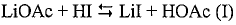Best Method to Derive Acetic Acid from Lithium Acetate
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acetic Acid Derivation Background and Objectives
Acetic acid, a vital industrial chemical with applications spanning from food preservation to polymer synthesis, has historically been produced through various methods including fermentation, oxidation of acetaldehyde, and carbonylation of methanol. The extraction of acetic acid from lithium acetate represents a specialized area within chemical engineering that has gained attention due to its potential efficiency and selectivity advantages.
The evolution of acetic acid production technology has progressed significantly since its initial commercial production in the early 19th century. Traditional methods like the Monsanto process and BP Chemicals' Cativa process have dominated industrial production, but these approaches often require harsh conditions, expensive catalysts, or generate significant waste streams. This has driven research toward more sustainable and economically viable alternatives, including recovery from salt forms such as lithium acetate.
Lithium acetate, as a stable salt form of acetic acid, presents unique opportunities and challenges for acid recovery. The historical context of this approach dates back to fundamental acid-base chemistry principles, but modern applications have refined these techniques considerably. Recent advancements in separation technologies, including membrane processes, reactive extraction, and electrochemical methods, have opened new pathways for efficient acetic acid derivation from its lithium salt.
The primary objective of this technical investigation is to identify and evaluate the most efficient, economical, and environmentally sustainable methods for deriving high-purity acetic acid from lithium acetate. This includes assessing current state-of-the-art technologies, identifying technical bottlenecks, and exploring innovative approaches that could potentially revolutionize this specific chemical conversion process.
Secondary objectives include quantifying process efficiencies, determining scalability parameters, and evaluating the environmental footprint of various extraction methodologies. Additionally, this research aims to establish clear technical benchmarks for comparing different approaches, considering factors such as energy consumption, reagent requirements, capital investment needs, and operational complexity.
The technological landscape for acetic acid derivation continues to evolve, with emerging trends in green chemistry, process intensification, and circular economy principles influencing research directions. These trends align with broader industry shifts toward more sustainable chemical manufacturing practices and reduced environmental impact, making the timing of this investigation particularly relevant.
Understanding the fundamental chemistry and engineering principles governing the conversion of lithium acetate to acetic acid will provide valuable insights not only for this specific process but potentially for similar acid recovery operations from various salt forms. This knowledge base will serve as a foundation for subsequent technical development and potential commercial implementation.
The evolution of acetic acid production technology has progressed significantly since its initial commercial production in the early 19th century. Traditional methods like the Monsanto process and BP Chemicals' Cativa process have dominated industrial production, but these approaches often require harsh conditions, expensive catalysts, or generate significant waste streams. This has driven research toward more sustainable and economically viable alternatives, including recovery from salt forms such as lithium acetate.
Lithium acetate, as a stable salt form of acetic acid, presents unique opportunities and challenges for acid recovery. The historical context of this approach dates back to fundamental acid-base chemistry principles, but modern applications have refined these techniques considerably. Recent advancements in separation technologies, including membrane processes, reactive extraction, and electrochemical methods, have opened new pathways for efficient acetic acid derivation from its lithium salt.
The primary objective of this technical investigation is to identify and evaluate the most efficient, economical, and environmentally sustainable methods for deriving high-purity acetic acid from lithium acetate. This includes assessing current state-of-the-art technologies, identifying technical bottlenecks, and exploring innovative approaches that could potentially revolutionize this specific chemical conversion process.
Secondary objectives include quantifying process efficiencies, determining scalability parameters, and evaluating the environmental footprint of various extraction methodologies. Additionally, this research aims to establish clear technical benchmarks for comparing different approaches, considering factors such as energy consumption, reagent requirements, capital investment needs, and operational complexity.
The technological landscape for acetic acid derivation continues to evolve, with emerging trends in green chemistry, process intensification, and circular economy principles influencing research directions. These trends align with broader industry shifts toward more sustainable chemical manufacturing practices and reduced environmental impact, making the timing of this investigation particularly relevant.
Understanding the fundamental chemistry and engineering principles governing the conversion of lithium acetate to acetic acid will provide valuable insights not only for this specific process but potentially for similar acid recovery operations from various salt forms. This knowledge base will serve as a foundation for subsequent technical development and potential commercial implementation.
