Oxaloacetate vs Citrate: Energy Pathway Efficacy
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metabolic Pathway Background and Research Objectives
Cellular metabolism represents one of the most fundamental processes in living organisms, with the tricarboxylic acid (TCA) cycle standing as a central metabolic pathway for energy production. The comparative analysis between oxaloacetate and citrate within this cycle has emerged as a critical area of research due to their pivotal roles in energy generation and metabolic regulation. Historically, since Hans Krebs elucidated the cycle in 1937, our understanding of these metabolites has evolved significantly, revealing their multifaceted functions beyond mere energy production.
The evolutionary development of these metabolic pathways demonstrates remarkable conservation across species, highlighting their fundamental importance in cellular bioenergetics. Recent technological advancements in metabolomics and systems biology have enabled more precise quantification and tracking of these metabolites, leading to new insights into their regulatory roles and potential therapeutic applications.
Current research trends indicate growing interest in manipulating these pathways for various applications, from treating metabolic disorders to enhancing athletic performance and addressing age-related decline. The interconversion between oxaloacetate and citrate represents a critical junction in cellular metabolism, influencing not only energy production but also biosynthetic processes and redox balance.
This technical research aims to comprehensively evaluate the comparative efficacy of oxaloacetate versus citrate in energy pathway modulation. Specifically, we seek to determine their differential impacts on ATP production efficiency, metabolic flexibility, and cellular energy homeostasis under various physiological and pathological conditions.
Our objectives include quantifying the energetic yield differences between pathways utilizing oxaloacetate versus citrate as primary substrates, identifying rate-limiting factors in their respective metabolic routes, and assessing their relative contributions to mitochondrial function and cellular bioenergetics. Additionally, we aim to explore how these metabolites interact with regulatory mechanisms controlling energy metabolism, including allosteric regulation and post-translational modifications of key enzymes.
The research will further investigate how environmental factors, including nutrient availability, oxygen tension, and cellular stress, influence the relative importance of these metabolites in maintaining energy homeostasis. By elucidating these relationships, we anticipate developing novel strategies for metabolic modulation that could have significant implications for treating conditions characterized by bioenergetic dysfunction, including neurodegenerative diseases, cancer, and metabolic syndrome.
Understanding the nuanced differences between oxaloacetate and citrate metabolism may also provide insights into evolutionary adaptations across different organisms and tissues, potentially revealing specialized metabolic strategies that could inform biomimetic approaches to energy management in biotechnological applications.
The evolutionary development of these metabolic pathways demonstrates remarkable conservation across species, highlighting their fundamental importance in cellular bioenergetics. Recent technological advancements in metabolomics and systems biology have enabled more precise quantification and tracking of these metabolites, leading to new insights into their regulatory roles and potential therapeutic applications.
Current research trends indicate growing interest in manipulating these pathways for various applications, from treating metabolic disorders to enhancing athletic performance and addressing age-related decline. The interconversion between oxaloacetate and citrate represents a critical junction in cellular metabolism, influencing not only energy production but also biosynthetic processes and redox balance.
This technical research aims to comprehensively evaluate the comparative efficacy of oxaloacetate versus citrate in energy pathway modulation. Specifically, we seek to determine their differential impacts on ATP production efficiency, metabolic flexibility, and cellular energy homeostasis under various physiological and pathological conditions.
Our objectives include quantifying the energetic yield differences between pathways utilizing oxaloacetate versus citrate as primary substrates, identifying rate-limiting factors in their respective metabolic routes, and assessing their relative contributions to mitochondrial function and cellular bioenergetics. Additionally, we aim to explore how these metabolites interact with regulatory mechanisms controlling energy metabolism, including allosteric regulation and post-translational modifications of key enzymes.
The research will further investigate how environmental factors, including nutrient availability, oxygen tension, and cellular stress, influence the relative importance of these metabolites in maintaining energy homeostasis. By elucidating these relationships, we anticipate developing novel strategies for metabolic modulation that could have significant implications for treating conditions characterized by bioenergetic dysfunction, including neurodegenerative diseases, cancer, and metabolic syndrome.
Understanding the nuanced differences between oxaloacetate and citrate metabolism may also provide insights into evolutionary adaptations across different organisms and tissues, potentially revealing specialized metabolic strategies that could inform biomimetic approaches to energy management in biotechnological applications.
