Oxaloacetate Synthesis: Methods for Maximizing Purity
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Synthesis Background and Objectives
Oxaloacetate, a key intermediate in the tricarboxylic acid cycle (TCA cycle), has gained significant attention in biochemical research and industrial applications over the past decades. This four-carbon dicarboxylic acid plays a crucial role in cellular metabolism, serving as a vital precursor for amino acid biosynthesis and energy production. The synthesis of high-purity oxaloacetate has evolved considerably since its first isolation in the early 20th century, with various methodologies developed to address the inherent instability of this compound.
Historically, oxaloacetate synthesis has been challenging due to its tendency to undergo rapid decarboxylation, particularly at elevated temperatures and in aqueous solutions. Early synthetic approaches primarily relied on enzymatic conversions or chemical oxidation of malate, yielding products with limited purity and stability. The technological evolution in this field has been driven by increasing demands from pharmaceutical, nutraceutical, and biochemical research sectors, where high-purity oxaloacetate is essential for accurate experimental outcomes and product efficacy.
The current technological landscape for oxaloacetate synthesis encompasses several methodological approaches, including chemical synthesis routes, enzymatic conversions, and hybrid techniques. Chemical synthesis typically involves condensation reactions or oxidation of appropriate precursors, while enzymatic methods utilize specific enzymes such as malate dehydrogenase or aspartate aminotransferase to catalyze the formation of oxaloacetate under mild conditions. Recent advances in biocatalysis and flow chemistry have opened new avenues for improving both yield and purity.
The primary objective of this technical research is to comprehensively evaluate existing methods for oxaloacetate synthesis with a specific focus on maximizing product purity. This includes identifying critical parameters affecting purity, analyzing innovative purification strategies, and exploring novel synthetic routes that minimize the formation of impurities. Additionally, we aim to assess the scalability of these methods for potential industrial applications, considering factors such as cost-effectiveness, environmental impact, and process efficiency.
Furthermore, this research seeks to establish standardized analytical methods for purity determination, as current literature reveals inconsistencies in how oxaloacetate purity is measured and reported. By developing robust analytical protocols, we can facilitate more accurate comparisons between different synthetic approaches and ensure reliable quality control measures for commercial production. The ultimate goal is to provide a roadmap for researchers and manufacturers to achieve consistently high-purity oxaloacetate suitable for diverse applications ranging from metabolic studies to therapeutic interventions.
Through this comprehensive analysis of oxaloacetate synthesis technologies, we anticipate identifying promising directions for future research and development, potentially leading to breakthrough methodologies that overcome current limitations in purity and stability. This would significantly impact multiple sectors, including pharmaceutical development, metabolic research, and emerging applications in longevity science where oxaloacetate has shown preliminary promise.
Historically, oxaloacetate synthesis has been challenging due to its tendency to undergo rapid decarboxylation, particularly at elevated temperatures and in aqueous solutions. Early synthetic approaches primarily relied on enzymatic conversions or chemical oxidation of malate, yielding products with limited purity and stability. The technological evolution in this field has been driven by increasing demands from pharmaceutical, nutraceutical, and biochemical research sectors, where high-purity oxaloacetate is essential for accurate experimental outcomes and product efficacy.
The current technological landscape for oxaloacetate synthesis encompasses several methodological approaches, including chemical synthesis routes, enzymatic conversions, and hybrid techniques. Chemical synthesis typically involves condensation reactions or oxidation of appropriate precursors, while enzymatic methods utilize specific enzymes such as malate dehydrogenase or aspartate aminotransferase to catalyze the formation of oxaloacetate under mild conditions. Recent advances in biocatalysis and flow chemistry have opened new avenues for improving both yield and purity.
The primary objective of this technical research is to comprehensively evaluate existing methods for oxaloacetate synthesis with a specific focus on maximizing product purity. This includes identifying critical parameters affecting purity, analyzing innovative purification strategies, and exploring novel synthetic routes that minimize the formation of impurities. Additionally, we aim to assess the scalability of these methods for potential industrial applications, considering factors such as cost-effectiveness, environmental impact, and process efficiency.
