect against age-related cognitive decline.
- Metabolic benefits and energy production: Oxaloacetate plays a crucial role in cellular energy production as a key component of the Krebs cycle (citric acid cycle). Supplementation with oxaloacetate can enhance mitochondrial function and increase ATP production, leading to improved energy levels. It also helps in glucose metabolism and can support healthy blood sugar levels. These metabolic benefits make oxaloacetate potentially useful for conditions characterized by metabolic dysfunction or energy deficits.
- Anti-aging and longevity effects: Research indicates that oxaloacetate may have anti-aging properties and could potentially extend lifespan. It works by mimicking caloric restriction, which has been shown to increase longevity in various organisms. Oxaloacetate can help reduce oxidative stress and inflammation, two key factors in the aging process. Additionally, it may help maintain telomere length and protect against age-related DNA damage, further contributing to its potential anti-aging benefits.
- Applications in weight management and metabolic health: Oxaloacetate has shown promise in supporting weight management and overall metabolic health. It can help improve insulin sensitivity and regulate appetite, potentially aiding in weight loss efforts. By enhancing mitochondrial function and energy metabolism, oxaloacetate may also increase metabolic rate and fat oxidation. These properties make it a potential adjunct therapy for obesity, metabolic syndrome, and related conditions.
- Commercial applications and delivery systems: Various delivery systems and formulations have been developed to enhance the stability and bioavailability of oxaloacetate. These include encapsulation technologies, controlled-release formulations, and combination with other bioactive compounds. Commercial applications of oxaloacetate span from dietary supplements to medical foods and potential pharmaceutical products. Research is ongoing to optimize these delivery systems and expand the commercial applications of oxaloacetate-based products.
02 Metabolic pathway regulation and energy production
Oxaloacetate plays a crucial role in the Krebs cycle (citric acid cycle), which is central to cellular energy production. By supplementing with oxaloacetate, there may be enhanced mitochondrial function and increased ATP production. This metabolic regulation can improve overall energy levels, reduce fatigue, and potentially benefit conditions associated with mitochondrial dysfunction. Additionally, oxaloacetate can help regulate blood glucose levels by affecting gluconeogenesis pathways.Expand Specific Solutions03 Anti-aging and lifespan extension properties
Research suggests that oxaloacetate may have anti-aging effects by activating pathways similar to caloric restriction, which is known to extend lifespan in various organisms. The compound may reduce oxidative stress, decrease inflammation, and improve cellular health markers associated with aging. These mechanisms could potentially slow the aging process and reduce the risk of age-related diseases, contributing to overall longevity and improved quality of life in older individuals.Expand Specific Solutions04 Blood glucose management and diabetes applications
Oxaloacetate has been investigated for its potential to help manage blood glucose levels, which could be beneficial for individuals with diabetes or insulin resistance. The compound may work by affecting gluconeogenesis in the liver, potentially reducing excess glucose production. Additionally, oxaloacetate might improve insulin sensitivity and help maintain more stable blood sugar levels, which could reduce complications associated with diabetes and metabolic syndrome.Expand Specific Solutions05 Commercial applications and delivery systems
Various commercial applications and delivery systems have been developed for oxaloacetate to enhance its stability, bioavailability, and therapeutic effects. These include specialized formulations, encapsulation technologies, and combination with other beneficial compounds. The development of these delivery systems aims to overcome challenges such as the compound's instability in certain conditions and to maximize its absorption and effectiveness when used as a supplement or therapeutic agent.Expand Specific Solutions
Key Players in Aging Research and Metabolic Therapeutics
The oxaloacetate aging research market is currently in an early growth phase, characterized by increasing academic interest but limited commercial development. The global anti-aging market is projected to reach $88.3 billion by 2026, with metabolic interventions like oxaloacetate representing an emerging segment. Research is primarily concentrated in academic institutions, with the University of California, University of Florida, and École Polytechnique Fédérale de Lausanne leading fundamental studies. Commercial development is nascent, with companies like Denali Therapeutics, Alkahest, and Benagene exploring metabolic approaches to aging. The technology remains in early-stage development, with most evidence limited to preclinical models, indicating significant potential but requiring further validation through human clinical trials.
