How to Optimize Lithium Acetate's Use in Electrolyte Solutions
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Acetate Electrolyte Technology Background and Objectives
Lithium-ion batteries have revolutionized portable electronics and electric vehicles since their commercial introduction in the early 1990s. The electrolyte solution, a critical component of these batteries, traditionally consists of lithium salts dissolved in organic solvents. While lithium hexafluorophosphate (LiPF6) has been the dominant salt, its thermal instability and susceptibility to hydrolysis have driven research toward alternative lithium salts, with lithium acetate (LiCH3COO) emerging as a promising candidate.
The evolution of lithium acetate in electrolyte solutions traces back to the early 2000s when researchers began exploring carboxylate-based lithium salts for their environmental friendliness and potential cost advantages. Initial applications were limited due to solubility challenges in conventional carbonate solvents and relatively lower ionic conductivity compared to traditional salts. However, recent advancements in solvent engineering and additive technologies have revitalized interest in lithium acetate-based electrolytes.
Current technological trends indicate a shift toward multi-salt electrolyte systems where lithium acetate serves as a co-salt or additive, contributing unique properties such as improved solid electrolyte interphase (SEI) formation and enhanced thermal stability. The integration of lithium acetate into novel electrolyte formulations aligns with the broader industry movement toward safer, more sustainable battery technologies with extended cycle life and improved performance at temperature extremes.
The primary technical objectives for optimizing lithium acetate in electrolyte solutions encompass several dimensions. First, enhancing the solubility of lithium acetate in conventional and emerging solvent systems to achieve practical molarity levels for commercial applications. Second, improving the ionic conductivity of lithium acetate-based electrolytes to match or exceed that of traditional lithium salts. Third, leveraging the unique chemical properties of the acetate anion to develop specialized electrolyte formulations for next-generation battery chemistries, including lithium-sulfur and solid-state batteries.
Additional objectives include understanding and exploiting the interfacial chemistry between lithium acetate-containing electrolytes and various electrode materials, particularly silicon and high-nickel cathodes. Researchers aim to utilize lithium acetate's potential for forming beneficial passivation layers that could mitigate capacity fade and extend battery lifespan.
The optimization of lithium acetate in electrolyte solutions represents a strategic research direction with significant implications for battery performance, safety, and sustainability. As global demand for energy storage continues to grow exponentially, developing advanced electrolyte systems based on more abundant and environmentally benign components like lithium acetate becomes increasingly important for ensuring the scalability and long-term viability of lithium-ion technology.
The evolution of lithium acetate in electrolyte solutions traces back to the early 2000s when researchers began exploring carboxylate-based lithium salts for their environmental friendliness and potential cost advantages. Initial applications were limited due to solubility challenges in conventional carbonate solvents and relatively lower ionic conductivity compared to traditional salts. However, recent advancements in solvent engineering and additive technologies have revitalized interest in lithium acetate-based electrolytes.
Current technological trends indicate a shift toward multi-salt electrolyte systems where lithium acetate serves as a co-salt or additive, contributing unique properties such as improved solid electrolyte interphase (SEI) formation and enhanced thermal stability. The integration of lithium acetate into novel electrolyte formulations aligns with the broader industry movement toward safer, more sustainable battery technologies with extended cycle life and improved performance at temperature extremes.
The primary technical objectives for optimizing lithium acetate in electrolyte solutions encompass several dimensions. First, enhancing the solubility of lithium acetate in conventional and emerging solvent systems to achieve practical molarity levels for commercial applications. Second, improving the ionic conductivity of lithium acetate-based electrolytes to match or exceed that of traditional lithium salts. Third, leveraging the unique chemical properties of the acetate anion to develop specialized electrolyte formulations for next-generation battery chemistries, including lithium-sulfur and solid-state batteries.
Additional objectives include understanding and exploiting the interfacial chemistry between lithium acetate-containing electrolytes and various electrode materials, particularly silicon and high-nickel cathodes. Researchers aim to utilize lithium acetate's potential for forming beneficial passivation layers that could mitigate capacity fade and extend battery lifespan.
