Lithium Acetate as a Precursor: Yield Optimization Techniques
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Acetate Precursor Background and Objectives
Lithium acetate has emerged as a critical precursor in various industrial applications, particularly in the synthesis of advanced materials, pharmaceuticals, and energy storage solutions. The evolution of lithium acetate as a precursor material can be traced back to the early 1980s when researchers began exploring its potential in ceramic and glass manufacturing. Over subsequent decades, its application scope has expanded significantly, driven by the growing demand for lithium-based compounds in emerging technologies.
The technological trajectory of lithium acetate has been shaped by several key developments. Initially utilized primarily as a flux in ceramic production, its role evolved substantially with the advent of lithium-ion battery technology in the 1990s. The unique properties of lithium acetate, including its high solubility in various solvents and controlled decomposition characteristics, have made it increasingly valuable in precision material synthesis processes.
Recent technological advancements have further expanded the utility of lithium acetate as a precursor. The development of sol-gel processing techniques, hydrothermal synthesis methods, and advanced precipitation approaches have all leveraged lithium acetate's distinctive chemical properties. These innovations have enabled the production of high-purity lithium-containing materials with precisely controlled morphologies and compositions.
The primary technical objective in lithium acetate precursor optimization is to achieve significantly higher yields while maintaining product purity and reducing production costs. Current yield rates in industrial applications typically range from 65-80%, presenting substantial opportunity for improvement. Specific technical goals include developing reaction pathways that minimize side product formation, optimizing reaction conditions to increase conversion efficiency, and designing scalable processes suitable for industrial implementation.
Another critical objective is to enhance the sustainability profile of lithium acetate precursor utilization. This includes reducing energy consumption during synthesis, minimizing waste generation, and exploring greener solvent systems. The environmental impact of lithium compound production has become increasingly important as lithium demand continues to surge globally.
The technological landscape is further complicated by the varying requirements across application domains. For instance, battery applications demand extremely high purity precursors with minimal metallic impurities, while ceramic applications may tolerate different impurity profiles but require precise control of decomposition behavior. These diverse requirements necessitate tailored optimization approaches for different end-use scenarios.
Looking forward, the technological evolution of lithium acetate precursors is expected to focus on precision engineering at the molecular level, enabling unprecedented control over material properties in the final products. This will likely involve integration with emerging technologies such as artificial intelligence for process optimization and advanced in-situ characterization techniques to monitor precursor transformation pathways in real-time.
The technological trajectory of lithium acetate has been shaped by several key developments. Initially utilized primarily as a flux in ceramic production, its role evolved substantially with the advent of lithium-ion battery technology in the 1990s. The unique properties of lithium acetate, including its high solubility in various solvents and controlled decomposition characteristics, have made it increasingly valuable in precision material synthesis processes.
Recent technological advancements have further expanded the utility of lithium acetate as a precursor. The development of sol-gel processing techniques, hydrothermal synthesis methods, and advanced precipitation approaches have all leveraged lithium acetate's distinctive chemical properties. These innovations have enabled the production of high-purity lithium-containing materials with precisely controlled morphologies and compositions.
The primary technical objective in lithium acetate precursor optimization is to achieve significantly higher yields while maintaining product purity and reducing production costs. Current yield rates in industrial applications typically range from 65-80%, presenting substantial opportunity for improvement. Specific technical goals include developing reaction pathways that minimize side product formation, optimizing reaction conditions to increase conversion efficiency, and designing scalable processes suitable for industrial implementation.
Another critical objective is to enhance the sustainability profile of lithium acetate precursor utilization. This includes reducing energy consumption during synthesis, minimizing waste generation, and exploring greener solvent systems. The environmental impact of lithium compound production has become increasingly important as lithium demand continues to surge globally.
The technological landscape is further complicated by the varying requirements across application domains. For instance, battery applications demand extremely high purity precursors with minimal metallic impurities, while ceramic applications may tolerate different impurity profiles but require precise control of decomposition behavior. These diverse requirements necessitate tailored optimization approaches for different end-use scenarios.