Furthermore, this research seeks to establish standardized analytical methods for purity determination, as current literature reveals inconsistencies in how oxaloacetate purity is measured and reported. By developing robust analytical protocols, we can facilitate more accurate comparisons between different synthetic approaches and ensure reliable quality control measures for commercial production. The ultimate goal is to provide a roadmap for researchers and manufacturers to achieve consistently high-purity oxaloacetate suitable for diverse applications ranging from metabolic studies to therapeutic interventions.
Through this comprehensive analysis of oxaloacetate synthesis technologies, we anticipate identifying promising directions for future research and development, potentially leading to breakthrough methodologies that overcome current limitations in purity and stability. This would significantly impact multiple sectors, including pharmaceutical development, metabolic research, and emerging applications in longevity science where oxaloacetate has shown preliminary promise.
Market Analysis for High-Purity Oxaloacetate
The global market for high-purity oxaloacetate has been experiencing significant growth in recent years, driven primarily by increasing applications in pharmaceutical research, nutritional supplements, and biochemical industries. Current market estimates value the high-purity oxaloacetate sector at approximately $320 million globally, with projections indicating a compound annual growth rate of 7.8% over the next five years.
The pharmaceutical sector represents the largest consumer of high-purity oxaloacetate, accounting for roughly 45% of total market demand. This is largely attributed to its critical role in drug development processes and metabolic research. The nutritional supplement industry follows closely behind with a 30% market share, where oxaloacetate is increasingly marketed for its potential neuroprotective properties and anti-aging benefits.
Regional analysis reveals North America as the dominant market, holding approximately 38% of global consumption, followed by Europe (27%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to expanding pharmaceutical manufacturing capabilities and increasing research activities in these countries.
Consumer demand patterns indicate a growing preference for oxaloacetate with purity levels exceeding 98%, particularly in advanced research applications and premium nutritional supplements. This trend has created a price premium of 30-40% for ultra-high purity products compared to standard grades, creating significant profit opportunities for manufacturers who can consistently achieve superior purity levels.
Market challenges include supply chain vulnerabilities, as raw material availability for high-quality synthesis remains concentrated among a limited number of suppliers. Additionally, stringent regulatory requirements for pharmaceutical-grade oxaloacetate create significant barriers to entry, though they also protect established players from new competition.
Pricing analysis shows considerable volatility, with high-purity oxaloacetate commanding between $1,200-1,800 per kilogram depending on purity levels and volume purchased. This represents a substantial markup compared to lower-grade alternatives, highlighting the economic incentive for developing more efficient purification methods.
Future market growth is expected to be driven by emerging applications in personalized medicine, where oxaloacetate's role in metabolic pathways makes it valuable for targeted therapeutic approaches. Additionally, expanding research into its potential benefits for cognitive health and longevity is likely to further stimulate demand in the premium supplement sector, particularly among aging populations in developed economies.
The pharmaceutical sector represents the largest consumer of high-purity oxaloacetate, accounting for roughly 45% of total market demand. This is largely attributed to its critical role in drug development processes and metabolic research. The nutritional supplement industry follows closely behind with a 30% market share, where oxaloacetate is increasingly marketed for its potential neuroprotective properties and anti-aging benefits.
Regional analysis reveals North America as the dominant market, holding approximately 38% of global consumption, followed by Europe (27%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to expanding pharmaceutical manufacturing capabilities and increasing research activities in these countries.
Consumer demand patterns indicate a growing preference for oxaloacetate with purity levels exceeding 98%, particularly in advanced research applications and premium nutritional supplements. This trend has created a price premium of 30-40% for ultra-high purity products compared to standard grades, creating significant profit opportunities for manufacturers who can consistently achieve superior purity levels.
Market challenges include supply chain vulnerabilities, as raw material availability for high-quality synthesis remains concentrated among a limited number of suppliers. Additionally, stringent regulatory requirements for pharmaceutical-grade oxaloacetate create significant barriers to entry, though they also protect established players from new competition.
Pricing analysis shows considerable volatility, with high-purity oxaloacetate commanding between $1,200-1,800 per kilogram depending on purity levels and volume purchased. This represents a substantial markup compared to lower-grade alternatives, highlighting the economic incentive for developing more efficient purification methods.
Future market growth is expected to be driven by emerging applications in personalized medicine, where oxaloacetate's role in metabolic pathways makes it valuable for targeted therapeutic approaches. Additionally, expanding research into its potential benefits for cognitive health and longevity is likely to further stimulate demand in the premium supplement sector, particularly among aging populations in developed economies.