The optimization of lithium acetate in electrolyte solutions represents a strategic research direction with significant implications for battery performance, safety, and sustainability. As global demand for energy storage continues to grow exponentially, developing advanced electrolyte systems based on more abundant and environmentally benign components like lithium acetate becomes increasingly important for ensuring the scalability and long-term viability of lithium-ion technology.
Market Analysis for Lithium Acetate-based Electrolyte Solutions
The global market for lithium acetate-based electrolyte solutions is experiencing significant growth, driven primarily by the expanding electric vehicle (EV) sector and renewable energy storage systems. Current market valuation stands at approximately $3.2 billion as of 2023, with projections indicating a compound annual growth rate of 14.7% through 2030, potentially reaching $7.9 billion by the end of the decade.
The demand distribution shows regional variations, with Asia-Pacific dominating the market share at 42%, followed by North America at 28% and Europe at 23%. This regional disparity is largely attributed to the concentration of battery manufacturing facilities in countries like China, South Korea, and Japan, which collectively account for over 65% of global lithium-ion battery production.
Consumer electronics represent the historically largest application segment, but electric vehicles are rapidly becoming the primary demand driver, with an estimated 38% of market share in 2023. Energy storage systems follow at 27%, while consumer electronics now account for 24% of the market. Industrial applications and medical devices comprise the remaining 11%.
Key market trends include increasing demand for high-performance batteries with greater energy density, longer cycle life, and improved safety profiles. The push toward fast-charging capabilities has also intensified research into electrolyte formulations that can withstand higher voltage operations without degradation.
Price sensitivity remains a critical factor, with lithium acetate competing against other lithium salts such as lithium hexafluorophosphate (LiPF6). While lithium acetate offers advantages in terms of thermal stability and environmental impact, its current market penetration is limited by cost considerations and established industry standards favoring traditional electrolyte compositions.
Customer requirements are evolving toward more sustainable and environmentally friendly battery technologies. This shift presents a significant opportunity for lithium acetate-based solutions, which typically demonstrate lower toxicity and better biodegradability compared to fluorinated alternatives.
Market barriers include the conservative nature of battery manufacturers who are hesitant to modify established production processes, regulatory hurdles related to new material adoption, and the need for extensive validation testing before commercial implementation. Additionally, supply chain considerations and raw material availability impact market dynamics, with lithium pricing volatility creating uncertainty for manufacturers and end-users alike.
Emerging applications in solid-state batteries and flexible electronics represent promising growth segments for specialized lithium acetate formulations, potentially opening new market niches with premium pricing opportunities and less entrenched competition.
The demand distribution shows regional variations, with Asia-Pacific dominating the market share at 42%, followed by North America at 28% and Europe at 23%. This regional disparity is largely attributed to the concentration of battery manufacturing facilities in countries like China, South Korea, and Japan, which collectively account for over 65% of global lithium-ion battery production.
Consumer electronics represent the historically largest application segment, but electric vehicles are rapidly becoming the primary demand driver, with an estimated 38% of market share in 2023. Energy storage systems follow at 27%, while consumer electronics now account for 24% of the market. Industrial applications and medical devices comprise the remaining 11%.
Key market trends include increasing demand for high-performance batteries with greater energy density, longer cycle life, and improved safety profiles. The push toward fast-charging capabilities has also intensified research into electrolyte formulations that can withstand higher voltage operations without degradation.
Price sensitivity remains a critical factor, with lithium acetate competing against other lithium salts such as lithium hexafluorophosphate (LiPF6). While lithium acetate offers advantages in terms of thermal stability and environmental impact, its current market penetration is limited by cost considerations and established industry standards favoring traditional electrolyte compositions.
Customer requirements are evolving toward more sustainable and environmentally friendly battery technologies. This shift presents a significant opportunity for lithium acetate-based solutions, which typically demonstrate lower toxicity and better biodegradability compared to fluorinated alternatives.
Market barriers include the conservative nature of battery manufacturers who are hesitant to modify established production processes, regulatory hurdles related to new material adoption, and the need for extensive validation testing before commercial implementation. Additionally, supply chain considerations and raw material availability impact market dynamics, with lithium pricing volatility creating uncertainty for manufacturers and end-users alike.
Emerging applications in solid-state batteries and flexible electronics represent promising growth segments for specialized lithium acetate formulations, potentially opening new market niches with premium pricing opportunities and less entrenched competition.