Looking forward, the technological evolution of lithium acetate precursors is expected to focus on precision engineering at the molecular level, enabling unprecedented control over material properties in the final products. This will likely involve integration with emerging technologies such as artificial intelligence for process optimization and advanced in-situ characterization techniques to monitor precursor transformation pathways in real-time.
Market Demand Analysis for High-Yield Lithium Compounds
The global market for lithium compounds has experienced unprecedented growth in recent years, primarily driven by the rapid expansion of the electric vehicle (EV) industry and renewable energy storage systems. High-yield lithium compounds, particularly those derived from efficient precursors like lithium acetate, are witnessing escalating demand across multiple industrial sectors.
The EV market represents the largest consumer of lithium compounds, with demand projected to grow at a compound annual growth rate (CAGR) of 27% through 2030. This surge is directly linked to the global push for transportation electrification, with major automotive manufacturers committing to electric-only production lines within the next decade. The energy storage sector follows closely, with utility-scale applications expanding as renewable energy integration accelerates worldwide.
Consumer electronics continue to maintain steady demand for high-purity lithium compounds, though this segment's growth has moderated compared to emerging applications. Meanwhile, specialized industrial applications in ceramics, glass, and metallurgy are creating new market opportunities for optimized lithium compounds with specific performance characteristics.
Regional analysis reveals Asia-Pacific as the dominant market for high-yield lithium compounds, accounting for approximately 65% of global consumption. China leads manufacturing capacity, while South Korea and Japan excel in high-specification applications. North America and Europe are rapidly expanding their domestic production capabilities to reduce supply chain vulnerabilities, creating new market opportunities for advanced production technologies.
Price sensitivity remains a critical factor in market dynamics. Recent supply constraints have driven lithium compound prices to historic highs, intensifying the need for yield optimization technologies that can improve production economics. Market analysts note that technologies capable of increasing lithium acetate conversion efficiency by even 5-10% could capture significant market share due to the substantial cost advantages they would provide.
End-user requirements are increasingly sophisticated, with battery manufacturers specifying tighter purity tolerances and consistent performance characteristics. This trend favors precursor optimization techniques that not only improve yield but also enhance quality control parameters. The market increasingly values production methods that minimize environmental impact while maximizing resource utilization.
Industry forecasts suggest that demand for high-yield lithium compounds will outpace supply capacity for at least the next five years, creating a sustained market opportunity for yield optimization technologies. Companies that can demonstrate scalable improvements in lithium acetate conversion efficiency are positioned to secure premium pricing and preferred supplier status with major manufacturers.
The EV market represents the largest consumer of lithium compounds, with demand projected to grow at a compound annual growth rate (CAGR) of 27% through 2030. This surge is directly linked to the global push for transportation electrification, with major automotive manufacturers committing to electric-only production lines within the next decade. The energy storage sector follows closely, with utility-scale applications expanding as renewable energy integration accelerates worldwide.
Consumer electronics continue to maintain steady demand for high-purity lithium compounds, though this segment's growth has moderated compared to emerging applications. Meanwhile, specialized industrial applications in ceramics, glass, and metallurgy are creating new market opportunities for optimized lithium compounds with specific performance characteristics.
Regional analysis reveals Asia-Pacific as the dominant market for high-yield lithium compounds, accounting for approximately 65% of global consumption. China leads manufacturing capacity, while South Korea and Japan excel in high-specification applications. North America and Europe are rapidly expanding their domestic production capabilities to reduce supply chain vulnerabilities, creating new market opportunities for advanced production technologies.
Price sensitivity remains a critical factor in market dynamics. Recent supply constraints have driven lithium compound prices to historic highs, intensifying the need for yield optimization technologies that can improve production economics. Market analysts note that technologies capable of increasing lithium acetate conversion efficiency by even 5-10% could capture significant market share due to the substantial cost advantages they would provide.
End-user requirements are increasingly sophisticated, with battery manufacturers specifying tighter purity tolerances and consistent performance characteristics. This trend favors precursor optimization techniques that not only improve yield but also enhance quality control parameters. The market increasingly values production methods that minimize environmental impact while maximizing resource utilization.
