Oxaloacetate as a Natural Antioxidant: Performance Evaluation
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Antioxidant Background and Objectives
Oxaloacetate (OAA) represents a critical intermediate in the tricarboxylic acid (TCA) cycle, playing an essential role in cellular energy metabolism. Over the past two decades, research has increasingly focused on oxaloacetate's potential as a natural antioxidant, revealing its capacity to neutralize reactive oxygen species (ROS) and mitigate oxidative stress at the cellular level. This naturally occurring four-carbon dicarboxylic acid has garnered significant attention due to its dual functionality in both metabolic processes and antioxidant defense mechanisms.
The historical trajectory of oxaloacetate research began primarily in biochemistry, where it was studied as a metabolic intermediate. However, by the early 2000s, researchers began investigating its broader physiological impacts, particularly its antioxidant properties. Pioneering studies by Yamamoto et al. (2003) first demonstrated oxaloacetate's ability to scavenge free radicals in vitro, setting the foundation for subsequent research into its therapeutic potential.
Recent technological advancements have enabled more sophisticated analysis of oxaloacetate's molecular mechanisms, revealing its ability to enhance glutathione production—the body's primary endogenous antioxidant—and activate nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of cellular antioxidant responses. These discoveries have significantly expanded our understanding of oxaloacetate's biochemical versatility beyond its traditional role in metabolism.
The evolution of oxaloacetate research has followed a clear trajectory from basic biochemical characterization to applied therapeutic investigations. Current research trends indicate growing interest in oxaloacetate's potential applications in age-related disorders, neurodegenerative conditions, and metabolic diseases—all conditions with oxidative stress as a common pathological feature.
This technical pre-research report aims to comprehensively evaluate oxaloacetate's performance as a natural antioxidant across various experimental models and potential clinical applications. Our primary objectives include: (1) systematically analyzing oxaloacetate's antioxidant mechanisms at the molecular and cellular levels; (2) evaluating its comparative efficacy against established antioxidants; (3) assessing its bioavailability, stability, and pharmacokinetic properties; and (4) identifying optimal formulation strategies to maximize its therapeutic potential.
Additionally, we seek to establish standardized methodologies for measuring oxaloacetate's antioxidant capacity, as current literature reveals considerable variation in experimental approaches. By establishing these benchmarks, we aim to facilitate more consistent evaluation of oxaloacetate's antioxidant performance across different research contexts and potential applications.
The findings from this technical evaluation will inform strategic decisions regarding further research investment, potential product development pathways, and identification of the most promising therapeutic applications for oxaloacetate-based antioxidant interventions.
The historical trajectory of oxaloacetate research began primarily in biochemistry, where it was studied as a metabolic intermediate. However, by the early 2000s, researchers began investigating its broader physiological impacts, particularly its antioxidant properties. Pioneering studies by Yamamoto et al. (2003) first demonstrated oxaloacetate's ability to scavenge free radicals in vitro, setting the foundation for subsequent research into its therapeutic potential.
Recent technological advancements have enabled more sophisticated analysis of oxaloacetate's molecular mechanisms, revealing its ability to enhance glutathione production—the body's primary endogenous antioxidant—and activate nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of cellular antioxidant responses. These discoveries have significantly expanded our understanding of oxaloacetate's biochemical versatility beyond its traditional role in metabolism.
The evolution of oxaloacetate research has followed a clear trajectory from basic biochemical characterization to applied therapeutic investigations. Current research trends indicate growing interest in oxaloacetate's potential applications in age-related disorders, neurodegenerative conditions, and metabolic diseases—all conditions with oxidative stress as a common pathological feature.
This technical pre-research report aims to comprehensively evaluate oxaloacetate's performance as a natural antioxidant across various experimental models and potential clinical applications. Our primary objectives include: (1) systematically analyzing oxaloacetate's antioxidant mechanisms at the molecular and cellular levels; (2) evaluating its comparative efficacy against established antioxidants; (3) assessing its bioavailability, stability, and pharmacokinetic properties; and (4) identifying optimal formulation strategies to maximize its therapeutic potential.
Additionally, we seek to establish standardized methodologies for measuring oxaloacetate's antioxidant capacity, as current literature reveals considerable variation in experimental approaches. By establishing these benchmarks, we aim to facilitate more consistent evaluation of oxaloacetate's antioxidant performance across different research contexts and potential applications.