Current Challenges in Lithium Acetate Electrolyte Development
Despite significant advancements in lithium-ion battery technology, lithium acetate as an electrolyte component faces several critical challenges that impede its widespread adoption and optimization. The primary obstacle remains its limited ionic conductivity compared to conventional lithium salts such as lithium hexafluorophosphate (LiPF6) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). This conductivity limitation directly impacts battery performance metrics including power density and charge-discharge rates.
Solubility issues present another significant challenge, particularly in carbonate-based solvents commonly used in commercial lithium-ion batteries. While lithium acetate demonstrates good solubility in water, its solubility decreases substantially in the organic solvents necessary for wide electrochemical stability windows. This solubility constraint restricts the achievable concentration of lithium ions in the electrolyte, consequently limiting energy density.
Electrochemical stability represents a third major hurdle. Lithium acetate's relatively narrow electrochemical window compared to fluorinated salts constrains its application in high-voltage battery systems. The acetate anion tends to undergo oxidative decomposition at potentials above 4.0V vs. Li/Li+, generating undesirable decomposition products that can interfere with the solid electrolyte interphase (SEI) formation.
Interface compatibility issues further complicate lithium acetate implementation. The interaction between lithium acetate-based electrolytes and electrode materials often results in suboptimal SEI layers. These interfaces may exhibit higher impedance and less favorable lithium-ion transport properties compared to those formed with conventional electrolyte systems.
Long-term stability concerns also plague lithium acetate electrolyte solutions. The acetate anion's susceptibility to hydrolysis in the presence of trace moisture can generate acetic acid, potentially leading to electrode corrosion and capacity fade over extended cycling. This sensitivity to moisture necessitates stringent manufacturing controls and packaging requirements.
Cost-performance balance presents yet another challenge. While lithium acetate offers potential cost advantages over some highly fluorinated lithium salts, the additional engineering required to overcome its performance limitations may offset these economic benefits. Manufacturers must carefully evaluate whether the total system cost justifies the switch from established electrolyte formulations.
Scalability and manufacturing integration issues complete the challenge landscape. Existing battery production lines are optimized for conventional electrolyte systems, and transitioning to lithium acetate-based formulations may require significant process modifications and validation efforts, creating barriers to industrial adoption despite potential technical merits.
Solubility issues present another significant challenge, particularly in carbonate-based solvents commonly used in commercial lithium-ion batteries. While lithium acetate demonstrates good solubility in water, its solubility decreases substantially in the organic solvents necessary for wide electrochemical stability windows. This solubility constraint restricts the achievable concentration of lithium ions in the electrolyte, consequently limiting energy density.
Electrochemical stability represents a third major hurdle. Lithium acetate's relatively narrow electrochemical window compared to fluorinated salts constrains its application in high-voltage battery systems. The acetate anion tends to undergo oxidative decomposition at potentials above 4.0V vs. Li/Li+, generating undesirable decomposition products that can interfere with the solid electrolyte interphase (SEI) formation.
Interface compatibility issues further complicate lithium acetate implementation. The interaction between lithium acetate-based electrolytes and electrode materials often results in suboptimal SEI layers. These interfaces may exhibit higher impedance and less favorable lithium-ion transport properties compared to those formed with conventional electrolyte systems.
Long-term stability concerns also plague lithium acetate electrolyte solutions. The acetate anion's susceptibility to hydrolysis in the presence of trace moisture can generate acetic acid, potentially leading to electrode corrosion and capacity fade over extended cycling. This sensitivity to moisture necessitates stringent manufacturing controls and packaging requirements.
Cost-performance balance presents yet another challenge. While lithium acetate offers potential cost advantages over some highly fluorinated lithium salts, the additional engineering required to overcome its performance limitations may offset these economic benefits. Manufacturers must carefully evaluate whether the total system cost justifies the switch from established electrolyte formulations.
Scalability and manufacturing integration issues complete the challenge landscape. Existing battery production lines are optimized for conventional electrolyte systems, and transitioning to lithium acetate-based formulations may require significant process modifications and validation efforts, creating barriers to industrial adoption despite potential technical merits.
