Measure Oxaloacetate Efficacy in Biochemical Pathways
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Biochemistry Background and Research Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in several fundamental biochemical pathways that sustain cellular energy production and biosynthesis. Historically, research on oxaloacetate dates back to the 1930s when Hans Krebs elucidated the citric acid cycle, where oxaloacetate serves as both the first substrate and final product. This four-carbon molecule occupies a unique position at the intersection of carbohydrate metabolism, amino acid synthesis, and gluconeogenesis, making it a pivotal compound in cellular bioenergetics.
The evolution of oxaloacetate research has progressed from basic metabolic understanding to exploring its therapeutic potential. Recent decades have witnessed growing interest in oxaloacetate's role beyond traditional metabolism, including its potential effects on longevity, neuroprotection, and metabolic health. Particularly noteworthy is the discovery that oxaloacetate can influence NAD+/NADH ratios, potentially impacting cellular energy status and aging processes.
Current technological advancements have significantly enhanced our ability to measure oxaloacetate concentrations and activity in biological systems. Mass spectrometry techniques, including LC-MS/MS, have revolutionized the precision with which we can quantify this metabolite in complex biological matrices. Additionally, isotope-labeling approaches have enabled researchers to track oxaloacetate flux through various metabolic pathways with unprecedented accuracy.
Despite these advances, significant challenges remain in accurately assessing oxaloacetate efficacy in living systems. The compound's inherent instability in aqueous solutions, rapid metabolism, and involvement in multiple interconnected pathways complicate measurement efforts. Furthermore, the transient nature of oxaloacetate in cellular environments necessitates sophisticated analytical approaches to capture its true biological impact.
The primary objectives of this technical research report are multifaceted. First, we aim to comprehensively evaluate current methodologies for measuring oxaloacetate concentrations and activity in biological systems, with particular emphasis on their sensitivity, specificity, and reproducibility. Second, we seek to identify key biochemical pathways where oxaloacetate intervention might yield significant therapeutic benefits, focusing on metabolic disorders, neurodegenerative conditions, and aging-related processes.
Additionally, this research intends to establish standardized protocols for assessing oxaloacetate efficacy across different experimental models, from cell cultures to animal studies and human clinical trials. By developing robust biomarkers and measurement techniques, we aim to create a foundation for reliable evaluation of oxaloacetate-based interventions. Finally, we will explore emerging technologies that might overcome current limitations in oxaloacetate measurement and delivery, potentially opening new avenues for therapeutic applications.
The evolution of oxaloacetate research has progressed from basic metabolic understanding to exploring its therapeutic potential. Recent decades have witnessed growing interest in oxaloacetate's role beyond traditional metabolism, including its potential effects on longevity, neuroprotection, and metabolic health. Particularly noteworthy is the discovery that oxaloacetate can influence NAD+/NADH ratios, potentially impacting cellular energy status and aging processes.
Current technological advancements have significantly enhanced our ability to measure oxaloacetate concentrations and activity in biological systems. Mass spectrometry techniques, including LC-MS/MS, have revolutionized the precision with which we can quantify this metabolite in complex biological matrices. Additionally, isotope-labeling approaches have enabled researchers to track oxaloacetate flux through various metabolic pathways with unprecedented accuracy.
Despite these advances, significant challenges remain in accurately assessing oxaloacetate efficacy in living systems. The compound's inherent instability in aqueous solutions, rapid metabolism, and involvement in multiple interconnected pathways complicate measurement efforts. Furthermore, the transient nature of oxaloacetate in cellular environments necessitates sophisticated analytical approaches to capture its true biological impact.
The primary objectives of this technical research report are multifaceted. First, we aim to comprehensively evaluate current methodologies for measuring oxaloacetate concentrations and activity in biological systems, with particular emphasis on their sensitivity, specificity, and reproducibility. Second, we seek to identify key biochemical pathways where oxaloacetate intervention might yield significant therapeutic benefits, focusing on metabolic disorders, neurodegenerative conditions, and aging-related processes.
Additionally, this research intends to establish standardized protocols for assessing oxaloacetate efficacy across different experimental models, from cell cultures to animal studies and human clinical trials. By developing robust biomarkers and measurement techniques, we aim to create a foundation for reliable evaluation of oxaloacetate-based interventions. Finally, we will explore emerging technologies that might overcome current limitations in oxaloacetate measurement and delivery, potentially opening new avenues for therapeutic applications.