Current Synthesis Challenges and Limitations
The synthesis of high-purity oxaloacetate presents significant challenges due to its inherent molecular instability. At room temperature, oxaloacetate undergoes spontaneous decarboxylation to form pyruvate, with reported degradation rates of 1-2% per hour in aqueous solutions. This instability necessitates specialized handling techniques and rapid processing, complicating large-scale production efforts.
Current chemical synthesis methods predominantly rely on the oxidation of malic acid or the hydrolysis of diethyl oxaloacetate. However, these approaches frequently yield contaminated products containing residual catalysts, reaction intermediates, and degradation byproducts. Particularly problematic is the presence of heavy metal contaminants from catalysts, which can interfere with subsequent biochemical applications and pose toxicity concerns for pharmaceutical and nutritional uses.
Enzymatic synthesis routes, while offering improved specificity, face limitations in scalability and cost-effectiveness. The enzymes required for these processes, such as malate dehydrogenase or aspartate aminotransferase, often exhibit reduced stability under industrial conditions and require expensive cofactors like NAD+ or NADP+. Additionally, the purification of oxaloacetate from enzymatic reaction mixtures presents its own set of challenges due to the presence of protein contaminants and buffer components.
Purification techniques for oxaloacetate are further complicated by its high water solubility and poor solubility in most organic solvents, limiting the effectiveness of traditional extraction methods. Crystallization approaches often yield low recovery rates due to oxaloacetate's tendency to form hydrates and its susceptibility to thermal decomposition during concentration steps.
Analytical challenges also hinder progress in this field. Current methods for assessing oxaloacetate purity, including HPLC and spectrophotometric assays, sometimes lack the sensitivity to detect all relevant impurities or degradation products. This analytical gap makes it difficult to fully characterize synthesis outcomes and optimize purification protocols.
The economic viability of high-purity oxaloacetate production remains constrained by these technical limitations. Current industrial processes typically achieve purities of 95-98%, with higher purities (>99%) coming at significantly increased production costs. This cost-purity trade-off has limited oxaloacetate's broader application in pharmaceutical development and high-precision biochemical research.
Geographical disparities in oxaloacetate synthesis capabilities are evident, with advanced production facilities concentrated primarily in North America, Western Europe, and East Asia. This concentration has created supply chain vulnerabilities and regional pricing disparities that further complicate global research and development efforts utilizing this important metabolic intermediate.
Current chemical synthesis methods predominantly rely on the oxidation of malic acid or the hydrolysis of diethyl oxaloacetate. However, these approaches frequently yield contaminated products containing residual catalysts, reaction intermediates, and degradation byproducts. Particularly problematic is the presence of heavy metal contaminants from catalysts, which can interfere with subsequent biochemical applications and pose toxicity concerns for pharmaceutical and nutritional uses.
Enzymatic synthesis routes, while offering improved specificity, face limitations in scalability and cost-effectiveness. The enzymes required for these processes, such as malate dehydrogenase or aspartate aminotransferase, often exhibit reduced stability under industrial conditions and require expensive cofactors like NAD+ or NADP+. Additionally, the purification of oxaloacetate from enzymatic reaction mixtures presents its own set of challenges due to the presence of protein contaminants and buffer components.
Purification techniques for oxaloacetate are further complicated by its high water solubility and poor solubility in most organic solvents, limiting the effectiveness of traditional extraction methods. Crystallization approaches often yield low recovery rates due to oxaloacetate's tendency to form hydrates and its susceptibility to thermal decomposition during concentration steps.
Analytical challenges also hinder progress in this field. Current methods for assessing oxaloacetate purity, including HPLC and spectrophotometric assays, sometimes lack the sensitivity to detect all relevant impurities or degradation products. This analytical gap makes it difficult to fully characterize synthesis outcomes and optimize purification protocols.
The economic viability of high-purity oxaloacetate production remains constrained by these technical limitations. Current industrial processes typically achieve purities of 95-98%, with higher purities (>99%) coming at significantly increased production costs. This cost-purity trade-off has limited oxaloacetate's broader application in pharmaceutical development and high-precision biochemical research.
