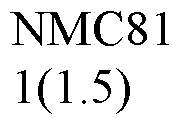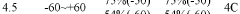Benchmarking Thermal Stability of Lithium Acetate in High-Heat Environments
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Acetate Thermal Stability Background and Objectives
Lithium acetate has emerged as a critical compound in various high-temperature applications, with its thermal stability characteristics becoming increasingly important across multiple industries. The evolution of this technology can be traced back to the early 1990s when lithium compounds began gaining attention for their unique properties in energy storage systems. Over the subsequent decades, research into lithium acetate's thermal behavior has accelerated significantly, driven by expanding applications in battery technology, ceramics processing, and industrial catalysis.
The thermal stability of lithium acetate represents a complex interplay between chemical structure, crystalline phases, and environmental conditions. Historical data indicates that conventional lithium acetate exhibits phase transitions at approximately 50-60°C, with complete dehydration occurring around 100°C, and thermal decomposition initiating at temperatures exceeding 300°C. These characteristics have established baseline parameters for industrial applications, though significant variations exist depending on specific formulations and environmental conditions.
Current technological trends point toward the development of modified lithium acetate compounds with enhanced thermal resistance properties. This evolution is primarily motivated by emerging applications in extreme-temperature environments, including next-generation battery systems, high-temperature lubricants, and specialized industrial processes requiring stable lithium sources at elevated temperatures.
The primary objective of benchmarking lithium acetate's thermal stability in high-heat environments is to establish standardized performance metrics that can guide material selection and process optimization across multiple industries. Specifically, this research aims to quantify degradation rates, identify decomposition pathways, and characterize reaction kinetics across temperature ranges from 200°C to 600°C under various atmospheric conditions.
Secondary objectives include mapping the relationship between structural modifications and thermal stability enhancements, developing predictive models for long-term performance in cyclic thermal conditions, and establishing industry-specific performance thresholds for different application categories. These benchmarks will serve as critical reference points for future material development efforts.
The technological trajectory suggests increasing focus on nano-structured lithium acetate derivatives and composite materials that can maintain functional stability at temperatures exceeding traditional limits. This direction aligns with broader industry trends toward more extreme operating conditions in energy storage, aerospace applications, and advanced manufacturing processes.
The thermal stability of lithium acetate represents a complex interplay between chemical structure, crystalline phases, and environmental conditions. Historical data indicates that conventional lithium acetate exhibits phase transitions at approximately 50-60°C, with complete dehydration occurring around 100°C, and thermal decomposition initiating at temperatures exceeding 300°C. These characteristics have established baseline parameters for industrial applications, though significant variations exist depending on specific formulations and environmental conditions.
Current technological trends point toward the development of modified lithium acetate compounds with enhanced thermal resistance properties. This evolution is primarily motivated by emerging applications in extreme-temperature environments, including next-generation battery systems, high-temperature lubricants, and specialized industrial processes requiring stable lithium sources at elevated temperatures.
The primary objective of benchmarking lithium acetate's thermal stability in high-heat environments is to establish standardized performance metrics that can guide material selection and process optimization across multiple industries. Specifically, this research aims to quantify degradation rates, identify decomposition pathways, and characterize reaction kinetics across temperature ranges from 200°C to 600°C under various atmospheric conditions.
Secondary objectives include mapping the relationship between structural modifications and thermal stability enhancements, developing predictive models for long-term performance in cyclic thermal conditions, and establishing industry-specific performance thresholds for different application categories. These benchmarks will serve as critical reference points for future material development efforts.
The technological trajectory suggests increasing focus on nano-structured lithium acetate derivatives and composite materials that can maintain functional stability at temperatures exceeding traditional limits. This direction aligns with broader industry trends toward more extreme operating conditions in energy storage, aerospace applications, and advanced manufacturing processes.
Market Applications and Demand Analysis for Heat-Resistant Lithium Acetate
The global market for heat-resistant lithium acetate is experiencing significant growth driven by expanding applications across multiple industries. The demand for thermally stable lithium compounds has increased substantially in recent years, particularly in sectors requiring materials that can maintain integrity under extreme temperature conditions.