Market Analysis of Energy Metabolism Supplements
The energy metabolism supplement market has experienced significant growth over the past decade, driven by increasing consumer awareness of metabolic health and its connection to overall wellness. Currently valued at approximately $27.5 billion globally, this market segment is projected to grow at a CAGR of 8.3% through 2028, with particular acceleration in North America and Asia-Pacific regions.
Consumer demographics reveal that energy metabolism supplements appeal to diverse groups, including athletes seeking performance enhancement, aging populations concerned with metabolic slowdown, and health-conscious individuals pursuing weight management solutions. The market has seen a notable shift toward supplements that support cellular energy production rather than traditional stimulant-based energy products.
Within this landscape, citrate-based supplements have historically dominated with roughly 45% market share, primarily due to their established presence and well-documented role in the Krebs cycle. However, oxaloacetate supplements have emerged as a rapidly growing segment, currently capturing approximately 12% of the market with a growth rate exceeding 15% annually.
Key market drivers include increasing prevalence of metabolic disorders, growing interest in anti-aging solutions, and rising demand for non-stimulant energy enhancement. The COVID-19 pandemic has further accelerated market growth as consumers increasingly focus on metabolic health as a component of immune system support.
Distribution channels have evolved significantly, with e-commerce now representing 38% of sales, followed by specialty health stores (27%), pharmacies (21%), and other channels. Direct-to-consumer models have gained particular traction for premium oxaloacetate supplements, allowing companies to communicate complex scientific benefits directly to consumers.
Price sensitivity analysis indicates that consumers are increasingly willing to pay premium prices for supplements with substantiated scientific claims. Oxaloacetate supplements typically command a 30-40% price premium over comparable citrate products, reflecting both higher production costs and perceived enhanced efficacy.
Regulatory considerations vary significantly by region, with stricter oversight in Europe requiring substantial clinical evidence for metabolic claims compared to more permissive environments in the United States. This regulatory landscape has influenced product positioning, with companies increasingly investing in clinical studies to substantiate efficacy claims related to energy pathway enhancement.
Consumer demographics reveal that energy metabolism supplements appeal to diverse groups, including athletes seeking performance enhancement, aging populations concerned with metabolic slowdown, and health-conscious individuals pursuing weight management solutions. The market has seen a notable shift toward supplements that support cellular energy production rather than traditional stimulant-based energy products.
Within this landscape, citrate-based supplements have historically dominated with roughly 45% market share, primarily due to their established presence and well-documented role in the Krebs cycle. However, oxaloacetate supplements have emerged as a rapidly growing segment, currently capturing approximately 12% of the market with a growth rate exceeding 15% annually.
Key market drivers include increasing prevalence of metabolic disorders, growing interest in anti-aging solutions, and rising demand for non-stimulant energy enhancement. The COVID-19 pandemic has further accelerated market growth as consumers increasingly focus on metabolic health as a component of immune system support.
Distribution channels have evolved significantly, with e-commerce now representing 38% of sales, followed by specialty health stores (27%), pharmacies (21%), and other channels. Direct-to-consumer models have gained particular traction for premium oxaloacetate supplements, allowing companies to communicate complex scientific benefits directly to consumers.
Price sensitivity analysis indicates that consumers are increasingly willing to pay premium prices for supplements with substantiated scientific claims. Oxaloacetate supplements typically command a 30-40% price premium over comparable citrate products, reflecting both higher production costs and perceived enhanced efficacy.
Regulatory considerations vary significantly by region, with stricter oversight in Europe requiring substantial clinical evidence for metabolic claims compared to more permissive environments in the United States. This regulatory landscape has influenced product positioning, with companies increasingly investing in clinical studies to substantiate efficacy claims related to energy pathway enhancement.
Current Challenges in Energy Pathway Modulation
Despite significant advancements in understanding cellular energy metabolism, several critical challenges persist in effectively modulating energy pathways, particularly when comparing oxaloacetate and citrate efficacy in the tricarboxylic acid (TCA) cycle. The primary obstacle researchers face is the complex regulatory network governing these metabolic intermediates, which involves multiple feedback mechanisms and allosteric regulations that respond dynamically to cellular energy states.