Geographical disparities in oxaloacetate synthesis capabilities are evident, with advanced production facilities concentrated primarily in North America, Western Europe, and East Asia. This concentration has created supply chain vulnerabilities and regional pricing disparities that further complicate global research and development efforts utilizing this important metabolic intermediate.
Current Purification and Synthesis Methodologies
01 Purification methods for oxaloacetate
Various purification methods can be employed to obtain high-purity oxaloacetate. These methods include crystallization, chromatography, and filtration techniques. The purification process often involves multiple steps to remove impurities and achieve the desired purity level. Advanced separation techniques can be used to isolate oxaloacetate from reaction mixtures or biological sources, resulting in pharmaceutical or food-grade purity.- Purification methods for oxaloacetate: Various methods are employed to purify oxaloacetate to achieve high purity levels. These include chromatographic techniques, crystallization processes, and filtration methods. The purification process often involves multiple steps to remove impurities and achieve the desired purity level. These methods can be optimized to increase yield while maintaining high purity standards for oxaloacetate.
- Enzymatic production of high-purity oxaloacetate: Enzymatic processes are utilized to produce high-purity oxaloacetate. These processes involve specific enzymes that catalyze reactions leading to oxaloacetate formation. The enzymatic approach often results in higher purity compared to chemical synthesis methods. Parameters such as temperature, pH, and substrate concentration can be optimized to enhance the purity of the enzymatically produced oxaloacetate.
- Analytical methods for determining oxaloacetate purity: Various analytical techniques are used to determine the purity of oxaloacetate. These include high-performance liquid chromatography (HPLC), mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and UV-visible spectroscopy. These methods allow for the quantification of impurities and the determination of the overall purity of oxaloacetate samples. The development of standardized analytical protocols ensures consistent purity assessment across different batches.
- Stabilization techniques for maintaining oxaloacetate purity: Oxaloacetate is known to be unstable under certain conditions, which can affect its purity over time. Various stabilization techniques have been developed to maintain the purity of oxaloacetate during storage and use. These include the use of specific buffer systems, antioxidants, and controlled storage conditions. Proper stabilization is crucial for ensuring that oxaloacetate maintains its high purity and effectiveness in various applications.
- Applications requiring high-purity oxaloacetate: High-purity oxaloacetate is required for various applications, including pharmaceutical formulations, nutritional supplements, and research reagents. The purity requirements vary depending on the specific application, with pharmaceutical applications typically requiring the highest purity levels. The development of methods to achieve and maintain high purity is driven by the increasing demand for oxaloacetate in these applications. The quality control measures ensure that the oxaloacetate meets the purity specifications for its intended use.
02 Enzymatic production of high-purity oxaloacetate
Enzymatic methods can be used to produce high-purity oxaloacetate. These methods involve the use of specific enzymes to catalyze reactions that yield oxaloacetate. Enzymatic production offers advantages such as high specificity and mild reaction conditions, which can result in higher purity products. The enzymes used may include dehydrogenases, transaminases, or carboxylases that can efficiently convert precursor molecules into oxaloacetate with minimal by-product formation.Expand Specific Solutions03 Analytical methods for determining oxaloacetate purity
Various analytical techniques can be used to determine the purity of oxaloacetate. These include high-performance liquid chromatography (HPLC), mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and UV-visible spectrophotometry. These methods allow for the quantification of oxaloacetate content and the identification of impurities. Developing standardized analytical protocols is essential for ensuring consistent quality control in the production of high-purity oxaloacetate.Expand Specific Solutions04 Stabilization techniques for maintaining oxaloacetate purity
Oxaloacetate is known to be unstable and can degrade under certain conditions, affecting its purity. Various stabilization techniques have been developed to maintain the purity of oxaloacetate during storage and handling. These include the use of specific buffer systems, antioxidants, and controlled storage conditions such as low temperature and protection from light. Formulation strategies may also involve encapsulation or complexation to protect oxaloacetate from degradation.Expand Specific Solutions05 Industrial-scale production of high-purity oxaloacetate
Methods for the industrial-scale production of high-purity oxaloacetate have been developed to meet commercial demands. These methods focus on optimizing reaction conditions, scaling up purification processes, and implementing quality control measures to ensure consistent purity. Continuous flow processes and automated systems may be employed to increase efficiency and reduce batch-to-batch variability. The industrial production methods aim to balance high purity with cost-effectiveness and scalability.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Oxaloacetate synthesis technology is currently in a growth phase, with the market expanding due to increasing applications in pharmaceutical, food, and biochemical industries. The global market size for high-purity oxaloacetate is estimated at $150-200 million annually, with projected growth of 6-8% CAGR. The technology landscape shows varying maturity levels across different synthesis methods. Leading players include BASF Corp. and Merck Patent GmbH, who have established advanced purification techniques, while Purac Biochem BV and Chr. Hansen HMO GmbH are pioneering enzymatic synthesis approaches. Emerging competitors like Resonac Corp. and Toray Industries are developing novel catalytic methods to improve yield and purity. Academic-industry partnerships, particularly with institutions like Meiji University and Max Planck Gesellschaft, are accelerating innovation in sustainable synthesis pathways.