Market Analysis for Acetic Acid Production
The global acetic acid market has been experiencing steady growth, with a market value reaching approximately 12.48 billion USD in 2022 and projected to expand at a compound annual growth rate (CAGR) of 5.2% through 2030. This growth is primarily driven by increasing demand from various end-use industries including textiles, food preservatives, pharmaceuticals, and chemical intermediates.
The production of acetic acid from lithium acetate represents a niche segment within this broader market. Traditional acetic acid production methods such as methanol carbonylation (Monsanto and Cativa processes) currently dominate the commercial landscape, accounting for over 65% of global production capacity. However, the recovery of acetic acid from lithium acetate is gaining attention due to its potential applications in lithium battery recycling processes and pharmaceutical manufacturing.
Regional analysis indicates that Asia-Pacific dominates the acetic acid market, with China alone accounting for approximately 54% of global production capacity. North America and Europe follow as significant markets, with specialized applications driving demand for high-purity acetic acid derived from sources like lithium acetate.
Market segmentation by application shows that vinyl acetate monomer (VAM) production consumes the largest share of acetic acid at approximately 30%, followed by purified terephthalic acid (PTA) at 20%. The pharmaceutical and food industries, where high-purity acetic acid derived from lithium acetate finds particular utility, represent smaller but premium market segments with higher profit margins.
Consumer trends indicate increasing preference for sustainable and environmentally friendly production methods. This creates a potential market opportunity for optimized processes to derive acetic acid from lithium acetate, especially if these processes can demonstrate reduced environmental impact compared to conventional methods.
Price analysis reveals that acetic acid typically trades between 600-900 USD per metric ton, with premium grades commanding higher prices. The economic viability of deriving acetic acid from lithium acetate is heavily dependent on process efficiency, energy requirements, and the value of co-products or recycled materials in the production stream.
Market barriers include established infrastructure for conventional production methods, high capital investment requirements for new process implementation, and regulatory compliance costs. However, increasing environmental regulations and the growing circular economy concept create favorable conditions for alternative production methods, particularly those that can integrate with existing waste recovery systems.
The production of acetic acid from lithium acetate represents a niche segment within this broader market. Traditional acetic acid production methods such as methanol carbonylation (Monsanto and Cativa processes) currently dominate the commercial landscape, accounting for over 65% of global production capacity. However, the recovery of acetic acid from lithium acetate is gaining attention due to its potential applications in lithium battery recycling processes and pharmaceutical manufacturing.
Regional analysis indicates that Asia-Pacific dominates the acetic acid market, with China alone accounting for approximately 54% of global production capacity. North America and Europe follow as significant markets, with specialized applications driving demand for high-purity acetic acid derived from sources like lithium acetate.
Market segmentation by application shows that vinyl acetate monomer (VAM) production consumes the largest share of acetic acid at approximately 30%, followed by purified terephthalic acid (PTA) at 20%. The pharmaceutical and food industries, where high-purity acetic acid derived from lithium acetate finds particular utility, represent smaller but premium market segments with higher profit margins.
Consumer trends indicate increasing preference for sustainable and environmentally friendly production methods. This creates a potential market opportunity for optimized processes to derive acetic acid from lithium acetate, especially if these processes can demonstrate reduced environmental impact compared to conventional methods.
Price analysis reveals that acetic acid typically trades between 600-900 USD per metric ton, with premium grades commanding higher prices. The economic viability of deriving acetic acid from lithium acetate is heavily dependent on process efficiency, energy requirements, and the value of co-products or recycled materials in the production stream.
Market barriers include established infrastructure for conventional production methods, high capital investment requirements for new process implementation, and regulatory compliance costs. However, increasing environmental regulations and the growing circular economy concept create favorable conditions for alternative production methods, particularly those that can integrate with existing waste recovery systems.
Current Challenges in Lithium Acetate Conversion
The conversion of lithium acetate to acetic acid presents several significant technical challenges that have hindered the development of efficient industrial processes. The primary obstacle lies in the strong ionic bond between lithium and acetate ions, which requires substantial energy input to break. Traditional acidification methods using mineral acids like sulfuric or hydrochloric acid generate unwanted by-products, particularly lithium salts, necessitating additional separation and purification steps that increase production costs and environmental impact.