Market Analysis for Oxaloacetate-Based Therapeutics
The global market for oxaloacetate-based therapeutics is experiencing significant growth, driven by increasing research into metabolic disorders, neurodegenerative diseases, and anti-aging applications. Current market estimates value the metabolic health supplement sector at approximately $42 billion globally, with compounds targeting mitochondrial function representing one of the fastest-growing segments at 8.3% CAGR.
Oxaloacetate's unique position as a key intermediate in the Krebs cycle has attracted substantial commercial interest, particularly for its potential applications in managing blood glucose levels, supporting mitochondrial health, and potentially extending lifespan. The North American market currently dominates consumption, accounting for roughly 45% of global sales, followed by Europe at 30% and Asia-Pacific at 18%.
Consumer demand patterns reveal growing interest in scientifically-validated supplements with demonstrable biochemical efficacy. This trend has been particularly pronounced in the 55+ demographic, where concerns about metabolic health and cognitive decline drive purchasing decisions. Market research indicates that consumers are increasingly willing to pay premium prices for supplements with robust clinical evidence, with the average price point for high-quality oxaloacetate supplements ranging from $60-120 per month's supply.
The therapeutic applications market segment shows even greater potential, with pharmaceutical companies investing in oxaloacetate-derived compounds for various indications. Particularly promising are developments in neurodegenerative disease management, where oxaloacetate's potential neuroprotective effects could address a market projected to reach $62 billion by 2030.
Competitive landscape analysis reveals approximately 15 significant players currently offering oxaloacetate supplements, with three pharmaceutical companies conducting advanced clinical trials for proprietary formulations. Market concentration remains relatively low, with the top five companies controlling approximately 57% of market share.
Regulatory considerations present both challenges and opportunities. While oxaloacetate faces relatively favorable regulatory pathways as a naturally occurring compound, demonstrating efficacy through standardized biochemical pathway measurements would significantly enhance market positioning and potentially enable premium pricing strategies.
Future market growth will likely be driven by advances in delivery systems that enhance bioavailability, as current formulations face challenges with stability and absorption. Companies investing in proprietary technologies to measure and demonstrate oxaloacetate efficacy in specific biochemical pathways stand to capture significant market share, particularly if they can establish clear connections between supplementation and measurable health outcomes.
Oxaloacetate's unique position as a key intermediate in the Krebs cycle has attracted substantial commercial interest, particularly for its potential applications in managing blood glucose levels, supporting mitochondrial health, and potentially extending lifespan. The North American market currently dominates consumption, accounting for roughly 45% of global sales, followed by Europe at 30% and Asia-Pacific at 18%.
Consumer demand patterns reveal growing interest in scientifically-validated supplements with demonstrable biochemical efficacy. This trend has been particularly pronounced in the 55+ demographic, where concerns about metabolic health and cognitive decline drive purchasing decisions. Market research indicates that consumers are increasingly willing to pay premium prices for supplements with robust clinical evidence, with the average price point for high-quality oxaloacetate supplements ranging from $60-120 per month's supply.
The therapeutic applications market segment shows even greater potential, with pharmaceutical companies investing in oxaloacetate-derived compounds for various indications. Particularly promising are developments in neurodegenerative disease management, where oxaloacetate's potential neuroprotective effects could address a market projected to reach $62 billion by 2030.
Competitive landscape analysis reveals approximately 15 significant players currently offering oxaloacetate supplements, with three pharmaceutical companies conducting advanced clinical trials for proprietary formulations. Market concentration remains relatively low, with the top five companies controlling approximately 57% of market share.
Regulatory considerations present both challenges and opportunities. While oxaloacetate faces relatively favorable regulatory pathways as a naturally occurring compound, demonstrating efficacy through standardized biochemical pathway measurements would significantly enhance market positioning and potentially enable premium pricing strategies.
Future market growth will likely be driven by advances in delivery systems that enhance bioavailability, as current formulations face challenges with stability and absorption. Companies investing in proprietary technologies to measure and demonstrate oxaloacetate efficacy in specific biochemical pathways stand to capture significant market share, particularly if they can establish clear connections between supplementation and measurable health outcomes.
Current Measurement Techniques and Technical Limitations
The measurement of oxaloacetate efficacy in biochemical pathways currently relies on several established techniques, each with specific advantages and limitations. High-Performance Liquid Chromatography (HPLC) remains one of the most widely used methods, offering good sensitivity and specificity for oxaloacetate detection in complex biological matrices. However, HPLC analysis requires extensive sample preparation, specialized equipment, and trained personnel, making it less suitable for high-throughput screening or point-of-care applications.