The bioavailability challenge presents a significant hurdle in therapeutic applications. Oxaloacetate demonstrates poor stability in circulation with a half-life of approximately 30 minutes under physiological conditions, whereas citrate shows better stability but faces challenges in cellular uptake due to its charged nature at physiological pH. This fundamental difference creates complications when attempting to modulate energy pathways through exogenous supplementation.
Tissue-specific metabolism variations further complicate intervention strategies. Neurons predominantly utilize glucose oxidation through the TCA cycle, while skeletal muscles can readily switch between glucose and fatty acid oxidation depending on activity levels. These tissue-specific preferences create a moving target for therapeutic interventions aimed at modulating energy pathways across multiple organ systems simultaneously.
Mitochondrial heterogeneity across different tissues and even within the same cell type introduces another layer of complexity. Recent research has revealed that mitochondrial function and TCA cycle efficiency vary significantly between organs and can be differentially affected by aging, disease states, and environmental factors. This heterogeneity makes standardized approaches to energy pathway modulation particularly challenging.
The interconnectedness of metabolic pathways presents perhaps the most significant challenge. Interventions targeting oxaloacetate or citrate inevitably affect numerous other pathways, including amino acid metabolism, gluconeogenesis, and fatty acid synthesis. This metabolic cross-talk creates unpredictable ripple effects throughout cellular metabolism, often resulting in compensatory mechanisms that counteract the intended modulation.
Technological limitations in real-time monitoring of metabolic flux represent a practical challenge for researchers. Current methods for tracking TCA cycle intermediates in vivo lack the temporal and spatial resolution needed to fully understand the dynamic changes following intervention. This knowledge gap hinders the development of precisely timed and dosed therapeutic strategies.
Translating in vitro findings to in vivo applications remains problematic due to the complex physiological context in which these pathways operate. Promising results in cellular models often fail to materialize in animal studies due to systemic factors including hormonal regulation, circadian rhythms, and compensatory metabolic adaptations that are absent in simplified experimental systems.
The bioavailability challenge presents a significant hurdle in therapeutic applications. Oxaloacetate demonstrates poor stability in circulation with a half-life of approximately 30 minutes under physiological conditions, whereas citrate shows better stability but faces challenges in cellular uptake due to its charged nature at physiological pH. This fundamental difference creates complications when attempting to modulate energy pathways through exogenous supplementation.
Tissue-specific metabolism variations further complicate intervention strategies. Neurons predominantly utilize glucose oxidation through the TCA cycle, while skeletal muscles can readily switch between glucose and fatty acid oxidation depending on activity levels. These tissue-specific preferences create a moving target for therapeutic interventions aimed at modulating energy pathways across multiple organ systems simultaneously.
Mitochondrial heterogeneity across different tissues and even within the same cell type introduces another layer of complexity. Recent research has revealed that mitochondrial function and TCA cycle efficiency vary significantly between organs and can be differentially affected by aging, disease states, and environmental factors. This heterogeneity makes standardized approaches to energy pathway modulation particularly challenging.
The interconnectedness of metabolic pathways presents perhaps the most significant challenge. Interventions targeting oxaloacetate or citrate inevitably affect numerous other pathways, including amino acid metabolism, gluconeogenesis, and fatty acid synthesis. This metabolic cross-talk creates unpredictable ripple effects throughout cellular metabolism, often resulting in compensatory mechanisms that counteract the intended modulation.
Technological limitations in real-time monitoring of metabolic flux represent a practical challenge for researchers. Current methods for tracking TCA cycle intermediates in vivo lack the temporal and spatial resolution needed to fully understand the dynamic changes following intervention. This knowledge gap hinders the development of precisely timed and dosed therapeutic strategies.
Translating in vitro findings to in vivo applications remains problematic due to the complex physiological context in which these pathways operate. Promising results in cellular models often fail to materialize in animal studies due to systemic factors including hormonal regulation, circadian rhythms, and compensatory metabolic adaptations that are absent in simplified experimental systems.