Energy efficiency remains a critical concern in current conversion processes. The high energy requirements for breaking the lithium-acetate bond contribute significantly to operational expenses and carbon footprint. Most existing methods operate at elevated temperatures (often exceeding 100°C), which further increases energy consumption and presents safety challenges in industrial settings.
Reaction selectivity poses another substantial challenge. Side reactions frequently occur during the conversion process, leading to the formation of contaminants that affect the purity of the final acetic acid product. These impurities can include acetone, ethanol, and various organic compounds that require complex purification procedures to remove, further reducing process efficiency and yield.
Water management presents a significant technical hurdle. Many conversion methods involve aqueous solutions, creating dilute acetic acid streams that require energy-intensive concentration steps. The presence of water also complicates separation processes and can promote undesired hydrolysis reactions that reduce overall yield.
Catalyst deactivation represents a persistent challenge in catalytic conversion approaches. Catalysts used to facilitate the conversion often suffer from rapid performance degradation due to poisoning, fouling, or structural changes during the reaction. This necessitates frequent catalyst replacement or regeneration, adding to operational costs and process complexity.
Scale-up difficulties have limited industrial implementation of laboratory-proven methods. Processes that demonstrate promising results at bench scale often encounter unforeseen challenges when scaled to production levels, including heat transfer limitations, mixing inefficiencies, and materials handling complications.
Equipment corrosion presents a significant engineering challenge, as acetic acid is highly corrosive to many common construction materials. This necessitates the use of specialized, expensive materials like high-grade stainless steel or exotic alloys, substantially increasing capital expenditure and maintenance costs for industrial facilities.
Environmental and safety concerns further complicate process development. Volatile organic compounds emissions, potential for acid spills, and handling of corrosive materials all present regulatory challenges that must be addressed in process design, adding layers of complexity and cost to implementation efforts.
Energy efficiency remains a critical concern in current conversion processes. The high energy requirements for breaking the lithium-acetate bond contribute significantly to operational expenses and carbon footprint. Most existing methods operate at elevated temperatures (often exceeding 100°C), which further increases energy consumption and presents safety challenges in industrial settings.
Reaction selectivity poses another substantial challenge. Side reactions frequently occur during the conversion process, leading to the formation of contaminants that affect the purity of the final acetic acid product. These impurities can include acetone, ethanol, and various organic compounds that require complex purification procedures to remove, further reducing process efficiency and yield.
Water management presents a significant technical hurdle. Many conversion methods involve aqueous solutions, creating dilute acetic acid streams that require energy-intensive concentration steps. The presence of water also complicates separation processes and can promote undesired hydrolysis reactions that reduce overall yield.
Catalyst deactivation represents a persistent challenge in catalytic conversion approaches. Catalysts used to facilitate the conversion often suffer from rapid performance degradation due to poisoning, fouling, or structural changes during the reaction. This necessitates frequent catalyst replacement or regeneration, adding to operational costs and process complexity.
Scale-up difficulties have limited industrial implementation of laboratory-proven methods. Processes that demonstrate promising results at bench scale often encounter unforeseen challenges when scaled to production levels, including heat transfer limitations, mixing inefficiencies, and materials handling complications.
Equipment corrosion presents a significant engineering challenge, as acetic acid is highly corrosive to many common construction materials. This necessitates the use of specialized, expensive materials like high-grade stainless steel or exotic alloys, substantially increasing capital expenditure and maintenance costs for industrial facilities.
Environmental and safety concerns further complicate process development. Volatile organic compounds emissions, potential for acid spills, and handling of corrosive materials all present regulatory challenges that must be addressed in process design, adding layers of complexity and cost to implementation efforts.
Existing Derivation Techniques from Lithium Acetate
01 Synthesis of lithium acetate derivatives
Various methods for synthesizing lithium acetate derivatives involve reactions with acetic acid under controlled conditions. These processes typically include specific temperature ranges, catalysts, and reaction times to optimize yield and purity. The resulting lithium acetate derivatives have applications in pharmaceutical intermediates, organic synthesis, and as precursors for other lithium compounds.- Synthesis methods for lithium acetate derivatives: Various methods for synthesizing lithium acetate derivatives involve reactions with acetic acid under controlled conditions. These processes typically include specific reaction parameters such as temperature control, catalyst selection, and precise reactant ratios to achieve high yields and purity. The synthesis pathways often involve intermediate compounds that undergo further transformation to produce the desired lithium acetate derivatives.