Mass spectrometry (MS), particularly when coupled with liquid chromatography (LC-MS), provides superior sensitivity and specificity compared to HPLC alone. This technique can detect oxaloacetate at nanomolar concentrations and distinguish it from structurally similar metabolites. The major limitations include high equipment costs, complex data interpretation, and the requirement for sample derivatization to enhance stability during analysis.
Enzymatic assays represent another approach, utilizing malate dehydrogenase to catalyze the conversion of oxaloacetate to malate while monitoring NADH oxidation spectrophotometrically. While these assays are relatively simple and cost-effective, they suffer from interference by other metabolites and limited sensitivity, particularly in complex biological samples.
Nuclear Magnetic Resonance (NMR) spectroscopy offers the advantage of non-destructive analysis and can provide structural information about oxaloacetate and its interactions within biochemical pathways. However, NMR has relatively poor sensitivity compared to other techniques, requiring higher sample concentrations, and the equipment is expensive and requires specialized expertise.
A significant technical challenge across all measurement techniques is oxaloacetate's inherent instability. With a half-life of approximately 30 minutes at physiological pH and temperature, rapid sample processing or stabilization methods are essential to prevent spontaneous decarboxylation to pyruvate, which can lead to substantial measurement errors.
Microfluidic devices and biosensors represent emerging technologies for oxaloacetate measurement, offering potential advantages in terms of reduced sample volume, faster analysis times, and potential for integration into portable devices. However, these approaches are still in developmental stages and face challenges related to sensitivity, specificity, and reproducibility.
The biological complexity of the samples presents another limitation, as oxaloacetate exists within intricate metabolic networks with rapid turnover rates. This dynamic nature makes it difficult to obtain accurate measurements that reflect true physiological conditions rather than artifacts of sample preparation or analysis delays.
Standardization across laboratories remains problematic, with different measurement techniques yielding varying results, complicating the comparison of data across studies and hindering the establishment of reference ranges for oxaloacetate in different biological contexts.
Mass spectrometry (MS), particularly when coupled with liquid chromatography (LC-MS), provides superior sensitivity and specificity compared to HPLC alone. This technique can detect oxaloacetate at nanomolar concentrations and distinguish it from structurally similar metabolites. The major limitations include high equipment costs, complex data interpretation, and the requirement for sample derivatization to enhance stability during analysis.
Enzymatic assays represent another approach, utilizing malate dehydrogenase to catalyze the conversion of oxaloacetate to malate while monitoring NADH oxidation spectrophotometrically. While these assays are relatively simple and cost-effective, they suffer from interference by other metabolites and limited sensitivity, particularly in complex biological samples.
Nuclear Magnetic Resonance (NMR) spectroscopy offers the advantage of non-destructive analysis and can provide structural information about oxaloacetate and its interactions within biochemical pathways. However, NMR has relatively poor sensitivity compared to other techniques, requiring higher sample concentrations, and the equipment is expensive and requires specialized expertise.
A significant technical challenge across all measurement techniques is oxaloacetate's inherent instability. With a half-life of approximately 30 minutes at physiological pH and temperature, rapid sample processing or stabilization methods are essential to prevent spontaneous decarboxylation to pyruvate, which can lead to substantial measurement errors.
Microfluidic devices and biosensors represent emerging technologies for oxaloacetate measurement, offering potential advantages in terms of reduced sample volume, faster analysis times, and potential for integration into portable devices. However, these approaches are still in developmental stages and face challenges related to sensitivity, specificity, and reproducibility.
The biological complexity of the samples presents another limitation, as oxaloacetate exists within intricate metabolic networks with rapid turnover rates. This dynamic nature makes it difficult to obtain accurate measurements that reflect true physiological conditions rather than artifacts of sample preparation or analysis delays.
Standardization across laboratories remains problematic, with different measurement techniques yielding varying results, complicating the comparison of data across studies and hindering the establishment of reference ranges for oxaloacetate in different biological contexts.