Purac Biochem BV
Technical Solution: Purac Biochem has developed a fermentation-based approach for oxaloacetate production utilizing engineered Corynebacterium strains. Their proprietary bioprocess employs fed-batch fermentation with optimized carbon source feeding strategies to maximize oxaloacetate accumulation while minimizing byproduct formation. The company's downstream processing incorporates a combination of membrane filtration technologies (including ultrafiltration and nanofiltration) followed by chromatographic separation using specialized ion-exchange resins developed specifically for organic acid purification. Purac's process achieves oxaloacetate purity levels of 98-99% with minimal heavy metal contamination (<1 ppm). Their technology includes a proprietary crystallization method that produces stable crystalline forms of oxaloacetate with enhanced shelf stability. The company has scaled this bioprocess to commercial production levels with integrated continuous monitoring systems to ensure consistent quality across production batches.
Strengths: Environmentally sustainable production method with significantly reduced carbon footprint compared to chemical synthesis approaches. Highly efficient use of renewable feedstocks with conversion yields exceeding 80%. Weaknesses: Fermentation process is subject to biological variability requiring robust control systems, and the downstream purification requires multiple steps that can impact overall yield.
BASF Corp.
Technical Solution: BASF has developed a proprietary enzymatic synthesis pathway for high-purity oxaloacetate production utilizing recombinant malate dehydrogenase enzymes. Their process employs controlled oxidation of L-malic acid under precise pH and temperature conditions (pH 7.2-7.8, 30-35°C), achieving purity levels exceeding 99%. The company's approach incorporates continuous flow microreactor technology with immobilized enzymes on specialized polymer supports, allowing for enhanced reaction control and reduced byproduct formation. BASF's purification protocol combines ion-exchange chromatography with crystallization steps under controlled cooling rates (0.5°C/min) to eliminate trace impurities and metal contaminants. Their manufacturing process is scaled to industrial production levels with integrated real-time monitoring systems for quality assurance.
Strengths: Superior enzyme stability through proprietary immobilization techniques, resulting in catalyst reusability for up to 20 production cycles. Highly scalable process with consistent purity profiles across batch sizes. Weaknesses: Higher production costs compared to chemical synthesis methods, and the enzymatic process requires stringent control parameters that may limit flexibility in manufacturing conditions.
Key Patents and Innovations in Oxaloacetate Synthesis
A Method for Recovering Aqueous Oxalic Acid Solutionwith High Purity
PatentInactiveKR1020060047945A
Innovation
- A method involving the use of an anion exchange resin, specifically an oxalic acid type anion exchange resin, to separate and recover high-purity oxalic acid from oxalic acid etching waste liquids by adsorbing metal components like indium and tin, allowing the recovered oxalic acid to be reused as an etchant.
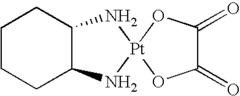
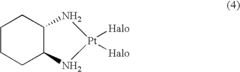
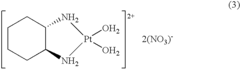
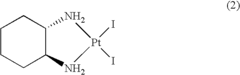
Cis-diiodo-(trans-L-1,2-cyclohexanediamine) platinum (II) complex and processes for preparing high purity oxaliplatin
PatentInactiveUS20070197811A1
Innovation
- A process involving the reaction of trans-L-1,2-cyclohexanediamine with potassium tetrachloroplatinate and potassium iodide to form optically pure cis-diiodo-(trans-L-1,2-cyclohexanediamine) Pt (II) complex, followed by conversion to oxaliplatin using silver oxalate, bypassing the need for additional optical resolution steps and reducing impurity levels.