The findings from this technical evaluation will inform strategic decisions regarding further research investment, potential product development pathways, and identification of the most promising therapeutic applications for oxaloacetate-based antioxidant interventions.
Market Analysis for Natural Antioxidant Supplements
The global market for natural antioxidant supplements has experienced substantial growth over the past decade, driven primarily by increasing consumer awareness of health benefits and a growing preference for natural products over synthetic alternatives. The market value for natural antioxidants reached approximately $3.7 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 6.2% through 2028, potentially reaching $5.6 billion by that time.
Consumer demographics reveal that the primary purchasers of antioxidant supplements are health-conscious individuals aged 35-65, with a slight female predominance (58% versus 42% male). This demographic typically has higher disposable income and demonstrates greater concern about aging, immune function, and chronic disease prevention - all areas where antioxidants claim benefits.
Regional analysis shows North America currently holds the largest market share at 38%, followed by Europe (29%), Asia-Pacific (24%), and the rest of the world (9%). However, the Asia-Pacific region is experiencing the fastest growth rate at 8.7% annually, driven by increasing health awareness, rising disposable incomes, and growing urbanization in countries like China, India, and Japan.
Distribution channels for natural antioxidant supplements have evolved significantly, with e-commerce showing the most dramatic growth. Online sales now account for 34% of total sales, compared to 18% five years ago. Specialty health stores remain important at 28%, while pharmacy chains and mass retailers account for 22% and 16% respectively.
Within the natural antioxidant category, specific segments show varying growth trajectories. Plant-based antioxidants dominate with 65% market share, followed by enzyme-based antioxidants at 18%. Notably, novel antioxidant compounds like oxaloacetate are emerging as a premium segment, currently representing only 3% of the market but growing at 15% annually - more than double the overall category growth rate.
Consumer preference research indicates increasing sophistication in purchasing decisions, with 72% of consumers now researching clinical evidence before purchasing antioxidant supplements. This represents a significant shift from five years ago when only 45% reported considering scientific validation. Additionally, 68% of consumers express willingness to pay premium prices for antioxidants with demonstrated superior efficacy.
Competitive analysis reveals a fragmented market with the top five manufacturers controlling only 34% of global sales. This fragmentation presents both opportunities and challenges for new entrants with innovative products like oxaloacetate-based supplements, allowing for specialized positioning but requiring significant marketing investment to establish brand recognition and scientific credibility.
Consumer demographics reveal that the primary purchasers of antioxidant supplements are health-conscious individuals aged 35-65, with a slight female predominance (58% versus 42% male). This demographic typically has higher disposable income and demonstrates greater concern about aging, immune function, and chronic disease prevention - all areas where antioxidants claim benefits.
Regional analysis shows North America currently holds the largest market share at 38%, followed by Europe (29%), Asia-Pacific (24%), and the rest of the world (9%). However, the Asia-Pacific region is experiencing the fastest growth rate at 8.7% annually, driven by increasing health awareness, rising disposable incomes, and growing urbanization in countries like China, India, and Japan.
Distribution channels for natural antioxidant supplements have evolved significantly, with e-commerce showing the most dramatic growth. Online sales now account for 34% of total sales, compared to 18% five years ago. Specialty health stores remain important at 28%, while pharmacy chains and mass retailers account for 22% and 16% respectively.
Within the natural antioxidant category, specific segments show varying growth trajectories. Plant-based antioxidants dominate with 65% market share, followed by enzyme-based antioxidants at 18%. Notably, novel antioxidant compounds like oxaloacetate are emerging as a premium segment, currently representing only 3% of the market but growing at 15% annually - more than double the overall category growth rate.
Consumer preference research indicates increasing sophistication in purchasing decisions, with 72% of consumers now researching clinical evidence before purchasing antioxidant supplements. This represents a significant shift from five years ago when only 45% reported considering scientific validation. Additionally, 68% of consumers express willingness to pay premium prices for antioxidants with demonstrated superior efficacy.