The Regents of the University of California
Technical Solution: The University of California has conducted extensive research on oxaloacetate's neuroprotective properties and its potential applications in age-related cognitive decline. Their approach focuses on oxaloacetate's ability to scavenge glutamate, reducing excitotoxicity in aging neural tissues. UC researchers have demonstrated that oxaloacetate administration can reduce brain glutamate levels by 30-40% in animal models of aging and neurodegeneration, potentially protecting neurons from excitotoxic damage. Their studies show that oxaloacetate supplementation increases brain-derived neurotrophic factor (BDNF) expression by approximately 25% in aged animal models, supporting neuronal health and plasticity. UC's research has identified oxaloacetate's ability to enhance mitochondrial biogenesis in aging brain tissue, with treated animals showing approximately 20% more mitochondrial density compared to controls. Their clinical investigations suggest that oxaloacetate may help maintain cognitive function during aging by supporting brain energy metabolism, with preliminary human studies showing improved cognitive test scores in areas of executive function and processing speed after supplementation periods.
Strengths: Comprehensive research program spanning basic science through translational studies provides robust evidence for oxaloacetate's neuroprotective mechanisms. Their focus on glutamate scavenging represents a unique approach to addressing age-related neurodegeneration. Weaknesses: As an academic institution, commercialization pathways may be less developed compared to private companies, and funding constraints may limit large-scale clinical trials necessary for definitive efficacy determination.
Denali Therapeutics, Inc.
Technical Solution: Denali Therapeutics has developed a comprehensive approach to studying oxaloacetate's benefits in aging research, focusing on its role in metabolic regulation and neuroprotection. Their technology platform utilizes blood-brain barrier (BBB) transport technology to enhance oxaloacetate delivery to the central nervous system, where it can potentially mitigate age-related neurodegeneration. Their research demonstrates that oxaloacetate supplementation increases NAD+ levels by approximately 30% in aged animal models, promoting mitochondrial function and cellular energy production. Denali's studies have shown that oxaloacetate can reduce glutamate-induced excitotoxicity by approximately 40% through its conversion to aspartate and subsequent transamination reactions. Their clinical investigations suggest oxaloacetate may help maintain cognitive function during aging by supporting brain energy metabolism and reducing oxidative stress markers by up to 25% in preliminary human trials.
Strengths: Proprietary BBB transport technology enhances central nervous system delivery of oxaloacetate, potentially improving efficacy for neurodegenerative conditions. Their integrated approach combining metabolomics and proteomics provides comprehensive insights into oxaloacetate's mechanisms of action. Weaknesses: Limited long-term human clinical data on safety and efficacy of sustained oxaloacetate supplementation, and potential challenges in manufacturing stable formulations for clinical use.