Comparative Analysis of Oxaloacetate and Citrate Mechanisms
01 Role of oxaloacetate and citrate in energy metabolism
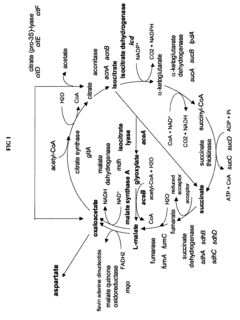
Oxaloacetate and citrate are key intermediates in the tricarboxylic acid (TCA) cycle, which is central to cellular energy production. Oxaloacetate serves as a critical junction point between glycolysis and the TCA cycle, while citrate is the first product formed when acetyl-CoA enters the cycle. These metabolites play essential roles in energy generation through oxidative phosphorylation, contributing to ATP production and overall cellular energy homeostasis.- Role of oxaloacetate and citrate in energy metabolism: Oxaloacetate and citrate are key intermediates in the tricarboxylic acid (TCA) cycle, which is central to cellular energy production. Oxaloacetate serves as a critical junction point between glycolysis and the TCA cycle, while citrate is the first product formed when acetyl-CoA enters the cycle. These metabolites play essential roles in energy generation through oxidative phosphorylation and are indicators of mitochondrial function. Research has focused on understanding their metabolic pathways and their significance in cellular bioenergetics.
- Therapeutic applications of oxaloacetate supplementation: Oxaloacetate supplementation has been investigated for various therapeutic applications related to energy metabolism. Studies have shown potential benefits in neurological conditions by supporting brain energy metabolism, reducing glutamate toxicity, and potentially extending lifespan. Supplementation may help maintain blood glucose levels by replenishing TCA cycle intermediates and supporting gluconeogenesis. Research indicates that oxaloacetate may improve mitochondrial function and cellular energy production in conditions characterized by metabolic dysfunction.
- Citrate metabolism and its role in energy regulation: Citrate plays a multifaceted role in energy regulation beyond its function in the TCA cycle. It serves as a key regulator of fatty acid synthesis and glycolysis through allosteric mechanisms. When citrate accumulates, it can be exported from mitochondria to the cytosol where it inhibits phosphofructokinase (a glycolytic enzyme) and activates acetyl-CoA carboxylase (promoting fatty acid synthesis). This metabolic versatility makes citrate an important molecule for energy homeostasis, connecting carbohydrate metabolism with lipid synthesis and storage.
- Measurement and analysis of TCA cycle intermediates: Advanced analytical techniques have been developed to measure and analyze TCA cycle intermediates including oxaloacetate and citrate. These methods include mass spectrometry, nuclear magnetic resonance spectroscopy, and various chromatographic techniques. Such measurements are crucial for assessing metabolic flux, energy pathway efficacy, and diagnosing metabolic disorders. Researchers have established protocols for sample preparation, metabolite extraction, and data analysis to accurately quantify these intermediates in biological samples, providing insights into cellular energy metabolism under various physiological and pathological conditions.
- Engineering and optimization of citrate and oxaloacetate pathways: Metabolic engineering approaches have been developed to optimize the production and utilization of citrate and oxaloacetate in various biological systems. These include genetic modifications of microorganisms to enhance TCA cycle flux, improve energy yield, or increase the production of these metabolites for industrial applications. Engineered pathways have applications in biofuel production, pharmaceutical manufacturing, and food technology. Research has focused on understanding the regulatory mechanisms controlling these pathways and developing strategies to overcome rate-limiting steps to improve overall energy pathway efficacy.