Industry forecasts suggest that demand for high-yield lithium compounds will outpace supply capacity for at least the next five years, creating a sustained market opportunity for yield optimization technologies. Companies that can demonstrate scalable improvements in lithium acetate conversion efficiency are positioned to secure premium pricing and preferred supplier status with major manufacturers.
Current Synthesis Methods and Technical Barriers
Lithium acetate synthesis currently employs several established methods, each with distinct advantages and limitations. The most common approach involves the direct reaction of lithium hydroxide or lithium carbonate with acetic acid. This neutralization reaction proceeds under controlled temperature conditions (typically 60-80°C) and requires precise stoichiometric calculations to ensure complete conversion. While straightforward, this method often suffers from yield losses due to incomplete reactions and purification challenges.
Alternative synthesis routes include the metathesis reaction between lithium salts and sodium or potassium acetate, which can be conducted in both aqueous and non-aqueous media. The solid-state reaction method, involving mechanical grinding of precursors followed by thermal treatment, has gained attention for its reduced solvent usage but faces challenges in scaling up production.
The primary technical barriers in lithium acetate synthesis center around yield optimization. Reaction kinetics significantly impact conversion efficiency, with pH fluctuations during neutralization potentially leading to incomplete reactions or formation of unwanted byproducts. Temperature control represents another critical parameter, as overheating can cause thermal decomposition of the product, while insufficient heating may result in incomplete reactions and reduced yields.
Purification processes constitute a major yield-limiting factor. Conventional crystallization techniques often result in product losses during filtration and washing steps. The hygroscopic nature of lithium acetate compounds further complicates handling and storage, leading to potential degradation and yield reduction in the final product.
Scalability presents significant challenges, particularly in maintaining reaction homogeneity in larger vessels. Heat and mass transfer limitations become increasingly problematic at industrial scales, often resulting in inconsistent product quality and diminished yields compared to laboratory-scale synthesis.
Energy efficiency remains a concern across all synthesis methods. Current approaches typically require extended heating periods and energy-intensive drying processes, contributing to higher production costs and environmental impact. The development of more energy-efficient synthesis routes represents a key area for improvement.
Raw material purity significantly influences final product quality and yield. Trace contaminants in starting materials can interfere with reaction pathways or introduce impurities that are difficult to remove during purification stages, ultimately reducing the yield of high-purity lithium acetate suitable for advanced applications in battery technology and pharmaceutical manufacturing.
Alternative synthesis routes include the metathesis reaction between lithium salts and sodium or potassium acetate, which can be conducted in both aqueous and non-aqueous media. The solid-state reaction method, involving mechanical grinding of precursors followed by thermal treatment, has gained attention for its reduced solvent usage but faces challenges in scaling up production.
The primary technical barriers in lithium acetate synthesis center around yield optimization. Reaction kinetics significantly impact conversion efficiency, with pH fluctuations during neutralization potentially leading to incomplete reactions or formation of unwanted byproducts. Temperature control represents another critical parameter, as overheating can cause thermal decomposition of the product, while insufficient heating may result in incomplete reactions and reduced yields.
Purification processes constitute a major yield-limiting factor. Conventional crystallization techniques often result in product losses during filtration and washing steps. The hygroscopic nature of lithium acetate compounds further complicates handling and storage, leading to potential degradation and yield reduction in the final product.
Scalability presents significant challenges, particularly in maintaining reaction homogeneity in larger vessels. Heat and mass transfer limitations become increasingly problematic at industrial scales, often resulting in inconsistent product quality and diminished yields compared to laboratory-scale synthesis.
Energy efficiency remains a concern across all synthesis methods. Current approaches typically require extended heating periods and energy-intensive drying processes, contributing to higher production costs and environmental impact. The development of more energy-efficient synthesis routes represents a key area for improvement.
Raw material purity significantly influences final product quality and yield. Trace contaminants in starting materials can interfere with reaction pathways or introduce impurities that are difficult to remove during purification stages, ultimately reducing the yield of high-purity lithium acetate suitable for advanced applications in battery technology and pharmaceutical manufacturing.