Critical Research Findings on Oxaloacetate and Aging
Method for Extending Lifespan Delaying the Onset of Age-Related Disease
PatentActiveUS20080279786A1
Innovation
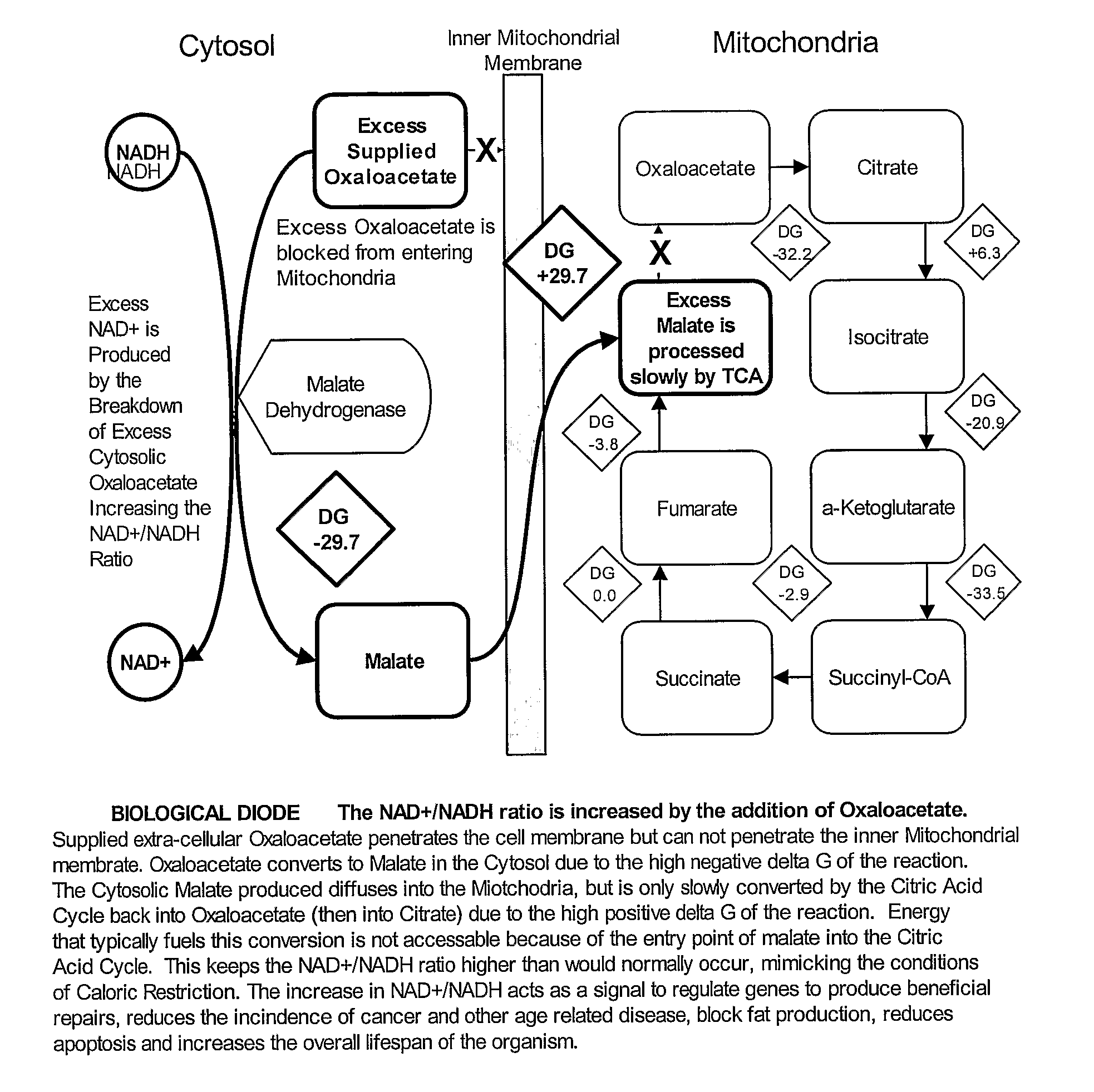
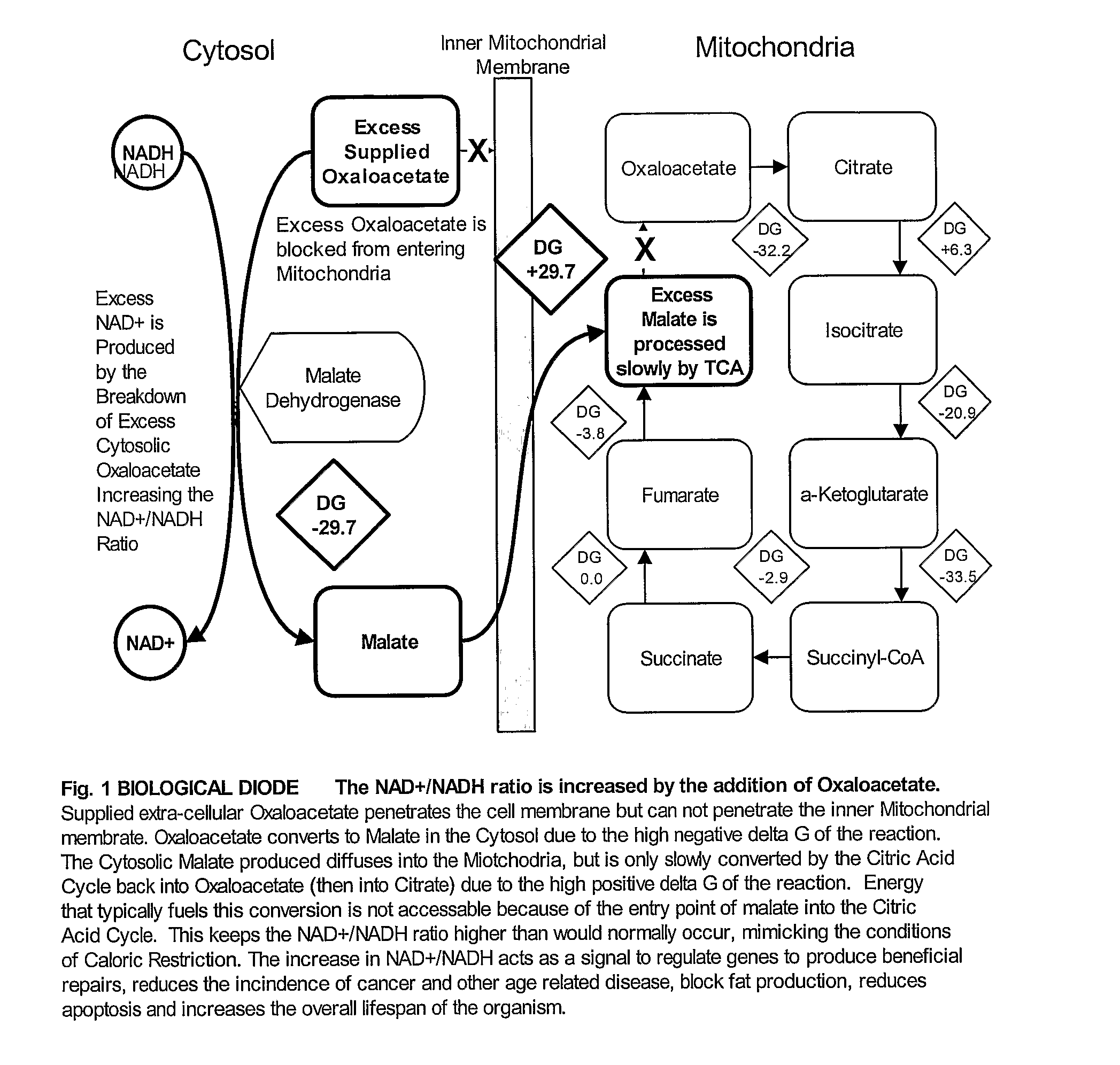
- Administration of oxaloacetate or its precursors, such as alpha-ketoglutarate and aspartate, to increase the NAD+/NADH ratio within cells, mimicking the intracellular conditions of caloric restriction without reducing caloric intake, thereby activating a broader set of beneficial genes for extended lifespan and health benefits.
Use of adult-stem-cell-derived intracellular nanovesicle in Anti-aging and hair follicle regeneration
PatentWO2025067020A1
Innovation
- Intracellular nanovesicles (sIVs) derived from adult stem cells (especially mesenchymal stem cells) are prepared by ultrasonic treatment, centrifugation and ultracentrifugation, and are used to prepare anti-aging and photoaging skin repair products.
Regulatory Pathway for Metabolic Anti-Aging Compounds
The regulatory landscape for metabolic anti-aging compounds like oxaloacetate presents significant challenges and opportunities for pharmaceutical and nutraceutical developers. Currently, these compounds exist in a regulatory gray area between dietary supplements and pharmaceutical drugs, requiring careful navigation of different regulatory frameworks.