Established Protocols for Oxaloacetate Pathway Analysis
01 Oxaloacetate as a therapeutic agent for neurological conditions
Oxaloacetate has shown efficacy in treating various neurological conditions by protecting neurons from excitotoxic damage. It functions by reducing glutamate levels in the brain, which is particularly beneficial in conditions like stroke, traumatic brain injury, and neurodegenerative diseases. The compound works by enhancing the blood glutamate scavenging effect, thereby decreasing glutamate concentration in the brain and providing neuroprotection.- Oxaloacetate as a therapeutic agent for neurological conditions: Oxaloacetate has shown efficacy in treating various neurological conditions by protecting neurons from glutamate-induced excitotoxicity. It works by scavenging blood glutamate, which reduces glutamate levels in the brain and prevents neuronal damage. This mechanism has proven effective in treating conditions such as traumatic brain injury, stroke, and neurodegenerative diseases. Clinical studies have demonstrated improved outcomes in patients with these conditions when treated with oxaloacetate.
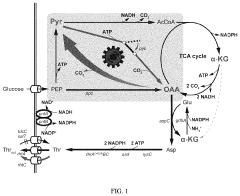
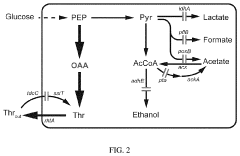
- Oxaloacetate in metabolic pathway regulation and energy production: Oxaloacetate plays a crucial role in cellular metabolism as a key intermediate in the Krebs cycle and gluconeogenesis. Research has shown its efficacy in enhancing energy production by facilitating the conversion of carbohydrates, fats, and proteins into ATP. It also helps regulate blood glucose levels by promoting glucose synthesis in the liver. These metabolic effects make oxaloacetate potentially valuable for treating conditions related to energy metabolism disorders and mitochondrial dysfunction.
- Oxaloacetate for anti-aging and lifespan extension: Studies have demonstrated oxaloacetate's efficacy in extending lifespan and slowing aging processes. It works by activating calorie restriction pathways, particularly through the regulation of NAD+/NADH ratios, which influences sirtuins and other longevity-associated proteins. Research has shown that oxaloacetate supplementation can reduce age-related biomarkers, improve mitochondrial function, and enhance cellular resistance to stress. These effects contribute to its potential as an anti-aging intervention.
- Oxaloacetate in cancer treatment and prevention: Oxaloacetate has shown efficacy in cancer treatment through multiple mechanisms. It can alter cancer cell metabolism by affecting the Warburg effect, where cancer cells preferentially use glycolysis for energy production. By modulating this metabolic pathway, oxaloacetate can inhibit cancer cell proliferation. Additionally, it has been found to enhance the effectiveness of conventional cancer treatments and reduce their side effects. Research indicates potential applications in various cancer types, including brain, breast, and colon cancers.
- Analytical methods for measuring oxaloacetate efficacy: Various analytical techniques have been developed to accurately measure oxaloacetate levels and assess its efficacy in biological systems. These include enzymatic assays, chromatographic methods, mass spectrometry, and biosensors. These techniques allow for precise quantification of oxaloacetate in biological samples, enabling researchers to correlate oxaloacetate levels with therapeutic outcomes. Advanced computational models have also been developed to predict oxaloacetate's efficacy in different physiological conditions and disease states.
02 Oxaloacetate in metabolic pathway regulation and energy production
Oxaloacetate plays a crucial role in cellular metabolism, particularly in the tricarboxylic acid (TCA) cycle and gluconeogenesis. Research has demonstrated its efficacy in enhancing energy production, regulating glucose metabolism, and improving mitochondrial function. These metabolic effects make oxaloacetate potentially valuable for treating conditions characterized by metabolic dysfunction, including diabetes and obesity.Expand Specific Solutions03 Oxaloacetate for lifespan extension and anti-aging applications
Studies have shown that oxaloacetate supplementation may contribute to increased lifespan and healthy aging. The compound appears to mimic some effects of caloric restriction, a well-established intervention for extending lifespan in various organisms. Its mechanisms include reducing oxidative stress, improving mitochondrial function, and activating pathways associated with longevity, such as AMPK and SIRT1 signaling pathways.Expand Specific Solutions04 Oxaloacetate in enzymatic assays and diagnostic applications
Oxaloacetate serves as an important substrate in various enzymatic assays used for diagnostic purposes. It is particularly valuable in assays measuring the activity of enzymes like aspartate aminotransferase (AST), which is important for liver function tests. The compound's efficacy in these applications relies on its specific chemical properties and its role in various metabolic pathways, allowing for accurate measurement of enzyme activities in biological samples.Expand Specific Solutions05 Oxaloacetate formulations and delivery methods
Various formulations and delivery methods have been developed to enhance the stability and bioavailability of oxaloacetate. These include specialized encapsulation techniques, combination with other compounds to improve stability, and modified release formulations. These approaches aim to overcome challenges related to the compound's inherent instability in certain conditions and to optimize its therapeutic efficacy by ensuring appropriate delivery to target tissues.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The oxaloacetate efficacy measurement market is in a growth phase, with increasing interest in metabolic pathway optimization across pharmaceutical and biotechnology sectors. The global market size for metabolic pathway analysis tools is expanding, driven by rising chronic disease prevalence and personalized medicine approaches. Technologically, the field shows moderate maturity with established players like Genomatica, DuPont, and METabolic EXplorer leading commercial applications, while research institutions such as Max Planck Society and University of Florida drive innovation. Pharmaceutical companies including Bristol Myers Squibb and Merck are investing in biochemical pathway analysis technologies, particularly for drug development applications. Academic-industry partnerships are accelerating technological advancement, with specialized biotechnology firms like Benagene focusing on niche applications in metabolic health.