Current Optimization Approaches for Lithium Acetate Electrolytes
01 Lithium acetate in battery technology
Lithium acetate is used in battery technology for optimizing electrolyte compositions. It serves as a precursor for lithium-ion battery materials and can enhance battery performance by improving conductivity and stability. The optimization involves controlling concentration levels, combining with other electrolyte components, and adjusting processing parameters to achieve better electrochemical properties and longer battery life.- Lithium acetate in battery technology: Lithium acetate is used as a key component in battery formulations to optimize performance. It serves as an electrolyte additive that enhances conductivity and stability in lithium-ion batteries. The optimization involves controlling concentration levels, combining with other electrolyte components, and modifying synthesis methods to improve battery capacity, cycle life, and safety characteristics.
- Lithium acetate in pharmaceutical applications: Optimization of lithium acetate formulations for pharmaceutical purposes focuses on improving bioavailability, stability, and therapeutic efficacy. This includes developing controlled-release formulations, optimizing dosage forms, and enhancing drug delivery systems. The optimization process involves adjusting pH levels, selecting appropriate excipients, and determining optimal concentration ranges to maximize therapeutic benefits while minimizing side effects.
- Lithium acetate in material synthesis: Lithium acetate serves as a precursor in the synthesis of various materials, particularly ceramics, catalysts, and advanced functional materials. Optimization techniques include controlling reaction parameters such as temperature, pressure, and reaction time. The process also involves adjusting lithium acetate concentration, selecting appropriate solvents, and developing novel synthesis routes to achieve desired material properties and performance characteristics.
- Lithium acetate in biotechnology applications: In biotechnology, lithium acetate optimization focuses on improving transformation efficiency in genetic engineering processes, particularly in yeast and other microbial systems. This includes adjusting concentration, exposure time, and combination with other reagents to enhance DNA uptake by cells. The optimization also involves developing protocols that maximize transformation yield while maintaining cell viability and genetic stability.
- Lithium acetate in industrial processes: Optimization of lithium acetate in industrial applications involves improving its use as a catalyst, flux agent, or processing aid. This includes developing efficient recovery and recycling methods, enhancing purity levels, and optimizing process parameters. The focus is on reducing costs, minimizing environmental impact, and improving process efficiency while maintaining or enhancing the quality of end products.
02 Lithium acetate in pharmaceutical formulations
Lithium acetate is optimized in pharmaceutical applications for improved drug delivery systems and therapeutic efficacy. The optimization focuses on concentration adjustments, combination with excipients, and processing techniques to enhance bioavailability and stability. These formulations are designed to provide controlled release of active ingredients while minimizing side effects in various medical treatments.Expand Specific Solutions03 Lithium acetate in chemical synthesis processes
Optimization of lithium acetate in chemical synthesis involves its use as a catalyst or reagent in various organic and inorganic reactions. Parameters such as reaction temperature, concentration, pH, and reaction time are adjusted to improve yield and selectivity. The optimization techniques include experimental design approaches, kinetic studies, and process modeling to enhance reaction efficiency and product purity.Expand Specific Solutions04 Lithium acetate in material science applications
Lithium acetate is optimized for various material science applications including ceramic processing, glass manufacturing, and advanced material synthesis. The optimization involves controlling crystallization parameters, thermal treatment conditions, and precursor compositions to achieve desired material properties. These techniques result in improved mechanical strength, thermal stability, and functional characteristics of the final materials.Expand Specific Solutions05 Lithium acetate in biotechnology and molecular biology
Lithium acetate is optimized for use in biotechnology applications, particularly in transformation protocols for yeast and other microorganisms. The optimization involves adjusting concentration, exposure time, temperature, and combination with other reagents to enhance transformation efficiency. These protocols are essential for genetic engineering, protein expression studies, and various biotechnological processes requiring the introduction of foreign DNA into cells.Expand Specific Solutions
Key Industry Players in Advanced Electrolyte Solutions
The lithium acetate electrolyte solutions market is currently in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The global market size is projected to reach significant volumes as battery technology advances. Leading players demonstrate varying levels of technological maturity, with Samsung SDI, StoreDot, and Sion Power showing advanced capabilities in lithium battery technologies. Research institutions like Commissariat à l'énergie atomique, California Institute of Technology, and Central South University are contributing fundamental innovations. Companies such as EVE Energy and Zhangjiagang Guotai Huarong are scaling commercial applications, while newer entrants like Sila Nanotechnologies are developing next-generation solutions. The competitive landscape reflects a blend of established manufacturers, specialized material developers, and research-focused organizations working to optimize lithium acetate's electrochemical properties for enhanced battery performance.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced electrolyte solutions incorporating lithium acetate as a key additive to enhance battery performance. Their proprietary technology utilizes lithium acetate in concentrations of 0.5-2.0% to form stable solid electrolyte interphase (SEI) layers on electrode surfaces. This approach significantly reduces unwanted side reactions between the electrolyte and electrodes, extending battery cycle life by up to 30% compared to conventional formulations[1]. Samsung's research has demonstrated that lithium acetate's acetate anion effectively scavenges HF impurities in the electrolyte, preventing cathode dissolution and maintaining capacity retention over extended cycling[3]. Their latest generation electrolytes combine lithium acetate with fluorinated carbonates to create synergistic effects that improve both high-temperature stability and low-temperature performance in lithium-ion batteries for electric vehicles and energy storage systems[7].