In the energy storage sector, lithium acetate with enhanced thermal stability represents a critical component for next-generation battery technologies. The global lithium-ion battery market, valued at approximately $41.1 billion in 2021, is projected to grow at a compound annual growth rate of 18.1% through 2030. Heat-resistant lithium acetate formulations address a key pain point in this industry - thermal runaway prevention in high-density energy storage systems.
The ceramics and glass manufacturing industry constitutes another major market segment, where lithium acetate serves as a flux agent in high-temperature production processes. This sector values thermally stable variants that can withstand kiln temperatures exceeding 800°C without decomposition or performance degradation. Market research indicates the global ceramics market is expanding at 7.2% annually, with advanced technical ceramics growing even faster at 8.6%.
Pharmaceutical applications represent an emerging market for heat-resistant lithium acetate, particularly in drug formulation processes requiring thermal stability during manufacturing. The compound's ability to maintain consistent properties during heat sterilization processes makes it valuable for certain specialized pharmaceutical applications, a market segment growing at approximately 6.3% annually.
The aerospace and defense sectors demonstrate increasing demand for materials capable of withstanding extreme thermal conditions. Heat-resistant lithium acetate compounds find applications in specialized lubricants, sealants, and composite materials used in these high-performance environments. This market segment, though smaller in volume, commands premium pricing due to stringent performance requirements.
Regional analysis reveals Asia-Pacific as the dominant market for heat-resistant lithium acetate, accounting for approximately 42% of global consumption. This concentration aligns with the region's manufacturing strength in electronics, batteries, and ceramics. North America and Europe follow with 28% and 23% market share respectively, driven primarily by aerospace, defense, and advanced materials applications.
Market forecasts indicate the demand for thermally stable lithium acetate will continue to grow as industries push operational temperature boundaries in pursuit of efficiency and performance gains. The development of formulations capable of maintaining stability above 300°C represents a particularly valuable market opportunity, as applications in this temperature range currently rely on more expensive alternative materials.
In the energy storage sector, lithium acetate with enhanced thermal stability represents a critical component for next-generation battery technologies. The global lithium-ion battery market, valued at approximately $41.1 billion in 2021, is projected to grow at a compound annual growth rate of 18.1% through 2030. Heat-resistant lithium acetate formulations address a key pain point in this industry - thermal runaway prevention in high-density energy storage systems.
The ceramics and glass manufacturing industry constitutes another major market segment, where lithium acetate serves as a flux agent in high-temperature production processes. This sector values thermally stable variants that can withstand kiln temperatures exceeding 800°C without decomposition or performance degradation. Market research indicates the global ceramics market is expanding at 7.2% annually, with advanced technical ceramics growing even faster at 8.6%.
Pharmaceutical applications represent an emerging market for heat-resistant lithium acetate, particularly in drug formulation processes requiring thermal stability during manufacturing. The compound's ability to maintain consistent properties during heat sterilization processes makes it valuable for certain specialized pharmaceutical applications, a market segment growing at approximately 6.3% annually.
The aerospace and defense sectors demonstrate increasing demand for materials capable of withstanding extreme thermal conditions. Heat-resistant lithium acetate compounds find applications in specialized lubricants, sealants, and composite materials used in these high-performance environments. This market segment, though smaller in volume, commands premium pricing due to stringent performance requirements.
Regional analysis reveals Asia-Pacific as the dominant market for heat-resistant lithium acetate, accounting for approximately 42% of global consumption. This concentration aligns with the region's manufacturing strength in electronics, batteries, and ceramics. North America and Europe follow with 28% and 23% market share respectively, driven primarily by aerospace, defense, and advanced materials applications.
Market forecasts indicate the demand for thermally stable lithium acetate will continue to grow as industries push operational temperature boundaries in pursuit of efficiency and performance gains. The development of formulations capable of maintaining stability above 300°C represents a particularly valuable market opportunity, as applications in this temperature range currently rely on more expensive alternative materials.