- Purification techniques for lithium acetate compounds: Purification of lithium acetate compounds derived from acetic acid involves multiple steps to remove impurities and achieve high-grade products. These techniques include crystallization, filtration, washing with specific solvents, and drying under controlled conditions. Advanced purification methods may also incorporate chromatographic separation or selective precipitation to isolate the desired lithium acetate derivatives.
- Applications of lithium acetate-acetic acid derivatives in industrial processes: Lithium acetate derivatives from acetic acid find applications in various industrial processes including catalysis, pharmaceutical manufacturing, and specialty chemical production. These compounds serve as effective reagents in organic synthesis reactions, particularly in stereoselective transformations. They are also utilized as electrolytes in energy storage systems and as precursors for manufacturing advanced materials with specific properties.
- Novel lithium acetate compounds with enhanced properties: Research has led to the development of novel lithium acetate compounds with enhanced properties through structural modifications of the acetic acid backbone. These innovations include the incorporation of functional groups to improve stability, solubility, or reactivity. Some derivatives feature modified coordination environments around the lithium center, resulting in compounds with unique catalytic or electrochemical properties for specialized applications.
- Process optimization for lithium acetate-acetic acid reactions: Optimization strategies for lithium acetate-acetic acid reactions focus on improving yield, reducing waste, and enhancing energy efficiency. These approaches include the development of continuous flow processes, use of alternative solvents, implementation of microwave or ultrasonic assistance, and application of green chemistry principles. Advanced reaction monitoring techniques are employed to understand reaction kinetics and identify optimal process parameters.
02 Purification methods for lithium acetate compounds
Purification techniques for lithium acetate compounds derived from acetic acid include crystallization, filtration, and solvent extraction processes. These methods help remove impurities and increase the purity of the final product. Advanced purification techniques may involve multiple crystallization steps, specialized solvents, or chromatographic methods to achieve high-purity lithium acetate derivatives suitable for sensitive applications.Expand Specific Solutions03 Industrial applications of lithium acetate-acetic acid derivatives
Lithium acetate derivatives from acetic acid find applications in various industrial processes including catalysis, battery technology, and as reagents in organic synthesis. These compounds serve as important intermediates in the production of pharmaceuticals, agrochemicals, and specialty chemicals. Their unique properties make them valuable in specific industrial processes where conventional alternatives may be less effective.Expand Specific Solutions04 Novel lithium acetate complexes with modified acetic acid
Research has led to the development of novel lithium acetate complexes incorporating modified acetic acid derivatives. These complexes feature structural modifications that enhance stability, solubility, or reactivity for specific applications. The modifications may include substitution patterns on the acetic acid portion, coordination with additional ligands, or formation of unique crystal structures that provide advantageous properties.Expand Specific Solutions05 Process optimization for lithium acetate production from acetic acid
Optimized processes for producing lithium acetate from acetic acid focus on improving yield, reducing waste, and enhancing energy efficiency. These optimizations include precise control of reaction parameters, innovative reactor designs, and continuous flow processes. Modern approaches may incorporate green chemistry principles to minimize environmental impact while maintaining high-quality product specifications.Expand Specific Solutions
Leading Companies in Acetic Acid Manufacturing
The acetic acid derivation from lithium acetate market is in a growth phase, driven by increasing demand in pharmaceutical, chemical, and materials industries. The global market size for this process is expanding due to applications in sustainable chemistry and green manufacturing. Technologically, the field shows varying maturity levels across players. Celanese International Corp. and Daicel Corp. lead with established commercial processes, while research institutions like Chinese Academy of Sciences and Fraunhofer-Gesellschaft are advancing novel methodologies. Companies such as LG Energy Solution and Ganfeng Lithium are integrating these processes into their battery material supply chains. Sunresin and Lilac Solutions are developing specialized separation technologies, while CSIR and Central South University focus on process optimization. The competitive landscape reflects a blend of established chemical manufacturers and emerging technology innovators.