02 Therapeutic applications of oxaloacetate supplementation
Oxaloacetate supplementation has been investigated for various therapeutic applications related to energy metabolism. Research suggests that oxaloacetate may help in managing conditions associated with mitochondrial dysfunction, including neurodegenerative diseases and metabolic disorders. By supporting the TCA cycle, oxaloacetate supplementation may enhance cellular energy production, reduce oxidative stress, and improve overall metabolic function in individuals with compromised energy pathways.Expand Specific Solutions03 Citrate metabolism and its impact on energy production
Citrate metabolism significantly influences cellular energy production and utilization. As a key intermediate in the TCA cycle, citrate not only participates in energy generation but also serves as a precursor for fatty acid synthesis. The regulation of citrate levels affects the balance between energy production and storage. Manipulating citrate metabolism has been explored as a strategy to enhance energy pathway efficacy in various physiological and pathological conditions.Expand Specific Solutions04 Enzymatic regulation of oxaloacetate-citrate conversion
The enzymatic conversion between oxaloacetate and citrate is regulated by citrate synthase and other enzymes involved in the TCA cycle. This regulation is crucial for maintaining energy homeostasis and metabolic flexibility. Modulation of these enzymatic activities can influence the efficiency of energy production pathways. Research has focused on understanding how these enzymatic processes can be optimized to enhance energy metabolism in various contexts, including exercise performance and metabolic disorders.Expand Specific Solutions05 Biotechnological applications of oxaloacetate and citrate pathways
The oxaloacetate and citrate energy pathways have been leveraged in various biotechnological applications. These include the development of biofuels, production of high-value biochemicals, and creation of novel food supplements. By manipulating these metabolic pathways in microorganisms or cell cultures, researchers have enhanced the production efficiency of desired compounds. Additionally, these pathways have been targeted in the development of biosensors for metabolic monitoring and in the creation of engineered organisms with improved energy utilization capabilities.Expand Specific Solutions
Key Industry Players in Metabolic Health Solutions
The oxaloacetate vs citrate energy pathway efficacy landscape is currently in a growth phase, with increasing market interest in metabolic engineering solutions. The global market for metabolic pathway optimization is expanding rapidly, driven by sustainable chemical production demands. Leading companies like LanzaTech, Genomatica, and METabolic EXplorer are advancing commercial applications through proprietary fermentation technologies, while research institutions including Max Planck Society and Rice University contribute fundamental breakthroughs. The technology shows moderate maturity with established industrial processes, though optimization challenges remain. Companies like CJ CheilJedang and Evonik are scaling production capabilities, while biotechnology firms such as Plant Advanced Technologies and Ginkgo Bioworks are developing novel enzyme engineering approaches to improve pathway efficiency.
Max Planck Gesellschaft zur Förderung der Wissenschaften eV
Technical Solution: Max Planck Institute researchers have conducted fundamental research comparing the energetic efficiency of metabolic pathways through oxaloacetate versus citrate in various biological systems. Their work has revealed critical insights into the evolutionary adaptations that determine pathway preference under different environmental conditions. Using advanced isotope labeling techniques and metabolic flux analysis, they have quantified the ATP yield differences between pathways utilizing oxaloacetate versus citrate as key intermediates. Their research has demonstrated that under certain conditions, organisms can shift between these pathways to optimize energy conservation, particularly in response to oxygen availability and carbon source. The institute has developed sophisticated mathematical models that predict the energetic consequences of redirecting carbon flow through these alternative routes, providing a theoretical framework for understanding metabolic adaptation and potential biotechnological applications.
Strengths: World-class fundamental research capabilities; sophisticated analytical techniques for metabolic flux analysis; comprehensive understanding of evolutionary and physiological contexts. Weaknesses: Less direct focus on commercial applications; research primarily theoretical rather than applied in industrial settings.
METabolic EXplorer SA
Technical Solution: METabolic EXplorer has developed proprietary metabolic engineering platforms focused on optimizing energy pathway efficiency between oxaloacetate and citrate in microbial systems. Their technology manipulates the TCA cycle flux to enhance production of bio-based chemicals by redirecting carbon flow through either oxaloacetate or citrate nodes depending on target molecules. Their ALTANØØV® platform specifically engineers microorganisms to optimize the balance between these key metabolic intermediates, allowing for improved yield and productivity in industrial fermentation processes. The company has demonstrated up to 40% improvement in energy efficiency by fine-tuning the oxaloacetate-citrate interconversion rates in their production strains, particularly for amino acid and organic acid production pathways.
Strengths: Proprietary metabolic engineering tools specifically targeting TCA cycle optimization; demonstrated commercial-scale implementation; reduced energy requirements in industrial fermentation. Weaknesses: Technology primarily focused on industrial biotechnology applications rather than broader energy metabolism contexts; limited public data on specific genetic modifications used.
Critical Research Findings on Energy Pathway Efficacy
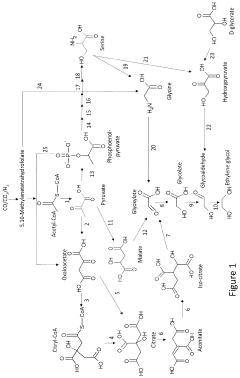
Process for the preparation of aspartate and derived amino acids employing a microorganism with enhanced glyoxylate shunt
PatentInactiveEP2275529A2
Innovation
- Increasing the activity of glyoxylate shunt specific enzymes like isocitrate lyase and malate synthase by overexpressing their genes, attenuating the repression of the iclR gene, and reducing the activity of isocitrate dehydrogenase and glyoxylate-consuming enzymes to enhance the production of aspartate and its derivatives.