Genomatica, Inc.
Technical Solution: Genomatica has pioneered a systems biology approach to measure oxaloacetate efficacy in biochemical pathways, particularly focusing on its role in sustainable chemical production. Their platform integrates multi-omics data (transcriptomics, proteomics, and metabolomics) to create comprehensive models of oxaloacetate metabolism in engineered microorganisms. The company has developed proprietary biosensors that can detect intracellular oxaloacetate fluctuations with temporal resolution of under 30 seconds, enabling dynamic pathway analysis. Genomatica's technology includes engineered strains with modified oxaloacetate metabolism that have achieved productivity improvements of 2-3 fold in target molecule production. Their measurement systems can distinguish between oxaloacetate pools in different cellular compartments, providing spatial resolution that conventional methods lack. The company has successfully applied this technology to optimize pathways for bio-based 1,4-butanediol production, where oxaloacetate serves as a critical intermediate.
Strengths: Comprehensive systems biology approach integrating multiple data types; industrial-scale validation of oxaloacetate pathway optimization; proven commercial applications in sustainable chemical production. Weaknesses: High technical complexity requiring specialized expertise; significant computational resources needed for data analysis and modeling.
Monsanto Technology LLC
Technical Solution: Monsanto (now part of Bayer) has developed a comprehensive platform for measuring oxaloacetate efficacy in plant biochemical pathways, with particular focus on crop improvement applications. Their technology combines metabolic flux analysis with advanced imaging techniques to visualize oxaloacetate distribution and utilization in plant tissues. The company has created transgenic plant lines with modified oxaloacetate metabolism, allowing for controlled studies of pathway efficiency under various environmental conditions. Their analytical platform can detect changes in oxaloacetate concentrations as small as 5% in plant tissues, enabling precise measurement of subtle metabolic shifts. Monsanto's approach integrates transcriptomic and metabolomic data to create predictive models of oxaloacetate utilization in C3 and C4 photosynthetic pathways, identifying key control points that influence crop productivity. The technology has been successfully applied to enhance nitrogen use efficiency in major crops by optimizing oxaloacetate-dependent transamination reactions, resulting in plants that require up to 20% less nitrogen fertilizer while maintaining yield.
Strengths: Specialized expertise in plant metabolism; integration of field-level phenotypic data with molecular analyses; practical applications in commercial crop improvement. Weaknesses: Technology primarily optimized for plant systems rather than microbial or mammalian applications; longer development cycles due to plant growth timelines.
Key Analytical Methods and Biomarker Technologies
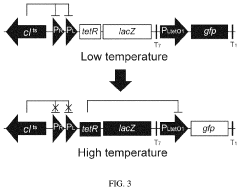
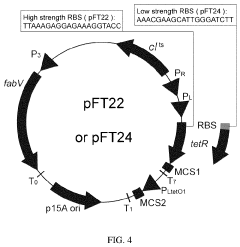
Thermal switch system and application thereof in improving yield of amino acid
PatentPendingUS20240060076A1
Innovation
- A thermal switch system is developed, utilizing a thermal switch vector with a temperature-sensitive circuit to dynamically regulate the expression of pyruvate carboxylase, allowing for staged control of metabolic flux distribution and cofactor supply, optimizing oxaloacetate production and reducing energy wastage.