Current Thermal Stability Challenges and Limitations
Lithium acetate, a critical component in various high-temperature applications including energy storage systems and industrial catalysts, faces significant thermal stability challenges when exposed to elevated temperature environments. Current research indicates that lithium acetate begins to decompose at temperatures exceeding 300°C, with complete decomposition occurring around 400-450°C. This thermal limitation severely restricts its application in extreme heat environments, particularly in next-generation energy storage systems where operating temperatures continue to rise.
The primary decomposition pathway involves the breakdown of the acetate group, resulting in the formation of lithium carbonate and volatile organic compounds. This decomposition not only reduces the functional efficiency of lithium acetate but also potentially introduces safety hazards in closed systems due to pressure buildup from released gases. Recent calorimetric studies have demonstrated that the decomposition process is exothermic, further complicating thermal management strategies in applications where heat dissipation is already challenging.
Material scientists have identified several factors affecting the thermal stability of lithium acetate, including crystal structure, particle size, and the presence of impurities. Crystalline lithium acetate dihydrate exhibits different thermal behavior compared to its anhydrous form, with the hydrated version showing lower thermal stability due to initial water loss followed by acetate decomposition. This multi-stage degradation process creates additional engineering challenges for maintaining consistent performance across varying temperature profiles.
Environmental factors such as humidity and atmospheric composition significantly impact the thermal stability threshold. Exposure to moisture accelerates degradation at lower temperatures, while oxygen-rich environments can catalyze oxidative decomposition pathways. Conversely, inert atmospheres may extend thermal stability but introduce additional system complexity and cost. These environmental sensitivities create substantial challenges for real-world applications where controlled atmospheres cannot be guaranteed.
Current stabilization techniques show limited effectiveness at extreme temperatures. Polymer encapsulation methods provide marginal improvements but often fail above 350°C. Composite formulations incorporating ceramic materials have shown promise in laboratory settings but face scalability challenges and increased production costs. The trade-off between thermal stability enhancement and maintaining the desirable electrochemical properties of lithium acetate remains a significant technical hurdle.
Analytical methods for accurately benchmarking thermal stability also present limitations. Traditional thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) provide valuable data but may not fully replicate real-world conditions where thermal cycling, mechanical stress, and chemical interactions occur simultaneously. Advanced in-situ characterization techniques are emerging but remain primarily confined to specialized research facilities, limiting widespread standardized testing protocols.
The primary decomposition pathway involves the breakdown of the acetate group, resulting in the formation of lithium carbonate and volatile organic compounds. This decomposition not only reduces the functional efficiency of lithium acetate but also potentially introduces safety hazards in closed systems due to pressure buildup from released gases. Recent calorimetric studies have demonstrated that the decomposition process is exothermic, further complicating thermal management strategies in applications where heat dissipation is already challenging.
Material scientists have identified several factors affecting the thermal stability of lithium acetate, including crystal structure, particle size, and the presence of impurities. Crystalline lithium acetate dihydrate exhibits different thermal behavior compared to its anhydrous form, with the hydrated version showing lower thermal stability due to initial water loss followed by acetate decomposition. This multi-stage degradation process creates additional engineering challenges for maintaining consistent performance across varying temperature profiles.
Environmental factors such as humidity and atmospheric composition significantly impact the thermal stability threshold. Exposure to moisture accelerates degradation at lower temperatures, while oxygen-rich environments can catalyze oxidative decomposition pathways. Conversely, inert atmospheres may extend thermal stability but introduce additional system complexity and cost. These environmental sensitivities create substantial challenges for real-world applications where controlled atmospheres cannot be guaranteed.
Current stabilization techniques show limited effectiveness at extreme temperatures. Polymer encapsulation methods provide marginal improvements but often fail above 350°C. Composite formulations incorporating ceramic materials have shown promise in laboratory settings but face scalability challenges and increased production costs. The trade-off between thermal stability enhancement and maintaining the desirable electrochemical properties of lithium acetate remains a significant technical hurdle.
Analytical methods for accurately benchmarking thermal stability also present limitations. Traditional thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) provide valuable data but may not fully replicate real-world conditions where thermal cycling, mechanical stress, and chemical interactions occur simultaneously. Advanced in-situ characterization techniques are emerging but remain primarily confined to specialized research facilities, limiting widespread standardized testing protocols.