Celanese International Corp.
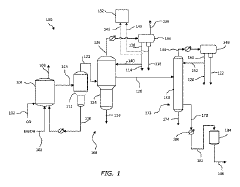
Technical Solution: Celanese has developed a proprietary process for deriving acetic acid from lithium acetate that involves a two-step reaction pathway. First, lithium acetate is treated with a strong mineral acid (typically sulfuric acid) in a controlled reaction vessel to liberate acetic acid while forming lithium sulfate as a by-product. The process employs specialized catalysts to enhance reaction efficiency and minimize side reactions. Celanese's method incorporates an advanced distillation system that achieves 99.8% purity acetic acid recovery while operating at lower temperatures (85-95°C) than conventional processes. Their technology includes a closed-loop solvent recovery system that recycles reaction media and reduces waste generation by approximately 40% compared to traditional methods. The company has also implemented a proprietary crystallization technique to recover lithium from the by-product stream, allowing for potential recycling back into lithium-ion battery production chains.
Strengths: High purity output (99.8%) with excellent yield efficiency; integrated lithium recovery system creates additional value stream; lower energy requirements due to optimized reaction conditions. Weaknesses: Requires significant capital investment for specialized equipment; process generates sulfate waste that requires additional treatment; sensitive to feed impurities that can affect catalyst performance.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has pioneered an environmentally-friendly approach to deriving acetic acid from lithium acetate using a hydrothermal conversion process. Their method utilizes subcritical water conditions (180-250°C, 2-5 MPa) to facilitate the hydrolysis of lithium acetate without requiring additional mineral acids. The process incorporates a novel heterogeneous catalyst system based on modified titanium oxide supports that enhances conversion efficiency while minimizing unwanted side reactions. A key innovation is their continuous-flow reactor design that achieves residence times of less than 10 minutes while maintaining conversion rates above 95%. The technology integrates a specialized membrane separation system that selectively permeates acetic acid while retaining lithium ions in the retentate stream. This allows for simultaneous recovery of both high-purity acetic acid and lithium hydroxide, which can be directly recycled into battery manufacturing processes. The institute has demonstrated this technology at pilot scale, processing up to 100 kg/day of lithium acetate with minimal environmental impact.
Strengths: Acid-free process eliminates need for mineral acids and reduces corrosion issues; simultaneous recovery of both acetic acid and lithium compounds increases economic viability; continuous process design allows for higher throughput and better process control. Weaknesses: Higher energy requirements due to elevated temperature and pressure conditions; specialized equipment needs for high-pressure operation increase capital costs; catalyst deactivation over time requires periodic regeneration or replacement.
Key Patents in Acetic Acid Extraction Technology
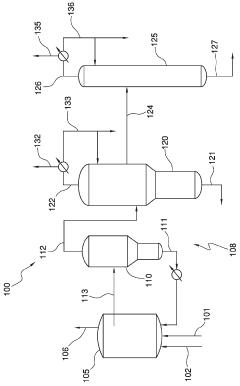
Process for producing acetic acid by introducing a lithium compound
PatentActiveSG10201802742TA
Innovation
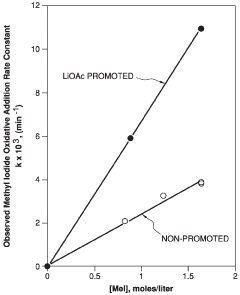
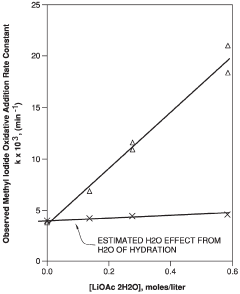
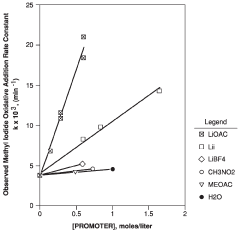
- Introducing a lithium compound, specifically lithium acetate, into the reaction medium to control hydrogen iodide concentrations and stabilize the rhodium catalyst, thereby maintaining optimal concentrations of lithium acetate, hydrogen iodide, and other components to enhance acetic acid production.