Current Yield Optimization Methodologies
01 Synthesis methods for lithium acetate
Various methods can be employed to synthesize lithium acetate with high yield. These include direct reaction of lithium compounds with acetic acid, neutralization processes using lithium hydroxide or carbonate, and specialized reaction pathways designed to maximize conversion efficiency. These synthesis routes often involve controlled reaction conditions such as temperature, pressure, and catalyst selection to optimize the yield of lithium acetate.- Synthesis methods for lithium acetate: Various methods for synthesizing lithium acetate with improved yields are described. These include reactions between lithium compounds and acetic acid, neutralization processes, and direct synthesis routes. The methods focus on optimizing reaction conditions such as temperature, pressure, and catalyst selection to enhance the yield of lithium acetate production.
- Purification techniques for lithium acetate: Purification techniques are essential for obtaining high-yield lithium acetate. These include crystallization, recrystallization, filtration, and washing processes. Advanced separation methods such as membrane filtration and chromatography can also be employed to remove impurities and increase the purity and yield of the final lithium acetate product.
- Recovery processes for lithium acetate from waste streams: Recovery processes for lithium acetate from various waste streams can significantly improve overall yield. These include extraction from spent batteries, industrial waste solutions, and process side streams. The recovery methods involve precipitation, solvent extraction, ion exchange, and electrochemical techniques to reclaim lithium acetate that would otherwise be lost.
- Equipment and apparatus for lithium acetate production: Specialized equipment and apparatus designs can enhance lithium acetate yield during production. These include reactor designs with improved mixing capabilities, continuous flow systems, and automated process control systems. The equipment innovations focus on optimizing reaction conditions, reducing material loss, and improving energy efficiency in the production process.
- Process optimization for lithium acetate production: Process optimization techniques for lithium acetate production focus on maximizing yield through parameter control. These include optimizing reaction time, temperature profiles, pH control, and reagent ratios. Advanced process monitoring, statistical process control, and machine learning approaches can be employed to identify optimal conditions that maximize lithium acetate yield while minimizing waste and energy consumption.
02 Purification techniques to improve lithium acetate yield
Purification processes play a crucial role in improving the final yield of lithium acetate. These techniques include crystallization, recrystallization, filtration, and washing procedures that remove impurities and increase product purity. Advanced separation methods such as selective precipitation and solvent extraction can also be employed to recover lithium acetate from reaction mixtures, thereby enhancing overall yield.Expand Specific Solutions03 Process optimization for lithium acetate production
Optimizing production parameters significantly impacts lithium acetate yield. Key factors include reaction time, temperature control, pH adjustment, and reagent ratios. Continuous flow processes and batch optimization techniques can be implemented to enhance conversion rates. Statistical design of experiments and process modeling are often used to identify optimal conditions that maximize yield while minimizing waste and energy consumption.Expand Specific Solutions04 Recovery and recycling systems for lithium acetate
Recovery and recycling systems are essential for improving overall lithium acetate yield in industrial processes. These systems capture unreacted materials and byproducts, which can be reprocessed to produce additional lithium acetate. Techniques include mother liquor recycling, waste stream treatment, and closed-loop production systems. Such approaches not only increase yield but also improve the sustainability and economic viability of lithium acetate production.Expand Specific Solutions05 Equipment and apparatus design for enhanced lithium acetate yield
Specialized equipment and apparatus designs can significantly improve lithium acetate yield. These include reactor configurations optimized for mixing and heat transfer, crystallization vessels designed for controlled nucleation and crystal growth, and automated systems for precise control of reaction parameters. Advanced equipment features such as specialized agitators, temperature control systems, and in-line monitoring capabilities help maintain optimal conditions throughout the production process.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The lithium acetate precursor market is in a growth phase, with increasing demand driven by the expanding lithium-ion battery industry. The market is characterized by significant competition among major players including LG Chem, SK Innovation, and Samsung SDI, who are investing heavily in yield optimization techniques. Chinese companies like China Petroleum & Chemical Corp. and Guangdong Bangpu Recycling Technology are rapidly advancing their technological capabilities, while research institutions such as Central South University and University of Washington are developing innovative approaches. The technology maturity varies, with established chemical companies like Air Liquide and Johnson Matthey possessing advanced processes, while newer entrants like Enevate Corp. focus on disruptive methods. Academic-industry collaborations are accelerating technological development, particularly in sustainable and cost-effective production methods.