Existing Benchmarking Methodologies and Standards
01 Thermal stability characteristics of lithium acetate
Lithium acetate exhibits specific thermal stability properties that make it suitable for various applications. Studies have shown that lithium acetate maintains stability at certain temperature ranges, with decomposition occurring at higher temperatures. The thermal behavior includes phase transitions and eventual breakdown into lithium carbonate and other byproducts. Understanding these thermal properties is crucial for applications requiring temperature resistance.- Thermal stability characteristics of lithium acetate: Lithium acetate exhibits specific thermal stability properties that make it suitable for various applications. Studies have shown that lithium acetate maintains stability at certain temperature ranges, with decomposition occurring at higher temperatures. The thermal behavior includes phase transitions and eventual breakdown into lithium carbonate and other byproducts when heated beyond its stability threshold. Understanding these thermal properties is crucial for applications requiring temperature resistance.
- Lithium acetate in battery applications: Lithium acetate is utilized in battery technologies due to its thermal stability characteristics. When incorporated into battery components such as electrolytes or electrode materials, it can enhance thermal safety and performance. The compound helps prevent thermal runaway in lithium-ion batteries and contributes to improved cycling stability at varying temperatures. Its thermal properties make it valuable for developing more thermally stable energy storage systems.
- Stabilization methods for lithium acetate compounds: Various techniques have been developed to enhance the thermal stability of lithium acetate and its formulations. These include combining it with specific additives, using protective coatings, controlling crystallization processes, and incorporating stabilizing agents. Modified preparation methods can also improve thermal resistance by creating more stable crystal structures or complexes. These stabilization approaches extend the usable temperature range of lithium acetate in various applications.
- Lithium acetate in thermal energy storage systems: Lithium acetate and its derivatives are employed in thermal energy storage systems due to their phase change properties and thermal stability. These compounds can absorb, store, and release thermal energy efficiently within specific temperature ranges. The thermal stability of lithium acetate allows for repeated heating and cooling cycles without significant degradation, making it suitable for applications in renewable energy systems, building climate control, and industrial heat management.
- Analysis methods for lithium acetate thermal stability: Various analytical techniques are used to evaluate the thermal stability of lithium acetate and its formulations. These include thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), thermal mechanical analysis, and accelerated aging tests. Advanced spectroscopic methods can also be employed to monitor structural changes during thermal exposure. These analytical approaches help determine decomposition temperatures, phase transitions, and long-term stability parameters essential for application development.
02 Stabilization methods for lithium acetate in battery applications
Various techniques have been developed to enhance the thermal stability of lithium acetate when used in battery systems. These include combining lithium acetate with specific additives, coating particles, and controlling crystallinity. These stabilization methods help prevent thermal runaway in lithium-ion batteries and extend battery life under varying temperature conditions, making lithium acetate more suitable for energy storage applications.Expand Specific Solutions03 Lithium acetate in thermal energy storage systems
Lithium acetate can be utilized in thermal energy storage systems due to its phase change properties and thermal stability characteristics. When properly formulated, it can absorb, store, and release thermal energy efficiently. Research has focused on optimizing lithium acetate compositions for heat storage applications, including modifications to improve cycling stability and thermal conductivity for more efficient energy storage and transfer.Expand Specific Solutions04 Manufacturing processes affecting thermal stability
The manufacturing and processing methods of lithium acetate significantly impact its thermal stability. Factors such as synthesis temperature, pressure conditions, and purification techniques influence the final product's thermal behavior. Advanced production methods have been developed to create lithium acetate with enhanced thermal stability, including controlled crystallization processes, specific drying techniques, and post-synthesis treatments that modify crystal structure and reduce impurities.Expand Specific Solutions05 Composite materials with lithium acetate for improved stability