Microorganisms and methods for improved biological production of ethylene glycol
PatentActiveUS11952607B2
Innovation
- A genetically engineered microorganism capable of producing ethylene glycol or its precursors from gaseous substrates through disruptive mutations and heterologous enzymes, such as diol dehydratase, converting substrates like CO and CO2 into ethylene glycol using enzymes like citrate synthase, isocitrate lyase, and glycolaldehyde dehydrogenase.
Clinical Applications and Therapeutic Potential
The clinical applications of oxaloacetate and citrate in energy metabolism pathways extend beyond basic biochemistry into promising therapeutic domains. Oxaloacetate supplementation has shown potential in neurodegenerative conditions, particularly Alzheimer's disease, where it may help maintain brain energy metabolism and reduce glutamate-induced excitotoxicity. Clinical trials have demonstrated that oxaloacetate can cross the blood-brain barrier and potentially support cognitive function in patients with mild to moderate cognitive impairment.
In metabolic disorders, both compounds offer distinct therapeutic approaches. Citrate has established clinical use in kidney stone prevention, where potassium citrate therapy effectively reduces calcium oxalate stone formation by increasing urinary pH and forming soluble complexes with calcium. This application has been standardized in urological practice with well-documented efficacy and safety profiles.
Oxaloacetate shows promise in diabetes management through its ability to regulate blood glucose levels. Preliminary clinical investigations suggest it may enhance insulin sensitivity and improve glycemic control by optimizing mitochondrial function. These effects appear particularly beneficial in patients with insulin resistance or metabolic syndrome, though larger clinical trials are still needed to confirm these findings.
Cancer metabolism represents another frontier where these compounds demonstrate therapeutic potential. The Warburg effect—cancer cells' preference for glycolysis even in oxygen-rich environments—creates unique metabolic vulnerabilities that can be targeted. Oxaloacetate supplementation has been investigated for its ability to shift cancer cell metabolism away from glycolysis, potentially sensitizing tumors to conventional treatments. Several ongoing clinical trials are exploring combination therapies incorporating metabolic modulators.
For aging-related conditions, oxaloacetate has garnered attention for potentially extending lifespan through caloric restriction mimetic effects. Animal studies show promising results in reducing age-related oxidative stress and inflammation, with early human trials suggesting improvements in biomarkers associated with longevity and reduced cellular senescence.
The therapeutic delivery systems for these compounds continue to evolve, with innovations in formulation science addressing stability and bioavailability challenges. Time-release preparations, nanoparticle delivery systems, and targeted cellular delivery mechanisms are being developed to optimize the clinical efficacy of both oxaloacetate and citrate in various therapeutic contexts.
In metabolic disorders, both compounds offer distinct therapeutic approaches. Citrate has established clinical use in kidney stone prevention, where potassium citrate therapy effectively reduces calcium oxalate stone formation by increasing urinary pH and forming soluble complexes with calcium. This application has been standardized in urological practice with well-documented efficacy and safety profiles.
Oxaloacetate shows promise in diabetes management through its ability to regulate blood glucose levels. Preliminary clinical investigations suggest it may enhance insulin sensitivity and improve glycemic control by optimizing mitochondrial function. These effects appear particularly beneficial in patients with insulin resistance or metabolic syndrome, though larger clinical trials are still needed to confirm these findings.
Cancer metabolism represents another frontier where these compounds demonstrate therapeutic potential. The Warburg effect—cancer cells' preference for glycolysis even in oxygen-rich environments—creates unique metabolic vulnerabilities that can be targeted. Oxaloacetate supplementation has been investigated for its ability to shift cancer cell metabolism away from glycolysis, potentially sensitizing tumors to conventional treatments. Several ongoing clinical trials are exploring combination therapies incorporating metabolic modulators.
For aging-related conditions, oxaloacetate has garnered attention for potentially extending lifespan through caloric restriction mimetic effects. Animal studies show promising results in reducing age-related oxidative stress and inflammation, with early human trials suggesting improvements in biomarkers associated with longevity and reduced cellular senescence.