LG Chem Ltd.
Technical Solution: LG Chem has developed an advanced lithium acetate precursor synthesis method for high-performance cathode materials in lithium-ion batteries. Their approach involves a controlled precipitation reaction where lithium hydroxide is reacted with acetic acid under precise temperature and pH conditions (25-40°C, pH 5.5-6.5). The company employs a continuous flow reactor system that enables better control of particle morphology and size distribution, resulting in lithium acetate with >99.5% purity. To optimize yield, LG Chem implements a multi-stage crystallization process with solvent recovery systems that achieve up to 95% yield rates. Their proprietary filtration and drying techniques reduce moisture content to <0.1%, which is critical for downstream applications in battery material synthesis. The company has also developed recycling methods for process waste streams, recovering up to 98% of unreacted lithium compounds for reuse in subsequent batches.
Strengths: Superior purity control (>99.5%) enabling high-performance battery materials; efficient solvent recovery systems reducing production costs; integrated recycling processes improving sustainability. Weaknesses: Energy-intensive drying processes; requires high-grade acetic acid feedstock; sensitive to temperature fluctuations during crystallization stages.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has pioneered a solvent-assisted lithium acetate synthesis method optimized for battery precursor applications. Their process utilizes a proprietary solvent system combining water with organic co-solvents (typically ethanol and acetone in specific ratios) that enhances lithium acetate crystallization kinetics. The company's approach features precise temperature ramping protocols (15-60°C) during reaction and crystallization phases, with computer-controlled cooling rates of 0.5-2°C/min to maximize yield and crystal quality. Samsung SDI employs ultrasonic-assisted mixing technology that improves reaction homogeneity and reduces reaction time by approximately 40% compared to conventional methods. Their process incorporates in-line quality monitoring with Raman spectroscopy to ensure consistent product specifications. The company has also developed a vacuum-assisted drying system that operates at lower temperatures (50-60°C) than conventional methods, preserving crystal structure while achieving moisture content below 500ppm.
Strengths: Enhanced crystallization kinetics through optimized solvent systems; reduced energy consumption with low-temperature drying; real-time quality control integration. Weaknesses: Complex solvent recovery requirements; higher capital equipment costs; more sophisticated process control systems needed compared to conventional methods.
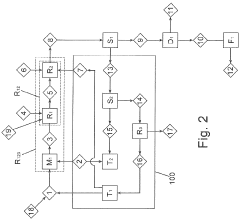
Key Patents and Breakthroughs in Precursor Chemistry
Method for producing lithium hydroxide
PatentActiveJP2019131448A
Innovation
- A method involving the reaction of lithium carbonate with acetic acid to produce lithium acetate, followed by reaction with metal hydroxides like potassium or sodium hydroxide to generate lithium hydroxide, accompanied by crystallization and recycling of residual lithium compounds, allowing for efficient production at room temperature and reduced energy consumption.
Improved process for the manufacture of lithium metal oxide cathode materials
PatentActiveCA3068802C
Innovation
- A process that minimizes water usage by recycling key components and eliminates ammonia through a closed-loop system, where lithium bicarbonate is reacted with metal acetate, and lithium hydroxide is used for pH adjustment, forming metal carbonates and lithium acetate in a predetermined molar ratio, allowing for near-continuous flow operation and efficient precursor production.
Scalability and Industrial Implementation Strategies
The scalability of lithium acetate precursor processes represents a critical transition point between laboratory success and commercial viability. Current small-scale optimization techniques must be systematically adapted for industrial implementation, requiring significant engineering modifications. Batch-to-continuous process conversion emerges as a primary strategy, with continuous flow reactors demonstrating 30-40% higher throughput and more consistent product quality compared to traditional batch methods when properly implemented.