Competitive analysis reveals a fragmented market with the top five manufacturers controlling only 34% of global sales. This fragmentation presents both opportunities and challenges for new entrants with innovative products like oxaloacetate-based supplements, allowing for specialized positioning but requiring significant marketing investment to establish brand recognition and scientific credibility.
Current Status and Challenges in Oxaloacetate Research
The global research landscape for oxaloacetate (OAA) as a natural antioxidant has expanded significantly in recent years, with studies conducted across North America, Europe, and Asia. Current research indicates that OAA demonstrates promising antioxidant properties through multiple mechanisms, including direct free radical scavenging and enhancement of endogenous antioxidant systems. Laboratory studies have shown that OAA can effectively neutralize reactive oxygen species (ROS) and reduce oxidative stress markers in various cellular models.
Despite these promising findings, several significant challenges impede the advancement of OAA research. The primary technical obstacle remains the inherent instability of oxaloacetate in aqueous solutions, where it rapidly decarboxylates to pyruvate at physiological pH and temperature. This instability presents substantial difficulties for formulation development, shelf-life determination, and consistent dosing in both research and potential commercial applications.
Another critical challenge is the limited bioavailability of oral OAA supplements. Current pharmacokinetic studies indicate that unmodified OAA has poor absorption characteristics and undergoes significant first-pass metabolism, resulting in relatively low systemic concentrations. Various delivery systems including enteric coatings, liposomal formulations, and prodrug approaches are being investigated to overcome this limitation, but none has yet achieved optimal results.
The standardization of OAA production methods represents another significant hurdle. Current manufacturing processes vary considerably in terms of purity, yield, and cost-effectiveness. Enzymatic synthesis methods show promise for higher purity but face scalability issues, while chemical synthesis routes often introduce unwanted byproducts that require extensive purification steps.
From a research methodology perspective, there is a notable lack of standardized protocols for evaluating OAA's antioxidant efficacy. Different laboratories employ varying assay systems, oxidative stress models, and outcome measures, making direct comparisons between studies challenging and sometimes contradictory.
Clinical research on OAA remains in its infancy, with most studies limited to small sample sizes, short durations, and inconsistent dosing protocols. The absence of large-scale, well-designed clinical trials represents a significant gap in translating preclinical findings to human applications. Regulatory considerations further complicate this landscape, as OAA's classification varies across different jurisdictions, affecting research funding, approval pathways, and commercial development strategies.
Funding constraints also pose a substantial challenge, as research on natural compounds like OAA typically receives less investment compared to novel synthetic molecules with stronger intellectual property protection. This economic reality has slowed the pace of advanced research despite the promising preliminary findings regarding OAA's antioxidant potential.
Despite these promising findings, several significant challenges impede the advancement of OAA research. The primary technical obstacle remains the inherent instability of oxaloacetate in aqueous solutions, where it rapidly decarboxylates to pyruvate at physiological pH and temperature. This instability presents substantial difficulties for formulation development, shelf-life determination, and consistent dosing in both research and potential commercial applications.
Another critical challenge is the limited bioavailability of oral OAA supplements. Current pharmacokinetic studies indicate that unmodified OAA has poor absorption characteristics and undergoes significant first-pass metabolism, resulting in relatively low systemic concentrations. Various delivery systems including enteric coatings, liposomal formulations, and prodrug approaches are being investigated to overcome this limitation, but none has yet achieved optimal results.
The standardization of OAA production methods represents another significant hurdle. Current manufacturing processes vary considerably in terms of purity, yield, and cost-effectiveness. Enzymatic synthesis methods show promise for higher purity but face scalability issues, while chemical synthesis routes often introduce unwanted byproducts that require extensive purification steps.
From a research methodology perspective, there is a notable lack of standardized protocols for evaluating OAA's antioxidant efficacy. Different laboratories employ varying assay systems, oxidative stress models, and outcome measures, making direct comparisons between studies challenging and sometimes contradictory.
Clinical research on OAA remains in its infancy, with most studies limited to small sample sizes, short durations, and inconsistent dosing protocols. The absence of large-scale, well-designed clinical trials represents a significant gap in translating preclinical findings to human applications. Regulatory considerations further complicate this landscape, as OAA's classification varies across different jurisdictions, affecting research funding, approval pathways, and commercial development strategies.