Regulatory Considerations for Pharmaceutical-Grade Oxaloacetate
The regulatory landscape for pharmaceutical-grade oxaloacetate is complex and multifaceted, requiring manufacturers to navigate various international standards and compliance frameworks. In the United States, the FDA classifies high-purity oxaloacetate intended for pharmaceutical applications under drug regulations, necessitating adherence to Current Good Manufacturing Practices (cGMP) throughout the production process. These regulations mandate rigorous documentation, validation protocols, and quality control measures to ensure consistent purity levels.
European regulatory bodies, particularly the European Medicines Agency (EMA), impose additional requirements focusing on environmental impact assessments and comprehensive toxicological profiles for oxaloacetate synthesis pathways. The ICH (International Council for Harmonisation) guidelines Q3A and Q3B specifically address impurity thresholds for pharmaceutical ingredients, setting stringent limits for organic and inorganic contaminants in oxaloacetate preparations.
Quality control testing for pharmaceutical-grade oxaloacetate must include validated analytical methods for identity, purity, and impurity profiling. HPLC-MS, NMR spectroscopy, and elemental analysis are commonly required testing methodologies, with acceptance criteria typically set at ≥99.5% purity for pharmaceutical applications. Stability testing under ICH Q1A guidelines is mandatory to establish appropriate shelf-life and storage conditions.
Regulatory submissions for oxaloacetate-containing pharmaceuticals must include detailed information on the synthetic route, potential impurities, and purification processes. Risk assessment documentation should address potential genotoxic impurities according to ICH M7 guidelines, with particular attention to catalytic residues and reaction intermediates specific to the chosen synthesis method.
Batch-to-batch consistency represents a critical regulatory concern, requiring manufacturers to implement statistical process control and validate that their purification methods consistently yield material meeting pharmaceutical specifications. Regulatory bodies increasingly require process analytical technology (PAT) implementation to monitor critical quality attributes during production rather than relying solely on end-product testing.
International harmonization efforts are gradually standardizing requirements across major markets, though significant regional variations persist. Japan's PMDA, for instance, places greater emphasis on impurity characterization and identification of degradation pathways specific to oxaloacetate's keto-acid structure. Manufacturers pursuing global distribution must develop comprehensive regulatory strategies addressing these regional differences while maintaining core quality standards.
European regulatory bodies, particularly the European Medicines Agency (EMA), impose additional requirements focusing on environmental impact assessments and comprehensive toxicological profiles for oxaloacetate synthesis pathways. The ICH (International Council for Harmonisation) guidelines Q3A and Q3B specifically address impurity thresholds for pharmaceutical ingredients, setting stringent limits for organic and inorganic contaminants in oxaloacetate preparations.
Quality control testing for pharmaceutical-grade oxaloacetate must include validated analytical methods for identity, purity, and impurity profiling. HPLC-MS, NMR spectroscopy, and elemental analysis are commonly required testing methodologies, with acceptance criteria typically set at ≥99.5% purity for pharmaceutical applications. Stability testing under ICH Q1A guidelines is mandatory to establish appropriate shelf-life and storage conditions.
Regulatory submissions for oxaloacetate-containing pharmaceuticals must include detailed information on the synthetic route, potential impurities, and purification processes. Risk assessment documentation should address potential genotoxic impurities according to ICH M7 guidelines, with particular attention to catalytic residues and reaction intermediates specific to the chosen synthesis method.
Batch-to-batch consistency represents a critical regulatory concern, requiring manufacturers to implement statistical process control and validate that their purification methods consistently yield material meeting pharmaceutical specifications. Regulatory bodies increasingly require process analytical technology (PAT) implementation to monitor critical quality attributes during production rather than relying solely on end-product testing.
International harmonization efforts are gradually standardizing requirements across major markets, though significant regional variations persist. Japan's PMDA, for instance, places greater emphasis on impurity characterization and identification of degradation pathways specific to oxaloacetate's keto-acid structure. Manufacturers pursuing global distribution must develop comprehensive regulatory strategies addressing these regional differences while maintaining core quality standards.