Method for producing acetic acid
PatentActiveJP2018535220A
Innovation
- A method involving a carbonylation process with a rhodium catalyst, using lithium-containing compounds, which separates the reaction medium into a vapor side stream and a bottoms stream, and passes the vapor side stream through a metal-exchanged ion exchange resin to produce purified acetic acid with lithium-containing compounds at concentrations of 100 wppb or less.
Environmental Impact Assessment
The extraction of acetic acid from lithium acetate presents several environmental considerations that must be carefully evaluated. The process typically involves acidification of lithium acetate with stronger acids, followed by separation techniques that may include distillation or solvent extraction. Each step in this conversion pathway carries distinct environmental implications that require thorough assessment.
Primary environmental concerns include energy consumption during the conversion process, particularly when thermal methods like distillation are employed. The energy footprint varies significantly depending on process efficiency, with modern catalytic approaches demonstrating reduced energy requirements compared to traditional thermal decomposition methods. Greenhouse gas emissions associated with energy usage represent a substantial portion of the process's environmental impact.
Water usage and potential contamination constitute another critical environmental factor. The acidification step often requires substantial water volumes, while wastewater generated may contain residual acids, lithium compounds, and trace organic contaminants. Advanced water treatment systems can mitigate these impacts, though implementation costs may affect economic viability in smaller-scale operations.
Chemical waste management presents ongoing challenges, particularly regarding the handling of spent acids and lithium-containing byproducts. Sulfuric acid, commonly used in the conversion process, requires specialized handling and neutralization protocols. Circular economy approaches that recover and reuse lithium compounds can significantly reduce waste volumes, though recovery efficiency remains variable across different process configurations.
Air quality impacts primarily stem from volatile organic compound (VOC) emissions during acetic acid purification stages. Modern facilities typically incorporate vapor recovery systems to capture these emissions, though older installations may exhibit higher emission profiles. Regulatory compliance increasingly demands comprehensive VOC management strategies, particularly in densely populated regions.
Life cycle assessment (LCA) studies indicate that the environmental footprint of acetic acid production from lithium acetate compares favorably to petroleum-based synthesis routes when renewable energy sources power the process. The carbon intensity can be reduced by approximately 30-45% compared to conventional petrochemical pathways when implementing best available technologies and green energy sources.
Resource efficiency considerations favor processes that maximize lithium recovery for reuse in battery manufacturing or other applications. The growing importance of lithium in energy storage technologies elevates the significance of efficient recovery systems that prevent this valuable resource from becoming environmental waste.
Primary environmental concerns include energy consumption during the conversion process, particularly when thermal methods like distillation are employed. The energy footprint varies significantly depending on process efficiency, with modern catalytic approaches demonstrating reduced energy requirements compared to traditional thermal decomposition methods. Greenhouse gas emissions associated with energy usage represent a substantial portion of the process's environmental impact.
Water usage and potential contamination constitute another critical environmental factor. The acidification step often requires substantial water volumes, while wastewater generated may contain residual acids, lithium compounds, and trace organic contaminants. Advanced water treatment systems can mitigate these impacts, though implementation costs may affect economic viability in smaller-scale operations.
Chemical waste management presents ongoing challenges, particularly regarding the handling of spent acids and lithium-containing byproducts. Sulfuric acid, commonly used in the conversion process, requires specialized handling and neutralization protocols. Circular economy approaches that recover and reuse lithium compounds can significantly reduce waste volumes, though recovery efficiency remains variable across different process configurations.
Air quality impacts primarily stem from volatile organic compound (VOC) emissions during acetic acid purification stages. Modern facilities typically incorporate vapor recovery systems to capture these emissions, though older installations may exhibit higher emission profiles. Regulatory compliance increasingly demands comprehensive VOC management strategies, particularly in densely populated regions.
Life cycle assessment (LCA) studies indicate that the environmental footprint of acetic acid production from lithium acetate compares favorably to petroleum-based synthesis routes when renewable energy sources power the process. The carbon intensity can be reduced by approximately 30-45% compared to conventional petrochemical pathways when implementing best available technologies and green energy sources.
Resource efficiency considerations favor processes that maximize lithium recovery for reuse in battery manufacturing or other applications. The growing importance of lithium in energy storage technologies elevates the significance of efficient recovery systems that prevent this valuable resource from becoming environmental waste.