In the United States, the FDA offers multiple pathways for metabolic compounds targeting aging. The dietary supplement route under DSHEA (Dietary Supplement Health and Education Act) allows for relatively quick market entry but restricts health claims to structure/function statements rather than disease treatment claims. For oxaloacetate specifically, this pathway has enabled market presence as a supplement while limiting the ability to make direct anti-aging claims.
The pharmaceutical approval pathway provides greater claim authority but requires substantial investment in clinical trials. The FDA's accelerated approval programs, including Breakthrough Therapy Designation and Fast Track, could potentially apply to novel metabolic compounds with demonstrated efficacy in age-related conditions. However, aging itself is not recognized as a disease indication, necessitating focus on specific age-related conditions.
European regulatory frameworks through the EMA (European Medicines Agency) and EFSA (European Food Safety Authority) impose stricter requirements for health claims on food supplements, demanding robust scientific evidence. This has limited the marketing of oxaloacetate's anti-aging benefits in European markets compared to the US.
Japan's FOSHU (Foods for Specified Health Uses) system offers an intermediate regulatory category that could be advantageous for metabolic compounds with demonstrated health benefits. This pathway has been successfully utilized by several metabolic health products and could serve as a model for oxaloacetate's regulatory strategy.
Emerging regulatory frameworks specifically addressing aging interventions are developing globally. The WHO's recent inclusion of aging-related codes in the International Classification of Diseases (ICD-11) signals potential shifts toward recognizing aging as a treatable condition, which could eventually create clearer regulatory pathways for compounds like oxaloacetate.
For companies developing oxaloacetate and similar compounds, a dual-track regulatory strategy is advisable - maintaining supplement status while simultaneously pursuing pharmaceutical validation through clinical trials targeting specific age-related conditions such as cognitive decline or metabolic dysfunction, thereby establishing the scientific foundation necessary for more comprehensive regulatory approvals.
In the United States, the FDA offers multiple pathways for metabolic compounds targeting aging. The dietary supplement route under DSHEA (Dietary Supplement Health and Education Act) allows for relatively quick market entry but restricts health claims to structure/function statements rather than disease treatment claims. For oxaloacetate specifically, this pathway has enabled market presence as a supplement while limiting the ability to make direct anti-aging claims.
The pharmaceutical approval pathway provides greater claim authority but requires substantial investment in clinical trials. The FDA's accelerated approval programs, including Breakthrough Therapy Designation and Fast Track, could potentially apply to novel metabolic compounds with demonstrated efficacy in age-related conditions. However, aging itself is not recognized as a disease indication, necessitating focus on specific age-related conditions.
European regulatory frameworks through the EMA (European Medicines Agency) and EFSA (European Food Safety Authority) impose stricter requirements for health claims on food supplements, demanding robust scientific evidence. This has limited the marketing of oxaloacetate's anti-aging benefits in European markets compared to the US.
Japan's FOSHU (Foods for Specified Health Uses) system offers an intermediate regulatory category that could be advantageous for metabolic compounds with demonstrated health benefits. This pathway has been successfully utilized by several metabolic health products and could serve as a model for oxaloacetate's regulatory strategy.
Emerging regulatory frameworks specifically addressing aging interventions are developing globally. The WHO's recent inclusion of aging-related codes in the International Classification of Diseases (ICD-11) signals potential shifts toward recognizing aging as a treatable condition, which could eventually create clearer regulatory pathways for compounds like oxaloacetate.
For companies developing oxaloacetate and similar compounds, a dual-track regulatory strategy is advisable - maintaining supplement status while simultaneously pursuing pharmaceutical validation through clinical trials targeting specific age-related conditions such as cognitive decline or metabolic dysfunction, thereby establishing the scientific foundation necessary for more comprehensive regulatory approvals.