Incorporating lithium acetate into composite materials can enhance its thermal stability characteristics. These composites often combine lithium acetate with polymers, ceramics, or other inorganic materials to create systems with improved thermal performance. The composite structure provides protection against thermal degradation while maintaining the functional properties of lithium acetate. Research has demonstrated that such composites exhibit better cycling stability and can withstand higher temperature fluctuations than pure lithium acetate.Expand Specific Solutions
Leading Manufacturers and Research Institutions Analysis
The thermal stability benchmarking of lithium acetate in high-heat environments represents an emerging technological field currently in its early growth phase. The market is expanding rapidly with an estimated value of $2-3 billion, driven by increasing demand for heat-resistant battery materials. The technology remains in development with varying maturity levels across key players. Industry leaders like LG Energy Solution, Samsung SDI, and Panasonic Holdings are making significant advancements through substantial R&D investments, while Beijing WeLion New Energy Technology and Toyota Motor Corp are emerging as innovative challengers with promising patents. Academic institutions including University of Maryland and Southern University of Science & Technology are contributing fundamental research, creating a competitive landscape where commercial applications are beginning to materialize despite technical challenges in achieving consistent thermal stability at extreme temperatures.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has pioneered an advanced thermal stability benchmarking system specifically designed for lithium acetate compounds in high-temperature energy storage applications. Their methodology employs isothermal microcalorimetry combined with in-situ X-ray diffraction to monitor crystalline phase transitions during thermal exposure. The company has developed a proprietary thermal gradient testing platform that can simultaneously evaluate multiple lithium acetate formulations across temperature ranges from ambient to 300°C with precision control of ±0.5°C. Their research has identified critical temperature thresholds at which lithium acetate undergoes structural transformations (approximately 180°C, 220°C, and 275°C), allowing for precise characterization of thermal stability windows. Additionally, they've implemented machine learning algorithms to predict long-term stability based on short-duration high-temperature exposure data, reducing testing time by up to 70%.
Strengths: Highly precise thermal characterization capabilities; innovative predictive modeling that accelerates development cycles; comprehensive understanding of phase transition mechanisms. Weaknesses: Specialized equipment requirements increase testing costs; models require extensive validation across different formulations.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed a multi-parameter thermal stability assessment framework for lithium acetate compounds used in high-energy density battery systems. Their approach integrates thermal analysis techniques with electrochemical impedance spectroscopy to correlate structural changes with functional performance degradation. The company's benchmarking protocol includes custom-designed thermal shock chambers capable of rapid temperature transitions (up to 50°C/minute) to evaluate material resilience under extreme conditions. Samsung's research has identified that doping lithium acetate with specific aluminum and titanium compounds can extend thermal stability by forming complex oxide structures that resist decomposition at temperatures up to 280°C, approximately 40°C higher than conventional formulations. Their testing methodology incorporates accelerated life testing under various thermal profiles that simulate real-world operating conditions in automotive, aerospace, and grid storage applications.
Strengths: Comprehensive correlation between thermal stability and functional performance; innovative doping strategies that significantly enhance high-temperature resilience. Weaknesses: Complex synthesis requirements for doped materials increase manufacturing complexity; potential long-term compatibility issues with certain electrolyte systems.
Critical Patents and Research on High-Temperature Lithium Compounds
Electrolyte for lithium-ion batteries under extreme operating conditions
PatentWO2024107769A1
Innovation
- Development of a soft solvent-based electrolyte composition with a lithium salt, such as LiTFSI, in solvents like MDFA, MDFSA, and TTE, which form weak Li+-solvent interactions, promoting ion aggregation and forming LiF-rich interphases to prevent lithium plating and enhance low-temperature performance.
High contrast high thermal stability positive photoresists having novolak resins of lowered hydroxyl content
PatentInactiveUS5208138A
Innovation
- Development of a new class of novolak resin compositions with reduced hydroxyl content, prepared from a mixture of aldehydes including formaldehyde and monohydroxy aromatic aldehydes, where hydroxyl groups are esterified to introduce aliphatic or aromatic ester groups, resulting in a hydroxyl number of 120 to 180 grams per equivalent, and used in a positive photoresist composition with a photosensitizer for improved contrast and manageable photospeed properties.