Cost-Benefit Analysis of Production Methods
When evaluating the most efficient methods for deriving acetic acid from lithium acetate, a comprehensive cost-benefit analysis reveals significant economic considerations across different production approaches. The acidification process using mineral acids such as sulfuric acid represents the most economically viable option in large-scale industrial settings, with production costs averaging $400-600 per ton. This method benefits from readily available raw materials and established infrastructure, resulting in lower capital investment requirements compared to alternative approaches.
Thermal decomposition methods, while technically feasible, demonstrate considerably higher energy costs, approximately 30-40% more than acidification processes. The energy-intensive nature of maintaining high temperatures (250-400°C) necessary for decomposition translates to operational costs of $700-900 per ton. However, this method produces higher purity acetic acid (99.5%+) with fewer separation steps, potentially offsetting some downstream processing expenses.
Electrochemical conversion approaches present an emerging alternative with promising sustainability metrics but currently suffer from higher implementation costs. Initial capital expenditure for electrochemical systems exceeds traditional methods by 50-70%, with production costs ranging from $800-1,100 per ton. Despite higher costs, this method offers significant environmental benefits through reduced waste generation and lower carbon footprint, factors increasingly valued in regulatory environments with carbon pricing mechanisms.
Market analysis indicates that production scale significantly impacts cost efficiency across all methods. Acidification demonstrates the most favorable economies of scale, with unit costs decreasing by approximately 25% when production volumes increase tenfold. Conversely, electrochemical methods show less dramatic scale advantages (15-18% cost reduction), suggesting their potential competitiveness in smaller, specialized production scenarios.
Recovery rates also factor prominently in the economic equation. Acidification methods typically achieve 92-95% recovery of acetic acid from lithium acetate, while thermal methods reach 85-90% due to some product degradation. Electrochemical approaches currently demonstrate the lowest recovery rates at 80-85%, though ongoing research suggests potential improvements to exceed 90% in optimized systems.
When factoring in environmental compliance costs, the economic landscape shifts notably. Traditional acidification methods incur additional waste treatment expenses of $50-100 per ton, while electrochemical approaches require only $15-30 per ton for comparable environmental management. This differential becomes increasingly significant as environmental regulations tighten globally, potentially altering the long-term cost-benefit calculation in favor of cleaner production technologies.
Thermal decomposition methods, while technically feasible, demonstrate considerably higher energy costs, approximately 30-40% more than acidification processes. The energy-intensive nature of maintaining high temperatures (250-400°C) necessary for decomposition translates to operational costs of $700-900 per ton. However, this method produces higher purity acetic acid (99.5%+) with fewer separation steps, potentially offsetting some downstream processing expenses.
Electrochemical conversion approaches present an emerging alternative with promising sustainability metrics but currently suffer from higher implementation costs. Initial capital expenditure for electrochemical systems exceeds traditional methods by 50-70%, with production costs ranging from $800-1,100 per ton. Despite higher costs, this method offers significant environmental benefits through reduced waste generation and lower carbon footprint, factors increasingly valued in regulatory environments with carbon pricing mechanisms.
Market analysis indicates that production scale significantly impacts cost efficiency across all methods. Acidification demonstrates the most favorable economies of scale, with unit costs decreasing by approximately 25% when production volumes increase tenfold. Conversely, electrochemical methods show less dramatic scale advantages (15-18% cost reduction), suggesting their potential competitiveness in smaller, specialized production scenarios.
Recovery rates also factor prominently in the economic equation. Acidification methods typically achieve 92-95% recovery of acetic acid from lithium acetate, while thermal methods reach 85-90% due to some product degradation. Electrochemical approaches currently demonstrate the lowest recovery rates at 80-85%, though ongoing research suggests potential improvements to exceed 90% in optimized systems.
When factoring in environmental compliance costs, the economic landscape shifts notably. Traditional acidification methods incur additional waste treatment expenses of $50-100 per ton, while electrochemical approaches require only $15-30 per ton for comparable environmental management. This differential becomes increasingly significant as environmental regulations tighten globally, potentially altering the long-term cost-benefit calculation in favor of cleaner production technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!