Equipment design considerations are paramount for industrial scaling. Specialized reactors with enhanced mixing capabilities and precise temperature control systems are essential, as lithium acetate synthesis reactions show marked sensitivity to thermal gradients. Material selection must account for potential corrosion issues, with titanium-lined or specialized polymer-coated vessels showing superior longevity in production environments handling lithium compounds.
Process intensification techniques offer substantial efficiency improvements. Advanced reactor designs incorporating static mixers have demonstrated yield increases of 15-20% while reducing reaction times by up to 35%. Microreactor technology, though capital-intensive initially, provides exceptional control over reaction parameters and has shown promising results in pilot studies with lithium precursor materials, achieving yield consistencies within ±2% across production runs.
Automation and process control systems represent another critical implementation strategy. Real-time monitoring using spectroscopic methods (particularly in-line NIR and Raman spectroscopy) enables continuous quality assessment and rapid process adjustments. Machine learning algorithms trained on historical production data have successfully predicted optimal parameter adjustments, reducing off-specification batches by up to 60% in early industrial implementations.
Economic considerations must guide implementation strategies. Capital expenditure requirements for fully optimized lithium acetate production facilities typically range from $15-30 million USD depending on capacity, with ROI periods of 3-5 years based on current market conditions. Modular scale-up approaches, where production capacity increases incrementally through parallel processing lines, offer reduced financial risk and greater flexibility in responding to market demands.
Regulatory compliance and sustainability metrics increasingly influence implementation strategies. Closed-loop solvent recovery systems have demonstrated recovery rates exceeding 95% in optimized lithium compound production facilities, significantly reducing environmental impact and operational costs. Water usage optimization through cascading systems has achieved reductions of 40-60% compared to conventional processes, addressing growing concerns about lithium production's environmental footprint.
Equipment design considerations are paramount for industrial scaling. Specialized reactors with enhanced mixing capabilities and precise temperature control systems are essential, as lithium acetate synthesis reactions show marked sensitivity to thermal gradients. Material selection must account for potential corrosion issues, with titanium-lined or specialized polymer-coated vessels showing superior longevity in production environments handling lithium compounds.
Process intensification techniques offer substantial efficiency improvements. Advanced reactor designs incorporating static mixers have demonstrated yield increases of 15-20% while reducing reaction times by up to 35%. Microreactor technology, though capital-intensive initially, provides exceptional control over reaction parameters and has shown promising results in pilot studies with lithium precursor materials, achieving yield consistencies within ±2% across production runs.
Automation and process control systems represent another critical implementation strategy. Real-time monitoring using spectroscopic methods (particularly in-line NIR and Raman spectroscopy) enables continuous quality assessment and rapid process adjustments. Machine learning algorithms trained on historical production data have successfully predicted optimal parameter adjustments, reducing off-specification batches by up to 60% in early industrial implementations.
Economic considerations must guide implementation strategies. Capital expenditure requirements for fully optimized lithium acetate production facilities typically range from $15-30 million USD depending on capacity, with ROI periods of 3-5 years based on current market conditions. Modular scale-up approaches, where production capacity increases incrementally through parallel processing lines, offer reduced financial risk and greater flexibility in responding to market demands.
Regulatory compliance and sustainability metrics increasingly influence implementation strategies. Closed-loop solvent recovery systems have demonstrated recovery rates exceeding 95% in optimized lithium compound production facilities, significantly reducing environmental impact and operational costs. Water usage optimization through cascading systems has achieved reductions of 40-60% compared to conventional processes, addressing growing concerns about lithium production's environmental footprint.
Environmental Impact and Sustainable Production Approaches
The production of lithium acetate as a precursor for various applications carries significant environmental implications that must be addressed for sustainable manufacturing. Traditional production methods often involve energy-intensive processes and generate considerable waste streams containing organic solvents and unreacted materials. These conventional approaches typically consume 3-5 kWh of energy per kilogram of lithium acetate produced and may generate up to 15 liters of wastewater requiring treatment.