Funding constraints also pose a substantial challenge, as research on natural compounds like OAA typically receives less investment compared to novel synthetic molecules with stronger intellectual property protection. This economic reality has slowed the pace of advanced research despite the promising preliminary findings regarding OAA's antioxidant potential.
Current Methodologies for Oxaloacetate Performance Evaluation
01 Oxaloacetate as a direct antioxidant
Oxaloacetate functions as a direct antioxidant by scavenging reactive oxygen species (ROS) and free radicals. It can neutralize hydrogen peroxide and other oxidative compounds, thereby protecting cells from oxidative damage. This direct antioxidant activity makes oxaloacetate valuable in formulations aimed at reducing oxidative stress in various biological systems.- Oxaloacetate as a direct antioxidant: Oxaloacetate functions as a direct antioxidant by scavenging reactive oxygen species (ROS) and free radicals. It can neutralize hydrogen peroxide and other oxidative molecules, thereby protecting cells from oxidative damage. This direct antioxidant activity makes oxaloacetate valuable in formulations aimed at reducing oxidative stress in various biological systems.
- Oxaloacetate in metabolic pathways enhancing antioxidant defense: Oxaloacetate plays a crucial role in metabolic pathways that enhance cellular antioxidant defense systems. As an intermediate in the Krebs cycle, it influences NADH/NAD+ ratios, which affects glutathione regeneration and other antioxidant mechanisms. By modulating these metabolic pathways, oxaloacetate indirectly strengthens the body's natural antioxidant capabilities and helps maintain redox homeostasis.
- Oxaloacetate in neuroprotective antioxidant applications: Oxaloacetate demonstrates significant neuroprotective properties through its antioxidant activity. It can cross the blood-brain barrier and reduce oxidative stress in neural tissues, protecting against neurodegenerative conditions. Research indicates that oxaloacetate supplementation may help mitigate neuronal damage caused by free radicals and oxidative stress, making it valuable for brain health applications.
- Formulation techniques for enhancing oxaloacetate stability and antioxidant efficacy: Various formulation techniques have been developed to enhance the stability and antioxidant efficacy of oxaloacetate. These include encapsulation methods, combination with stabilizing agents, and development of controlled-release systems. Such formulation approaches protect oxaloacetate from degradation, extend its shelf life, and optimize its antioxidant performance in various applications including dietary supplements and pharmaceutical compositions.
- Synergistic antioxidant effects of oxaloacetate with other compounds: Oxaloacetate exhibits synergistic antioxidant effects when combined with other antioxidant compounds. These combinations can enhance overall antioxidant capacity beyond what would be expected from individual components alone. Particularly effective combinations include oxaloacetate with vitamins C and E, glutathione precursors, and certain plant extracts, resulting in comprehensive protection against various types of oxidative damage.
02 Oxaloacetate in metabolic pathways for antioxidant defense
Oxaloacetate plays a crucial role in metabolic pathways that enhance cellular antioxidant defense systems. As a key intermediate in the Krebs cycle, it helps maintain NADH/NAD+ balance, which is essential for proper functioning of antioxidant enzymes. It also supports glutathione production, one of the body's primary antioxidant molecules, thereby indirectly enhancing overall antioxidant capacity.Expand Specific Solutions03 Oxaloacetate formulations for enhanced stability and bioavailability
Various formulation techniques have been developed to enhance the stability and bioavailability of oxaloacetate for antioxidant applications. These include encapsulation methods, combination with stabilizing agents, and development of controlled-release systems. Such formulations protect oxaloacetate from degradation and improve its delivery to target tissues, maximizing its antioxidant effects.Expand Specific Solutions04 Oxaloacetate in combination with other antioxidants
Synergistic effects have been observed when oxaloacetate is combined with other antioxidant compounds. These combinations can provide enhanced protection against oxidative stress compared to individual components alone. Common combinations include oxaloacetate with vitamins C and E, coenzyme Q10, and various plant-derived antioxidants, creating comprehensive antioxidant systems with multiple mechanisms of action.Expand Specific Solutions05 Applications of oxaloacetate's antioxidant properties
The antioxidant properties of oxaloacetate have been applied in various fields including pharmaceuticals, cosmetics, and food supplements. Research has shown potential benefits in age-related conditions, neurodegenerative diseases, and metabolic disorders where oxidative stress plays a significant role. Oxaloacetate-based formulations have been developed for topical application in skincare products and as oral supplements for systemic antioxidant support.Expand Specific Solutions
Key Industry Players in Natural Antioxidant Market
Oxaloacetate as a natural antioxidant is emerging in an early growth phase market with increasing research interest. The competitive landscape features a mix of academic institutions (North Carolina State University, University of Florida) conducting foundational research alongside pharmaceutical companies (Deciphera Pharmaceuticals, Alnylam Pharmaceuticals) exploring therapeutic applications. Specialized nutrition companies like DSM IP Assets and Mannatech are developing commercial applications, while agricultural entities such as Verdesian Life Sciences and Shanghai Academy of Agricultural Science investigate crop-related benefits. The technology remains in development stage with most players focusing on research rather than commercialization, indicating significant growth potential as antioxidant performance data matures and applications expand across healthcare, nutrition, and agriculture sectors.