Purification and isolation of recombinant oxalate degrading enzymes
PatentInactiveHK1152967A
Innovation
- Development of spray-dried particles containing oxalate degrading enzymes, such as oxalate decarboxylase, which are delivered to the stomach to actively degrade oxalate, combined with a method for isolating and purifying recombinant proteins that are insoluble in host cells, using specific mutations to enhance solubility and activity.
Regulatory Framework for Metabolic Pathway Interventions
The regulatory landscape governing metabolic pathway interventions has evolved significantly in response to advances in biochemical research, particularly concerning compounds like oxaloacetate. Regulatory bodies worldwide have established frameworks that address the unique challenges of evaluating metabolic modulators, which operate at the intersection of pharmaceuticals, nutraceuticals, and dietary supplements.
In the United States, the FDA has developed specific guidance for metabolic pathway interventions, requiring rigorous clinical evidence demonstrating biochemical efficacy before market approval. For oxaloacetate specifically, regulatory classification depends on its intended use and claims - whether positioned as a drug targeting specific metabolic disorders or as a dietary supplement supporting normal metabolic function.
The European Medicines Agency (EMA) employs a more stratified approach, with dedicated pathways for metabolic modulators based on their mechanism of action and therapeutic claims. Their framework emphasizes the need for standardized biomarker validation when measuring efficacy in biochemical pathways, particularly for Krebs cycle intermediates like oxaloacetate.
Regulatory requirements typically mandate demonstration of both safety and efficacy through standardized biochemical assays. For oxaloacetate, this includes validated methods for measuring its impact on NAD+/NADH ratios, mitochondrial function, and downstream metabolic effects. These requirements have driven the development of standardized protocols for measuring oxaloacetate's bioavailability and metabolic impact.
International harmonization efforts through ICH (International Council for Harmonisation) have established guidelines for bioanalytical method validation specific to metabolic pathway interventions. These guidelines provide a framework for consistent evaluation of compounds like oxaloacetate across different regulatory jurisdictions, facilitating global research and development.
Recent regulatory trends indicate a shift toward adaptive licensing approaches for metabolic modulators, allowing for staged approval based on evolving evidence of biochemical efficacy. This approach recognizes the complex nature of metabolic interventions and the challenges in establishing definitive clinical endpoints for compounds that influence fundamental biochemical pathways.
Compliance with these regulatory frameworks requires sophisticated analytical capabilities for measuring oxaloacetate's effects on biochemical pathways, including standardized methods for quantifying metabolic flux, enzyme activity, and downstream biomarkers. Organizations developing oxaloacetate-based interventions must navigate these requirements while demonstrating consistent quality, purity, and biochemical activity of their products.
In the United States, the FDA has developed specific guidance for metabolic pathway interventions, requiring rigorous clinical evidence demonstrating biochemical efficacy before market approval. For oxaloacetate specifically, regulatory classification depends on its intended use and claims - whether positioned as a drug targeting specific metabolic disorders or as a dietary supplement supporting normal metabolic function.
The European Medicines Agency (EMA) employs a more stratified approach, with dedicated pathways for metabolic modulators based on their mechanism of action and therapeutic claims. Their framework emphasizes the need for standardized biomarker validation when measuring efficacy in biochemical pathways, particularly for Krebs cycle intermediates like oxaloacetate.
Regulatory requirements typically mandate demonstration of both safety and efficacy through standardized biochemical assays. For oxaloacetate, this includes validated methods for measuring its impact on NAD+/NADH ratios, mitochondrial function, and downstream metabolic effects. These requirements have driven the development of standardized protocols for measuring oxaloacetate's bioavailability and metabolic impact.
International harmonization efforts through ICH (International Council for Harmonisation) have established guidelines for bioanalytical method validation specific to metabolic pathway interventions. These guidelines provide a framework for consistent evaluation of compounds like oxaloacetate across different regulatory jurisdictions, facilitating global research and development.
Recent regulatory trends indicate a shift toward adaptive licensing approaches for metabolic modulators, allowing for staged approval based on evolving evidence of biochemical efficacy. This approach recognizes the complex nature of metabolic interventions and the challenges in establishing definitive clinical endpoints for compounds that influence fundamental biochemical pathways.
Compliance with these regulatory frameworks requires sophisticated analytical capabilities for measuring oxaloacetate's effects on biochemical pathways, including standardized methods for quantifying metabolic flux, enzyme activity, and downstream biomarkers. Organizations developing oxaloacetate-based interventions must navigate these requirements while demonstrating consistent quality, purity, and biochemical activity of their products.