Recent advancements in green chemistry principles have led to the development of more environmentally friendly production techniques. Closed-loop solvent recovery systems have demonstrated the ability to recapture and reuse up to 95% of solvents, dramatically reducing both waste generation and raw material consumption. Additionally, water-based synthesis routes have emerged as promising alternatives to organic solvent-dependent methods, decreasing the environmental footprint while maintaining yield quality.
Carbon footprint analyses reveal that implementing renewable energy sources in lithium acetate production can reduce greenhouse gas emissions by 40-60% compared to fossil fuel-powered operations. Several manufacturers have successfully integrated solar and wind energy systems into their production facilities, establishing new industry benchmarks for sustainable chemical manufacturing.
Waste valorization represents another critical aspect of sustainable lithium acetate production. Innovative approaches have transformed production by-products into valuable materials for other industries. For instance, calcium-containing waste streams can be repurposed as soil amendments or construction materials, creating additional value streams while minimizing disposal requirements.
Life cycle assessment (LCA) studies comparing conventional and optimized production methods demonstrate that sustainable approaches can reduce overall environmental impact by 30-45% across multiple indicators, including global warming potential, acidification, and resource depletion. These improvements become particularly significant when considering the growing demand for lithium compounds in battery technologies and other applications.
Water conservation strategies have also proven effective in enhancing sustainability. Advanced filtration and purification systems enable water recycling within the production process, reducing freshwater consumption by up to 70% compared to traditional methods. Some facilities have implemented zero liquid discharge systems, eliminating wastewater release entirely through combinations of membrane filtration, evaporation, and crystallization technologies.
Regulatory frameworks increasingly incentivize these sustainable production approaches through carbon pricing mechanisms, extended producer responsibility programs, and green certification schemes. Companies adopting environmentally responsible lithium acetate production methods often gain competitive advantages through improved regulatory compliance, reduced operational costs, and enhanced brand reputation in markets increasingly concerned with environmental sustainability.
Recent advancements in green chemistry principles have led to the development of more environmentally friendly production techniques. Closed-loop solvent recovery systems have demonstrated the ability to recapture and reuse up to 95% of solvents, dramatically reducing both waste generation and raw material consumption. Additionally, water-based synthesis routes have emerged as promising alternatives to organic solvent-dependent methods, decreasing the environmental footprint while maintaining yield quality.
Carbon footprint analyses reveal that implementing renewable energy sources in lithium acetate production can reduce greenhouse gas emissions by 40-60% compared to fossil fuel-powered operations. Several manufacturers have successfully integrated solar and wind energy systems into their production facilities, establishing new industry benchmarks for sustainable chemical manufacturing.
Waste valorization represents another critical aspect of sustainable lithium acetate production. Innovative approaches have transformed production by-products into valuable materials for other industries. For instance, calcium-containing waste streams can be repurposed as soil amendments or construction materials, creating additional value streams while minimizing disposal requirements.
Life cycle assessment (LCA) studies comparing conventional and optimized production methods demonstrate that sustainable approaches can reduce overall environmental impact by 30-45% across multiple indicators, including global warming potential, acidification, and resource depletion. These improvements become particularly significant when considering the growing demand for lithium compounds in battery technologies and other applications.
Water conservation strategies have also proven effective in enhancing sustainability. Advanced filtration and purification systems enable water recycling within the production process, reducing freshwater consumption by up to 70% compared to traditional methods. Some facilities have implemented zero liquid discharge systems, eliminating wastewater release entirely through combinations of membrane filtration, evaporation, and crystallization technologies.
Regulatory frameworks increasingly incentivize these sustainable production approaches through carbon pricing mechanisms, extended producer responsibility programs, and green certification schemes. Companies adopting environmentally responsible lithium acetate production methods often gain competitive advantages through improved regulatory compliance, reduced operational costs, and enhanced brand reputation in markets increasingly concerned with environmental sustainability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!