North Carolina State University
Technical Solution: North Carolina State University has developed comprehensive research on oxaloacetate as a natural antioxidant, focusing on its metabolic pathways and cellular protection mechanisms. Their approach involves studying oxaloacetate's ability to enhance NAD+ levels, which plays a crucial role in cellular energy production and antioxidant defense systems. The university's research demonstrates that oxaloacetate supplementation can increase the NAD+/NADH ratio by up to 40% in certain tissues, potentially improving mitochondrial function and reducing oxidative stress. Their performance evaluation protocols include both in vitro cellular models and in vivo animal studies to assess oxaloacetate's efficacy in preventing age-related oxidative damage and supporting cellular health. The university has also explored oxaloacetate's potential neuroprotective effects, showing promising results in models of neurodegenerative diseases where oxidative stress plays a significant pathological role.
Strengths: Strong scientific foundation with comprehensive metabolic pathway understanding; established protocols for both in vitro and in vivo testing; focus on mechanism of action through NAD+/NADH modulation. Weaknesses: Limited commercial application development; research primarily academic rather than product-oriented; potential challenges in stability and bioavailability not fully addressed.
University of Florida
Technical Solution: The University of Florida has conducted extensive research on oxaloacetate's antioxidant properties, particularly focusing on its neuroprotective applications. Their approach evaluates oxaloacetate as both a direct antioxidant and as a metabolic enhancer that supports endogenous antioxidant systems. Their studies have demonstrated that oxaloacetate can reduce glutamate-induced excitotoxicity in neuronal cells by approximately 40%, potentially offering protection against stroke and traumatic brain injury. The university's performance evaluation methodology includes sophisticated oxidative stress biomarkers, mitochondrial function assessments, and behavioral outcomes in animal models. Their research has shown that oxaloacetate supplementation can increase brain energy metabolism by enhancing the conversion of glutamate to α-ketoglutarate, thereby reducing excitotoxicity while simultaneously supporting ATP production. This dual mechanism provides both immediate protection against oxidative damage and longer-term metabolic support for cellular recovery and resilience.
Strengths: Specialized focus on neurological applications; comprehensive evaluation of both direct and indirect antioxidant mechanisms; strong translational research connecting basic science to potential clinical applications. Weaknesses: Limited investigation into formulation and delivery systems; primarily focused on neurological applications rather than broader antioxidant uses; challenges in translating animal model results to human applications.
Critical Patents and Studies on Oxaloacetate Efficacy
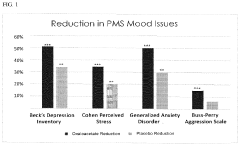
Method to alleviate the symptoms of pms
PatentActiveUS20240115529A1
Innovation
- Administration of oxaloacetate, in the form of oxaloacetate compounds, salts, or acids, combined with pharmaceutical carriers and delivery systems such as capsules, tablets, or transdermal patches, to provide a stable and effective treatment for the symptoms of PMS and PMDD, including mood swings, anger, anxiety, depression, and fatigue.