Clinical Translation Challenges and Opportunities
Translating oxaloacetate research from laboratory to clinical applications presents significant challenges that must be addressed systematically. The metabolic complexity of oxaloacetate's role in biochemical pathways necessitates sophisticated measurement techniques that can reliably quantify its efficacy in human subjects. Current analytical methods often struggle with oxaloacetate's inherent instability in biological samples, creating obstacles for consistent clinical assessment protocols.
Bioavailability represents a primary translational hurdle, as oxaloacetate undergoes rapid degradation in the gastrointestinal tract. Various delivery systems including enteric coatings, nanoparticle encapsulation, and prodrug formulations are being explored to enhance stability and absorption. However, standardization of these approaches across clinical settings remains problematic, contributing to variability in patient outcomes and complicating efficacy measurements.
Interindividual metabolic differences significantly impact oxaloacetate metabolism, creating challenges for establishing universal dosing guidelines. Genetic polymorphisms affecting key enzymes in the TCA cycle, variations in gut microbiota composition, and differences in baseline metabolic states all contribute to heterogeneous responses. Personalized medicine approaches incorporating metabolomic profiling may offer opportunities to predict individual responses and optimize treatment protocols.
Regulatory considerations present additional complexities, as oxaloacetate exists in a classification gray area between dietary supplement and therapeutic agent. This ambiguity affects clinical trial design requirements, endpoint selection, and ultimately the pathway to market approval. Collaborative efforts between researchers, industry stakeholders, and regulatory bodies are essential to establish appropriate frameworks for evaluating oxaloacetate-based interventions.
Despite these challenges, several promising opportunities exist for clinical translation. The growing field of metabolic medicine provides a receptive clinical environment for oxaloacetate applications, particularly in age-related conditions, neurodegenerative disorders, and metabolic syndromes. Advances in metabolomic technologies now enable more precise measurement of oxaloacetate's effects on downstream metabolites and pathway flux, offering surrogate markers for clinical efficacy.
Strategic partnerships between academic institutions and pharmaceutical companies are accelerating translational research through shared resources and expertise. These collaborations facilitate larger, more robust clinical trials with sophisticated biomarker analysis capabilities. Additionally, patient advocacy groups increasingly recognize the potential of metabolic interventions, creating opportunities for targeted clinical programs addressing specific disease populations where oxaloacetate shows particular promise.
Bioavailability represents a primary translational hurdle, as oxaloacetate undergoes rapid degradation in the gastrointestinal tract. Various delivery systems including enteric coatings, nanoparticle encapsulation, and prodrug formulations are being explored to enhance stability and absorption. However, standardization of these approaches across clinical settings remains problematic, contributing to variability in patient outcomes and complicating efficacy measurements.
Interindividual metabolic differences significantly impact oxaloacetate metabolism, creating challenges for establishing universal dosing guidelines. Genetic polymorphisms affecting key enzymes in the TCA cycle, variations in gut microbiota composition, and differences in baseline metabolic states all contribute to heterogeneous responses. Personalized medicine approaches incorporating metabolomic profiling may offer opportunities to predict individual responses and optimize treatment protocols.
Regulatory considerations present additional complexities, as oxaloacetate exists in a classification gray area between dietary supplement and therapeutic agent. This ambiguity affects clinical trial design requirements, endpoint selection, and ultimately the pathway to market approval. Collaborative efforts between researchers, industry stakeholders, and regulatory bodies are essential to establish appropriate frameworks for evaluating oxaloacetate-based interventions.
Despite these challenges, several promising opportunities exist for clinical translation. The growing field of metabolic medicine provides a receptive clinical environment for oxaloacetate applications, particularly in age-related conditions, neurodegenerative disorders, and metabolic syndromes. Advances in metabolomic technologies now enable more precise measurement of oxaloacetate's effects on downstream metabolites and pathway flux, offering surrogate markers for clinical efficacy.
Strategic partnerships between academic institutions and pharmaceutical companies are accelerating translational research through shared resources and expertise. These collaborations facilitate larger, more robust clinical trials with sophisticated biomarker analysis capabilities. Additionally, patient advocacy groups increasingly recognize the potential of metabolic interventions, creating opportunities for targeted clinical programs addressing specific disease populations where oxaloacetate shows particular promise.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!