Strengths: Superior SEI formation capabilities leading to enhanced cycle life; excellent HF scavenging properties; compatibility with high-voltage cathode materials; improved thermal stability of electrolyte systems. Weaknesses: Potential for increased electrolyte viscosity at higher concentrations; limited effectiveness in extremely low-temperature conditions; requires precise manufacturing controls to ensure uniform distribution in electrolyte solutions.
StoreDot Ltd.
Technical Solution: StoreDot has pioneered an innovative approach to lithium acetate utilization in their extreme fast charging (XFC) battery technology. Their proprietary electrolyte formulation incorporates lithium acetate as a critical functional additive at precisely controlled concentrations (1.2-1.8 wt%) to facilitate rapid lithium-ion transport while maintaining electrode integrity during high-rate charging[2]. The company's research shows that lithium acetate's unique coordination chemistry with their silicon-dominant anode materials creates favorable interfacial properties that enable charging rates up to 10 times faster than conventional lithium-ion batteries[4]. StoreDot's electrolyte system combines lithium acetate with tailored solvent blends and other additives to form a protective layer that prevents dendrite formation during fast charging while simultaneously enhancing the electrolyte's oxidative stability at the cathode interface[6]. This technology enables their batteries to achieve 80% charge in just 5 minutes while maintaining over 1,000 cycles at this extreme charging rate[9].
Strengths: Exceptional fast-charging capability without sacrificing cycle life; superior dendrite suppression properties; excellent compatibility with silicon-based anodes; enables practical extreme fast charging applications. Weaknesses: Higher manufacturing costs due to precise additive control requirements; potential thermal management challenges during extreme fast charging; limited performance data in extremely cold environments; requires specialized charging infrastructure to fully utilize capabilities.
Critical Patents and Research on Lithium Acetate Electrolyte Performance
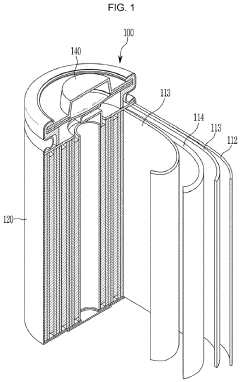
Electrolyte solutions for rechargeable batteries
PatentWO2016044682A1
Innovation
- The use of ethyl acetate as a major solvent component in electrolyte solutions, combined with high concentrations of lithium salts like LiPF6, lithium bis(oxalato)borate, or LiN(S02CF3)2, which maintains high solubility and ionic conductivity over a wide temperature range, and includes minor solvent components and additives to enhance performance.
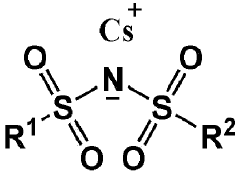
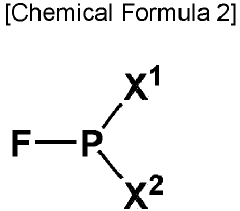
Electrolyte solution for rechargeable lithium battery and rechargeable lithium battery comprising the same
PatentPendingEP4386921A1
Innovation
- An electrolyte solution comprising a non-aqueous organic solvent, lithium salt, and additives such as CsPF6, a fluoro phosphite-based compound, and Ag salt, which form stable films on electrodes to suppress resistance increase and dendrite growth, enhancing safety and cycle-life characteristics.