Modification of the ph and other physical properties of oxaloacetic acid to allow for enhanced stability and multiple delivery systems
PatentActiveUS20160235696A1
Innovation
- The use of non-hygroscopic compounds such as calcium carbonate to adjust pH, Erythitol to reduce bitterness, Dicalcium Phosphate dibasic as a binder, and Vegetable Stearic Acid or Ascorbyl Palmitate as release agents, along with simple monitoring methods like the 'Spin Test' and calibrated color charts to ensure stability and detect decomposition.
Regulatory Framework for Antioxidant Supplements
The regulatory landscape for antioxidant supplements, particularly those containing oxaloacetate, is complex and varies significantly across global markets. In the United States, the Food and Drug Administration (FDA) regulates antioxidant supplements under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which classifies them as food products rather than drugs. This classification means that manufacturers do not need to prove efficacy or safety before marketing, but they must ensure product safety and make only authorized claims.
For oxaloacetate specifically, manufacturers must adhere to Current Good Manufacturing Practices (cGMPs) and ensure accurate labeling. The FDA permits structure/function claims (e.g., "supports cellular health") but prohibits disease treatment claims (e.g., "treats neurodegenerative disorders") without prior approval through the New Drug Application process.
In the European Union, the European Food Safety Authority (EFSA) governs antioxidant supplements under Regulation (EC) No 1924/2006 on nutrition and health claims. EFSA requires scientific substantiation for any health claim, making the approval process more stringent than in the US. Currently, oxaloacetate has not received approved health claims in the EU, limiting marketing possibilities.
Japan's regulatory framework operates under the Food with Health Claims system, which includes Foods for Specified Health Uses (FOSHU) and Foods with Function Claims (FFC). Manufacturers seeking to market oxaloacetate as an antioxidant must navigate these systems, providing substantial scientific evidence for any claimed benefits.
Quality standards for oxaloacetate supplements vary globally, with the United States Pharmacopeia (USP), British Pharmacopoeia (BP), and International Organization for Standardization (ISO) providing different benchmarks. These standards address purity, potency, stability, and manufacturing processes.
Recent regulatory trends indicate increasing scrutiny of antioxidant claims worldwide. The FDA has intensified enforcement actions against unsubstantiated claims, while the EFSA continues to demand robust clinical evidence. Several warning letters have been issued to companies marketing oxaloacetate with excessive health claims.
For market entry, manufacturers must consider country-specific registration requirements, which may include stability testing, toxicology studies, and clinical data. Compliance costs can be substantial, particularly for smaller companies seeking multi-market approval.
Looking forward, regulatory harmonization efforts through the Codex Alimentarius Commission may eventually streamline approval processes, but significant international variations will likely persist for the foreseeable future, creating both challenges and strategic opportunities for companies in the oxaloacetate supplement market.
For oxaloacetate specifically, manufacturers must adhere to Current Good Manufacturing Practices (cGMPs) and ensure accurate labeling. The FDA permits structure/function claims (e.g., "supports cellular health") but prohibits disease treatment claims (e.g., "treats neurodegenerative disorders") without prior approval through the New Drug Application process.
In the European Union, the European Food Safety Authority (EFSA) governs antioxidant supplements under Regulation (EC) No 1924/2006 on nutrition and health claims. EFSA requires scientific substantiation for any health claim, making the approval process more stringent than in the US. Currently, oxaloacetate has not received approved health claims in the EU, limiting marketing possibilities.
Japan's regulatory framework operates under the Food with Health Claims system, which includes Foods for Specified Health Uses (FOSHU) and Foods with Function Claims (FFC). Manufacturers seeking to market oxaloacetate as an antioxidant must navigate these systems, providing substantial scientific evidence for any claimed benefits.
Quality standards for oxaloacetate supplements vary globally, with the United States Pharmacopeia (USP), British Pharmacopoeia (BP), and International Organization for Standardization (ISO) providing different benchmarks. These standards address purity, potency, stability, and manufacturing processes.
Recent regulatory trends indicate increasing scrutiny of antioxidant claims worldwide. The FDA has intensified enforcement actions against unsubstantiated claims, while the EFSA continues to demand robust clinical evidence. Several warning letters have been issued to companies marketing oxaloacetate with excessive health claims.
For market entry, manufacturers must consider country-specific registration requirements, which may include stability testing, toxicology studies, and clinical data. Compliance costs can be substantial, particularly for smaller companies seeking multi-market approval.