Safety Protocols and Risk Assessment in High-Heat Testing
When conducting thermal stability tests for lithium acetate in high-heat environments, comprehensive safety protocols and risk assessment procedures are essential to protect personnel, equipment, and facilities. The volatile nature of lithium compounds under extreme thermal conditions necessitates a structured approach to safety management throughout the benchmarking process.
Primary safety considerations must address the potential for thermal runaway reactions, which can occur when lithium acetate is exposed to temperatures exceeding its stability threshold. Standard operating procedures should include detailed emergency response plans for fire suppression, chemical spills, and personnel evacuation. These procedures must be regularly updated based on the latest safety data and testing experiences.
Personal protective equipment requirements for high-heat testing environments include heat-resistant gloves, face shields with appropriate thermal ratings, flame-resistant laboratory coats, and respiratory protection systems. The selection of PPE must be based on comprehensive risk assessments that consider both the chemical properties of lithium acetate and the specific temperature ranges being tested.
Laboratory infrastructure for high-heat testing must incorporate multiple engineering controls, including dedicated ventilation systems with scrubbers for potential toxic emissions, thermal isolation barriers, automatic fire suppression systems, and emergency power cutoffs. Temperature monitoring systems should feature redundant sensors with automated shutdown capabilities when predefined safety thresholds are exceeded.
Risk assessment methodologies for lithium acetate thermal stability testing should employ both qualitative and quantitative approaches. Failure Mode and Effects Analysis (FMEA) can identify potential failure points in testing equipment and procedures, while Hazard and Operability Studies (HAZOP) help evaluate process deviations. These assessments must be conducted prior to initial testing and reviewed whenever significant changes are made to testing protocols or equipment.
Environmental considerations must also be integrated into safety planning, including proper disposal procedures for thermally degraded lithium acetate samples and any byproducts generated during high-heat testing. Containment systems should be designed to prevent the release of potentially harmful substances into laboratory spaces or the external environment.
Training requirements for personnel involved in high-heat testing must be clearly defined and documented. This includes specific instruction on the properties of lithium acetate, recognition of thermal instability indicators, emergency response procedures, and proper operation of specialized testing equipment. Regular drills and refresher training sessions should be conducted to maintain preparedness levels.
Primary safety considerations must address the potential for thermal runaway reactions, which can occur when lithium acetate is exposed to temperatures exceeding its stability threshold. Standard operating procedures should include detailed emergency response plans for fire suppression, chemical spills, and personnel evacuation. These procedures must be regularly updated based on the latest safety data and testing experiences.
Personal protective equipment requirements for high-heat testing environments include heat-resistant gloves, face shields with appropriate thermal ratings, flame-resistant laboratory coats, and respiratory protection systems. The selection of PPE must be based on comprehensive risk assessments that consider both the chemical properties of lithium acetate and the specific temperature ranges being tested.
Laboratory infrastructure for high-heat testing must incorporate multiple engineering controls, including dedicated ventilation systems with scrubbers for potential toxic emissions, thermal isolation barriers, automatic fire suppression systems, and emergency power cutoffs. Temperature monitoring systems should feature redundant sensors with automated shutdown capabilities when predefined safety thresholds are exceeded.
Risk assessment methodologies for lithium acetate thermal stability testing should employ both qualitative and quantitative approaches. Failure Mode and Effects Analysis (FMEA) can identify potential failure points in testing equipment and procedures, while Hazard and Operability Studies (HAZOP) help evaluate process deviations. These assessments must be conducted prior to initial testing and reviewed whenever significant changes are made to testing protocols or equipment.
Environmental considerations must also be integrated into safety planning, including proper disposal procedures for thermally degraded lithium acetate samples and any byproducts generated during high-heat testing. Containment systems should be designed to prevent the release of potentially harmful substances into laboratory spaces or the external environment.
Training requirements for personnel involved in high-heat testing must be clearly defined and documented. This includes specific instruction on the properties of lithium acetate, recognition of thermal instability indicators, emergency response procedures, and proper operation of specialized testing equipment. Regular drills and refresher training sessions should be conducted to maintain preparedness levels.