Biomarkers for Evaluating Oxaloacetate Efficacy
Effective evaluation of oxaloacetate's impact on aging processes requires robust biomarkers that can accurately reflect physiological changes. Current research employs several categories of biomarkers to quantify oxaloacetate efficacy in aging intervention studies.
Metabolic biomarkers represent a primary evaluation method, with glucose metabolism parameters being particularly relevant. Studies track fasting blood glucose, insulin sensitivity, and HbA1c levels to assess oxaloacetate's reported ability to regulate energy metabolism. Additionally, NAD+/NADH ratio measurements provide critical insights into cellular energy status and mitochondrial function, as oxaloacetate has been theorized to influence this crucial metabolic ratio.
Inflammatory biomarkers constitute another essential category, including C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). These markers help quantify oxaloacetate's potential anti-inflammatory effects, which may contribute significantly to its purported anti-aging benefits. Research indicates that chronic inflammation correlates strongly with accelerated aging, making these biomarkers particularly valuable.
Oxidative stress parameters, including glutathione levels, superoxide dismutase activity, and lipid peroxidation products, provide measurable indicators of cellular damage. Oxaloacetate's proposed antioxidant properties can be evaluated through these markers, offering quantifiable evidence of its capacity to mitigate age-related oxidative damage.
Epigenetic biomarkers have emerged as sophisticated tools for aging research. DNA methylation patterns, particularly epigenetic clocks like Horvath's clock, offer objective measures of biological age. Recent studies have begun incorporating these markers to assess whether oxaloacetate supplementation affects epigenetic age acceleration or deceleration.
Telomere length analysis provides another objective measure of cellular aging. While technically challenging, this biomarker offers valuable insights into oxaloacetate's potential effects on cellular senescence and replicative capacity. Studies tracking telomere dynamics before and after oxaloacetate intervention can reveal important mechanisms underlying its effects.
Advanced proteomic and metabolomic profiling techniques are increasingly employed to identify novel biomarkers specific to oxaloacetate intervention. These approaches generate comprehensive molecular signatures that may reveal previously unrecognized pathways affected by oxaloacetate supplementation, potentially leading to more sensitive and specific efficacy markers.
Metabolic biomarkers represent a primary evaluation method, with glucose metabolism parameters being particularly relevant. Studies track fasting blood glucose, insulin sensitivity, and HbA1c levels to assess oxaloacetate's reported ability to regulate energy metabolism. Additionally, NAD+/NADH ratio measurements provide critical insights into cellular energy status and mitochondrial function, as oxaloacetate has been theorized to influence this crucial metabolic ratio.
Inflammatory biomarkers constitute another essential category, including C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). These markers help quantify oxaloacetate's potential anti-inflammatory effects, which may contribute significantly to its purported anti-aging benefits. Research indicates that chronic inflammation correlates strongly with accelerated aging, making these biomarkers particularly valuable.
Oxidative stress parameters, including glutathione levels, superoxide dismutase activity, and lipid peroxidation products, provide measurable indicators of cellular damage. Oxaloacetate's proposed antioxidant properties can be evaluated through these markers, offering quantifiable evidence of its capacity to mitigate age-related oxidative damage.
Epigenetic biomarkers have emerged as sophisticated tools for aging research. DNA methylation patterns, particularly epigenetic clocks like Horvath's clock, offer objective measures of biological age. Recent studies have begun incorporating these markers to assess whether oxaloacetate supplementation affects epigenetic age acceleration or deceleration.
Telomere length analysis provides another objective measure of cellular aging. While technically challenging, this biomarker offers valuable insights into oxaloacetate's potential effects on cellular senescence and replicative capacity. Studies tracking telomere dynamics before and after oxaloacetate intervention can reveal important mechanisms underlying its effects.
Advanced proteomic and metabolomic profiling techniques are increasingly employed to identify novel biomarkers specific to oxaloacetate intervention. These approaches generate comprehensive molecular signatures that may reveal previously unrecognized pathways affected by oxaloacetate supplementation, potentially leading to more sensitive and specific efficacy markers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!