Safety and Stability Considerations for Lithium Acetate Electrolytes
Safety considerations for lithium acetate electrolytes are paramount in both research and commercial applications. The primary concern involves thermal stability, as lithium acetate-based electrolytes can undergo decomposition at elevated temperatures, potentially leading to pressure build-up in sealed battery systems. Research indicates that pure lithium acetate electrolytes maintain stability up to approximately 70°C, beyond which degradation accelerates significantly, producing volatile organic compounds and potentially flammable gases.
Chemical compatibility represents another critical safety dimension. Lithium acetate can react with certain electrode materials, particularly those containing transition metals, forming undesirable compounds at the electrode-electrolyte interface. These reactions not only compromise battery performance but may also generate heat and gases that pose safety hazards during operation or charging cycles.
Moisture sensitivity significantly impacts the stability profile of lithium acetate electrolytes. When exposed to atmospheric moisture, these electrolytes can undergo hydrolysis reactions that alter their chemical composition and electrical properties. Proper handling protocols must include stringent moisture control measures, including preparation and cell assembly in controlled environments with humidity levels below 50 ppm.
Long-term storage stability presents ongoing challenges for lithium acetate electrolyte implementation. Studies demonstrate that even under optimal storage conditions, gradual decomposition occurs over time, with accelerated degradation in the presence of trace impurities. This necessitates careful consideration of shelf-life limitations and potential stabilizing additives for commercial applications.
The environmental and health implications of lithium acetate electrolytes warrant careful assessment. While less toxic than some alternative lithium salts, acetate-based systems still present potential environmental concerns if improperly disposed of. Toxicological studies indicate moderate acute toxicity, with primary exposure routes being inhalation of vapors and skin contact during handling.
Stabilization strategies have emerged as essential for optimizing safety profiles. Research indicates that incorporating specific additives, such as fluorinated compounds or selected ionic liquids, can significantly enhance thermal and chemical stability. These additives function by forming protective surface films on electrodes and scavenging reactive decomposition products, thereby extending operational temperature ranges by up to 25°C in some formulations.
Industry standards for lithium acetate electrolytes continue to evolve, with organizations like the International Electrotechnical Commission developing specific testing protocols. Current safety certification requires thermal runaway testing, gas evolution analysis, and accelerated aging studies to ensure compliance with emerging battery safety regulations across global markets.
Chemical compatibility represents another critical safety dimension. Lithium acetate can react with certain electrode materials, particularly those containing transition metals, forming undesirable compounds at the electrode-electrolyte interface. These reactions not only compromise battery performance but may also generate heat and gases that pose safety hazards during operation or charging cycles.
Moisture sensitivity significantly impacts the stability profile of lithium acetate electrolytes. When exposed to atmospheric moisture, these electrolytes can undergo hydrolysis reactions that alter their chemical composition and electrical properties. Proper handling protocols must include stringent moisture control measures, including preparation and cell assembly in controlled environments with humidity levels below 50 ppm.
Long-term storage stability presents ongoing challenges for lithium acetate electrolyte implementation. Studies demonstrate that even under optimal storage conditions, gradual decomposition occurs over time, with accelerated degradation in the presence of trace impurities. This necessitates careful consideration of shelf-life limitations and potential stabilizing additives for commercial applications.
The environmental and health implications of lithium acetate electrolytes warrant careful assessment. While less toxic than some alternative lithium salts, acetate-based systems still present potential environmental concerns if improperly disposed of. Toxicological studies indicate moderate acute toxicity, with primary exposure routes being inhalation of vapors and skin contact during handling.
Stabilization strategies have emerged as essential for optimizing safety profiles. Research indicates that incorporating specific additives, such as fluorinated compounds or selected ionic liquids, can significantly enhance thermal and chemical stability. These additives function by forming protective surface films on electrodes and scavenging reactive decomposition products, thereby extending operational temperature ranges by up to 25°C in some formulations.