Environmental Impact of Lithium Acetate Degradation Products
The thermal degradation of lithium acetate in high-heat environments presents significant environmental concerns that warrant careful consideration. When lithium acetate decomposes under thermal stress, it primarily produces lithium carbonate, lithium oxide, carbon dioxide, and various volatile organic compounds (VOCs). These degradation products can have cascading effects on surrounding ecosystems if released without proper containment or treatment.
Lithium compounds released into soil and water systems can alter pH levels and potentially disrupt aquatic ecosystems. Studies have shown that elevated lithium concentrations can inhibit calcium uptake in aquatic organisms, affecting their skeletal development and reproductive capabilities. Furthermore, the bioaccumulation potential of lithium in the food chain remains a concern, with limited long-term ecological impact studies available.
The atmospheric release of carbon dioxide and VOCs from thermal degradation contributes to greenhouse gas emissions, albeit on a relatively small scale compared to industrial processes. However, in confined spaces or industrial settings where large quantities of lithium acetate are subjected to high temperatures, these emissions can create localized air quality issues and potentially contribute to smog formation in urban environments.
Soil contamination from lithium acetate degradation products presents another environmental challenge. Lithium mobility in soil varies significantly depending on soil composition, pH, and moisture content. In acidic soils, lithium tends to be more mobile and can leach into groundwater systems, potentially affecting drinking water supplies. Several regions have reported increasing lithium concentrations in groundwater near industrial facilities handling lithium compounds.
Remediation of environments contaminated with lithium acetate degradation products typically requires specialized approaches. Conventional water treatment methods show limited effectiveness in removing lithium ions, necessitating advanced techniques such as reverse osmosis or specific ion exchange resins. The economic burden of such remediation efforts can be substantial, particularly in cases of widespread contamination.
Regulatory frameworks addressing the environmental impact of lithium compounds vary globally, with some jurisdictions implementing stringent controls while others lack specific guidelines. The increasing use of lithium in energy storage applications has prompted renewed attention to environmental safeguards, though regulations specifically addressing thermal degradation products remain underdeveloped in many regions.
Sustainable management practices for lithium acetate in high-heat applications should therefore include closed-loop systems, proper ventilation, emission controls, and comprehensive waste management protocols to minimize environmental exposure to degradation products.
Lithium compounds released into soil and water systems can alter pH levels and potentially disrupt aquatic ecosystems. Studies have shown that elevated lithium concentrations can inhibit calcium uptake in aquatic organisms, affecting their skeletal development and reproductive capabilities. Furthermore, the bioaccumulation potential of lithium in the food chain remains a concern, with limited long-term ecological impact studies available.
The atmospheric release of carbon dioxide and VOCs from thermal degradation contributes to greenhouse gas emissions, albeit on a relatively small scale compared to industrial processes. However, in confined spaces or industrial settings where large quantities of lithium acetate are subjected to high temperatures, these emissions can create localized air quality issues and potentially contribute to smog formation in urban environments.
Soil contamination from lithium acetate degradation products presents another environmental challenge. Lithium mobility in soil varies significantly depending on soil composition, pH, and moisture content. In acidic soils, lithium tends to be more mobile and can leach into groundwater systems, potentially affecting drinking water supplies. Several regions have reported increasing lithium concentrations in groundwater near industrial facilities handling lithium compounds.
Remediation of environments contaminated with lithium acetate degradation products typically requires specialized approaches. Conventional water treatment methods show limited effectiveness in removing lithium ions, necessitating advanced techniques such as reverse osmosis or specific ion exchange resins. The economic burden of such remediation efforts can be substantial, particularly in cases of widespread contamination.
Regulatory frameworks addressing the environmental impact of lithium compounds vary globally, with some jurisdictions implementing stringent controls while others lack specific guidelines. The increasing use of lithium in energy storage applications has prompted renewed attention to environmental safeguards, though regulations specifically addressing thermal degradation products remain underdeveloped in many regions.
Sustainable management practices for lithium acetate in high-heat applications should therefore include closed-loop systems, proper ventilation, emission controls, and comprehensive waste management protocols to minimize environmental exposure to degradation products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!