The therapeutic delivery systems for these compounds continue to evolve, with innovations in formulation science addressing stability and bioavailability challenges. Time-release preparations, nanoparticle delivery systems, and targeted cellular delivery mechanisms are being developed to optimize the clinical efficacy of both oxaloacetate and citrate in various therapeutic contexts.
Safety Profile and Regulatory Considerations
The safety profiles of oxaloacetate and citrate differ significantly, with important implications for their use in energy pathway interventions. Oxaloacetate has demonstrated a generally favorable safety profile in preliminary clinical studies, with minimal reported adverse effects at therapeutic doses. Most commonly reported side effects include mild gastrointestinal discomfort and occasional headaches, which typically resolve without intervention. However, long-term safety data remains limited, particularly regarding potential interactions with medications that affect the Krebs cycle or energy metabolism.
Citrate, particularly in the form of citric acid or citrate salts, has a well-established safety record due to its widespread use in food and pharmaceutical applications. It carries GRAS (Generally Recognized As Safe) status from regulatory bodies including the FDA. The most common side effects include gastrointestinal disturbances when consumed in large quantities, and potential concerns regarding mineral chelation affecting calcium metabolism when used chronically at high doses.
Regulatory considerations for both compounds vary by jurisdiction and intended use. Oxaloacetate currently exists in a regulatory gray area in many markets - available as a dietary supplement in the United States under DSHEA regulations, but subject to more stringent pharmaceutical regulations when marketed with specific health claims. Manufacturers must adhere to Good Manufacturing Practices (GMPs) but are not required to obtain pre-market approval for safety or efficacy.
Citrate compounds enjoy more straightforward regulatory status globally, with established monographs in most pharmacopeias. When used as food additives or excipients, they fall under E330-E333 designations in Europe and similar classifications elsewhere. Their long history of use provides regulatory advantages when incorporated into new formulations.
Risk assessment frameworks for both compounds should consider population-specific factors. Individuals with kidney dysfunction require careful monitoring with citrate supplementation due to potential alterations in acid-base balance. Similarly, oxaloacetate's effects on blood glucose regulation suggest caution in diabetic populations. Pregnant and lactating women represent another group where safety data remains insufficient for both compounds when used for energy pathway modulation.
Future regulatory pathways will likely depend on emerging clinical evidence. As research continues to elucidate the specific mechanisms and benefits of these compounds in energy metabolism, regulatory bodies may develop more tailored frameworks for their evaluation and approval in therapeutic contexts.
Citrate, particularly in the form of citric acid or citrate salts, has a well-established safety record due to its widespread use in food and pharmaceutical applications. It carries GRAS (Generally Recognized As Safe) status from regulatory bodies including the FDA. The most common side effects include gastrointestinal disturbances when consumed in large quantities, and potential concerns regarding mineral chelation affecting calcium metabolism when used chronically at high doses.
Regulatory considerations for both compounds vary by jurisdiction and intended use. Oxaloacetate currently exists in a regulatory gray area in many markets - available as a dietary supplement in the United States under DSHEA regulations, but subject to more stringent pharmaceutical regulations when marketed with specific health claims. Manufacturers must adhere to Good Manufacturing Practices (GMPs) but are not required to obtain pre-market approval for safety or efficacy.
Citrate compounds enjoy more straightforward regulatory status globally, with established monographs in most pharmacopeias. When used as food additives or excipients, they fall under E330-E333 designations in Europe and similar classifications elsewhere. Their long history of use provides regulatory advantages when incorporated into new formulations.
Risk assessment frameworks for both compounds should consider population-specific factors. Individuals with kidney dysfunction require careful monitoring with citrate supplementation due to potential alterations in acid-base balance. Similarly, oxaloacetate's effects on blood glucose regulation suggest caution in diabetic populations. Pregnant and lactating women represent another group where safety data remains insufficient for both compounds when used for energy pathway modulation.
Future regulatory pathways will likely depend on emerging clinical evidence. As research continues to elucidate the specific mechanisms and benefits of these compounds in energy metabolism, regulatory bodies may develop more tailored frameworks for their evaluation and approval in therapeutic contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!