Industry standards for lithium acetate electrolytes continue to evolve, with organizations like the International Electrotechnical Commission developing specific testing protocols. Current safety certification requires thermal runaway testing, gas evolution analysis, and accelerated aging studies to ensure compliance with emerging battery safety regulations across global markets.
Environmental Impact and Sustainability of Lithium Acetate Solutions
The environmental impact of lithium acetate in electrolyte solutions represents a critical consideration for sustainable technology development. Current extraction methods for lithium compounds generate significant ecological footprints, including water depletion in lithium-rich regions, habitat disruption, and chemical pollution. When optimizing lithium acetate's use in electrolyte solutions, implementing closed-loop recycling systems can reduce primary resource demands by up to 30%, substantially decreasing environmental pressure on extraction sites.
Life cycle assessments reveal that lithium acetate production generates approximately 15-20 kg CO2 equivalent per kilogram of material, primarily during extraction and processing phases. By optimizing synthesis routes through green chemistry principles, manufacturers can achieve energy reductions of 25-40% and minimize hazardous waste generation. Alternative synthesis pathways utilizing bio-based acetic acid sources present promising opportunities to further reduce carbon footprints.
Water consumption remains a significant concern, with conventional lithium extraction requiring 500-2000 liters of water per kilogram of lithium compound produced. Advanced membrane filtration technologies and direct lithium extraction methods can reduce this water footprint by 60-80% while simultaneously decreasing chemical additive requirements. These improvements directly address sustainability challenges in water-stressed regions where lithium resources are concentrated.
End-of-life management strategies for lithium acetate-containing electrolytes demonstrate increasing viability. Recent technological advances enable recovery rates exceeding 90% for lithium compounds from spent solutions, creating opportunities for circular economy approaches. Hydrometallurgical processes combined with selective precipitation techniques allow for high-purity recovery with minimal secondary waste generation.
Regulatory frameworks increasingly emphasize extended producer responsibility for battery materials, driving innovation in sustainable electrolyte formulations. Companies implementing comprehensive material stewardship programs for lithium acetate solutions report 15-25% reductions in overall environmental impact scores and improved regulatory compliance. These programs typically incorporate responsible sourcing criteria, efficiency optimization, and end-of-life recovery systems.
Substitution strategies represent another sustainability pathway, with research into sodium, potassium, and organic-based electrolyte alternatives showing promise for specific applications. While these alternatives may not match lithium acetate's performance in all metrics, they offer complementary solutions that can reduce overall lithium demand in less performance-critical applications, creating a more diversified and resilient material ecosystem.
Life cycle assessments reveal that lithium acetate production generates approximately 15-20 kg CO2 equivalent per kilogram of material, primarily during extraction and processing phases. By optimizing synthesis routes through green chemistry principles, manufacturers can achieve energy reductions of 25-40% and minimize hazardous waste generation. Alternative synthesis pathways utilizing bio-based acetic acid sources present promising opportunities to further reduce carbon footprints.
Water consumption remains a significant concern, with conventional lithium extraction requiring 500-2000 liters of water per kilogram of lithium compound produced. Advanced membrane filtration technologies and direct lithium extraction methods can reduce this water footprint by 60-80% while simultaneously decreasing chemical additive requirements. These improvements directly address sustainability challenges in water-stressed regions where lithium resources are concentrated.
End-of-life management strategies for lithium acetate-containing electrolytes demonstrate increasing viability. Recent technological advances enable recovery rates exceeding 90% for lithium compounds from spent solutions, creating opportunities for circular economy approaches. Hydrometallurgical processes combined with selective precipitation techniques allow for high-purity recovery with minimal secondary waste generation.
Regulatory frameworks increasingly emphasize extended producer responsibility for battery materials, driving innovation in sustainable electrolyte formulations. Companies implementing comprehensive material stewardship programs for lithium acetate solutions report 15-25% reductions in overall environmental impact scores and improved regulatory compliance. These programs typically incorporate responsible sourcing criteria, efficiency optimization, and end-of-life recovery systems.
Substitution strategies represent another sustainability pathway, with research into sodium, potassium, and organic-based electrolyte alternatives showing promise for specific applications. While these alternatives may not match lithium acetate's performance in all metrics, they offer complementary solutions that can reduce overall lithium demand in less performance-critical applications, creating a more diversified and resilient material ecosystem.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!