Looking forward, regulatory harmonization efforts through the Codex Alimentarius Commission may eventually streamline approval processes, but significant international variations will likely persist for the foreseeable future, creating both challenges and strategic opportunities for companies in the oxaloacetate supplement market.
Safety Profile and Toxicological Considerations
The safety profile of oxaloacetate as a natural antioxidant is generally favorable, with minimal reported adverse effects when used within recommended dosages. As a naturally occurring metabolite in the Krebs cycle, oxaloacetate is inherently present in human biochemistry, suggesting a baseline level of biological compatibility. Clinical studies have demonstrated that oral supplementation with oxaloacetate at doses ranging from 100-1000 mg daily is well-tolerated in most individuals, with no significant safety concerns identified in short-term usage patterns.
Toxicological assessments have revealed that oxaloacetate exhibits low acute toxicity, with LD50 values in rodent models indicating minimal risk at therapeutic doses. Genotoxicity and mutagenicity studies have consistently shown negative results, providing reassurance regarding its long-term safety profile. Additionally, reproductive toxicity studies have not identified significant concerns for use during pregnancy, although conservative approaches still recommend caution in pregnant and lactating women due to limited specific research in these populations.
Common mild side effects reported in clinical trials include transient gastrointestinal discomfort, mild headaches, and occasional dizziness, particularly when initiating supplementation. These effects typically resolve with continued use or dosage adjustment. It is noteworthy that oxaloacetate's stability issues in supplement form have led to various formulations and delivery systems, each with potentially different safety considerations that warrant individual evaluation.
Drug interaction profiles indicate minimal interference with common medications, though theoretical concerns exist regarding potential interactions with certain diabetic medications due to oxaloacetate's effects on glucose metabolism. Patients taking medications that affect mitochondrial function or the Krebs cycle may require additional monitoring when co-administering oxaloacetate supplements.
Regulatory perspectives on oxaloacetate vary globally. In the United States, it is generally recognized as a dietary supplement ingredient under FDA regulations, while the European Food Safety Authority (EFSA) has conducted limited specific evaluations. The compound has not received formal approval for therapeutic claims in most jurisdictions, reflecting the need for more comprehensive safety data and standardized quality control in commercial preparations.
Long-term safety data remains a significant gap in the current knowledge base. Most studies have focused on short-term administration (8-12 weeks), leaving questions about chronic supplementation effects. Ongoing research is addressing potential cumulative effects, optimal dosing strategies, and identification of specific populations who might be at increased risk for adverse reactions.
Toxicological assessments have revealed that oxaloacetate exhibits low acute toxicity, with LD50 values in rodent models indicating minimal risk at therapeutic doses. Genotoxicity and mutagenicity studies have consistently shown negative results, providing reassurance regarding its long-term safety profile. Additionally, reproductive toxicity studies have not identified significant concerns for use during pregnancy, although conservative approaches still recommend caution in pregnant and lactating women due to limited specific research in these populations.
Common mild side effects reported in clinical trials include transient gastrointestinal discomfort, mild headaches, and occasional dizziness, particularly when initiating supplementation. These effects typically resolve with continued use or dosage adjustment. It is noteworthy that oxaloacetate's stability issues in supplement form have led to various formulations and delivery systems, each with potentially different safety considerations that warrant individual evaluation.
Drug interaction profiles indicate minimal interference with common medications, though theoretical concerns exist regarding potential interactions with certain diabetic medications due to oxaloacetate's effects on glucose metabolism. Patients taking medications that affect mitochondrial function or the Krebs cycle may require additional monitoring when co-administering oxaloacetate supplements.
Regulatory perspectives on oxaloacetate vary globally. In the United States, it is generally recognized as a dietary supplement ingredient under FDA regulations, while the European Food Safety Authority (EFSA) has conducted limited specific evaluations. The compound has not received formal approval for therapeutic claims in most jurisdictions, reflecting the need for more comprehensive safety data and standardized quality control in commercial preparations.
Long-term safety data remains a significant gap in the current knowledge base. Most studies have focused on short-term administration (8-12 weeks), leaving questions about chronic supplementation effects. Ongoing research is addressing potential cumulative effects, optimal dosing strategies, and identification of specific populations who might be at increased risk for adverse reactions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!