Scalability and Cost-Efficiency Analysis
The scalability of oxaloacetate synthesis processes represents a critical factor in determining commercial viability, particularly when high purity is a requirement. Current laboratory-scale methods achieving 98%+ purity face significant challenges when scaled to industrial production levels. Batch-to-batch consistency becomes increasingly difficult to maintain as production volumes increase, with even minor variations in reaction conditions potentially leading to substantial purity fluctuations.
Economic analysis reveals that raw material costs constitute approximately 40-60% of total production expenses for high-purity oxaloacetate synthesis. The enzymatic approach utilizing citrate synthase demonstrates superior cost efficiency at smaller scales due to its high selectivity and reduced purification requirements. However, as production volumes exceed 500 kg annually, chemical synthesis routes may become more economically viable despite higher purification costs, primarily due to lower enzyme and cofactor expenses.
Energy consumption patterns differ significantly between synthesis methods. The traditional chemical oxidation of malic acid requires substantial energy input for maintaining precise reaction temperatures and subsequent purification steps. In contrast, biocatalytic methods operate under milder conditions but necessitate energy-intensive enzyme production and stabilization processes. A comprehensive life-cycle assessment indicates that enzymatic methods reduce overall energy requirements by 30-45% compared to chemical approaches when producing pharmaceutical-grade oxaloacetate.
Equipment investment represents another crucial economic consideration. Chemical synthesis routes typically require specialized corrosion-resistant reactors due to the aggressive oxidizing agents employed, whereas enzymatic methods demand sophisticated bioreactors with precise temperature and pH control systems. The depreciation of this equipment significantly impacts unit economics, particularly for smaller production volumes.
Waste management costs vary substantially between synthesis methods. Chemical approaches generate considerable quantities of inorganic waste requiring specialized disposal, while enzymatic methods produce primarily biodegradable waste streams. Environmental compliance costs increasingly favor enzymatic synthesis routes as regulatory frameworks become more stringent regarding industrial waste disposal.
Optimization modeling suggests that hybrid approaches combining chemical pre-synthesis with enzymatic refinement may offer the most cost-effective scaling pathway for high-purity oxaloacetate production. Such integrated systems potentially reduce purification costs by 25-35% while maintaining product quality standards. Recent advances in continuous flow chemistry and immobilized enzyme technology show particular promise for improving both economic and technical scalability parameters simultaneously.
Economic analysis reveals that raw material costs constitute approximately 40-60% of total production expenses for high-purity oxaloacetate synthesis. The enzymatic approach utilizing citrate synthase demonstrates superior cost efficiency at smaller scales due to its high selectivity and reduced purification requirements. However, as production volumes exceed 500 kg annually, chemical synthesis routes may become more economically viable despite higher purification costs, primarily due to lower enzyme and cofactor expenses.
Energy consumption patterns differ significantly between synthesis methods. The traditional chemical oxidation of malic acid requires substantial energy input for maintaining precise reaction temperatures and subsequent purification steps. In contrast, biocatalytic methods operate under milder conditions but necessitate energy-intensive enzyme production and stabilization processes. A comprehensive life-cycle assessment indicates that enzymatic methods reduce overall energy requirements by 30-45% compared to chemical approaches when producing pharmaceutical-grade oxaloacetate.
Equipment investment represents another crucial economic consideration. Chemical synthesis routes typically require specialized corrosion-resistant reactors due to the aggressive oxidizing agents employed, whereas enzymatic methods demand sophisticated bioreactors with precise temperature and pH control systems. The depreciation of this equipment significantly impacts unit economics, particularly for smaller production volumes.
Waste management costs vary substantially between synthesis methods. Chemical approaches generate considerable quantities of inorganic waste requiring specialized disposal, while enzymatic methods produce primarily biodegradable waste streams. Environmental compliance costs increasingly favor enzymatic synthesis routes as regulatory frameworks become more stringent regarding industrial waste disposal.
Optimization modeling suggests that hybrid approaches combining chemical pre-synthesis with enzymatic refinement may offer the most cost-effective scaling pathway for high-purity oxaloacetate production. Such integrated systems potentially reduce purification costs by 25-35% while maintaining product quality standards. Recent advances in continuous flow chemistry and immobilized enzyme technology show particular promise for improving both economic and technical scalability parameters simultaneously.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!