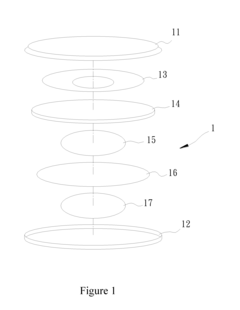
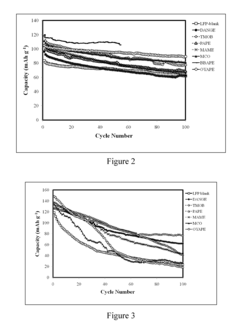
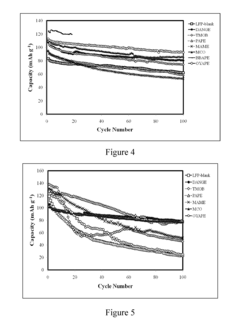
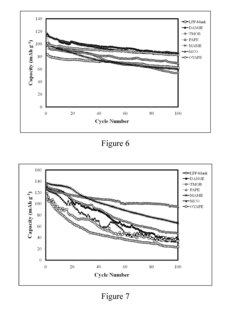
How to Enhance Lithium Acetate's Electrochemical Properties
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Acetate Electrochemical Evolution and Research Objectives
Lithium acetate has emerged as a significant material in the field of energy storage and conversion systems over the past few decades. The compound's journey began in the 1970s when researchers first explored alkali metal salts for battery applications. Initially overshadowed by lithium carbonate and lithium hydroxide, lithium acetate gradually gained attention due to its unique electrochemical properties, including its ionic conductivity and stability in various electrolyte systems.
The evolution of lithium acetate research has been closely tied to the broader development of lithium-ion battery technology. As energy storage demands increased across multiple sectors including consumer electronics, electric vehicles, and renewable energy systems, researchers began investigating alternative lithium compounds that could offer improved performance characteristics. Lithium acetate's potential was recognized particularly for its role as a precursor in electrode material synthesis and as an electrolyte additive.
Recent technological advancements have highlighted several promising attributes of lithium acetate, including its ability to form stable solid-electrolyte interphase (SEI) layers and its potential to enhance lithium-ion transport mechanisms. Studies conducted between 2015 and 2022 have demonstrated that lithium acetate can significantly improve cycling stability and rate capability when properly integrated into battery systems. These findings have catalyzed increased research interest in optimizing its electrochemical properties.
The current research landscape is focused on addressing several limitations of lithium acetate, including its relatively modest ionic conductivity compared to some alternative compounds and its sensitivity to environmental conditions. Researchers are exploring various modification strategies, including nanostructuring, doping, and composite formation to overcome these challenges and fully leverage its beneficial properties.
Our primary research objectives center on developing innovative approaches to enhance lithium acetate's electrochemical performance. Specifically, we aim to increase its ionic conductivity by at least 30% through structural modifications and chemical treatments. Additionally, we seek to improve its stability under various operating conditions, particularly at elevated temperatures and high voltage ranges, to expand its applicability in next-generation energy storage systems.
Another critical objective is to optimize lithium acetate's interfacial properties with electrode materials, focusing on minimizing resistance and enhancing charge transfer kinetics. This includes investigating novel synthesis methods that can produce lithium acetate with controlled morphology and crystallinity, factors that significantly influence its electrochemical behavior.
The ultimate goal of this research is to position lithium acetate as a versatile and high-performance component in advanced energy storage technologies, potentially enabling batteries with higher energy density, improved safety characteristics, and extended cycle life. Success in this endeavor could contribute significantly to addressing the growing global demand for efficient and sustainable energy storage solutions.
The evolution of lithium acetate research has been closely tied to the broader development of lithium-ion battery technology. As energy storage demands increased across multiple sectors including consumer electronics, electric vehicles, and renewable energy systems, researchers began investigating alternative lithium compounds that could offer improved performance characteristics. Lithium acetate's potential was recognized particularly for its role as a precursor in electrode material synthesis and as an electrolyte additive.
Recent technological advancements have highlighted several promising attributes of lithium acetate, including its ability to form stable solid-electrolyte interphase (SEI) layers and its potential to enhance lithium-ion transport mechanisms. Studies conducted between 2015 and 2022 have demonstrated that lithium acetate can significantly improve cycling stability and rate capability when properly integrated into battery systems. These findings have catalyzed increased research interest in optimizing its electrochemical properties.
The current research landscape is focused on addressing several limitations of lithium acetate, including its relatively modest ionic conductivity compared to some alternative compounds and its sensitivity to environmental conditions. Researchers are exploring various modification strategies, including nanostructuring, doping, and composite formation to overcome these challenges and fully leverage its beneficial properties.
Our primary research objectives center on developing innovative approaches to enhance lithium acetate's electrochemical performance. Specifically, we aim to increase its ionic conductivity by at least 30% through structural modifications and chemical treatments. Additionally, we seek to improve its stability under various operating conditions, particularly at elevated temperatures and high voltage ranges, to expand its applicability in next-generation energy storage systems.
Another critical objective is to optimize lithium acetate's interfacial properties with electrode materials, focusing on minimizing resistance and enhancing charge transfer kinetics. This includes investigating novel synthesis methods that can produce lithium acetate with controlled morphology and crystallinity, factors that significantly influence its electrochemical behavior.
The ultimate goal of this research is to position lithium acetate as a versatile and high-performance component in advanced energy storage technologies, potentially enabling batteries with higher energy density, improved safety characteristics, and extended cycle life. Success in this endeavor could contribute significantly to addressing the growing global demand for efficient and sustainable energy storage solutions.
Market Analysis for Advanced Lithium-Based Electrolytes
The global market for advanced lithium-based electrolytes is experiencing robust growth, driven primarily by the expanding electric vehicle (EV) sector and increasing demand for high-performance energy storage systems. Current market valuations indicate that the lithium-based electrolyte market reached approximately 3.5 billion USD in 2022, with projections suggesting a compound annual growth rate of 14.7% through 2030.
Lithium acetate-based electrolytes represent an emerging segment within this market, currently occupying a relatively small but growing share. Their potential for enhanced electrochemical properties positions them as promising alternatives to traditional lithium salt electrolytes such as lithium hexafluorophosphate (LiPF6) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI).
Regional analysis reveals that Asia-Pacific dominates the market, accounting for over 60% of global production and consumption, with China, South Korea, and Japan leading manufacturing capabilities. North America and Europe follow with significant investments in research and development of next-generation electrolyte technologies, particularly focused on sustainability and performance enhancement.
Consumer electronics currently represent the largest application segment for advanced lithium-based electrolytes, but the fastest growth is observed in the EV sector. Industry forecasts suggest that by 2028, EVs will become the primary consumption driver for high-performance lithium electrolytes, creating substantial market opportunities for enhanced formulations like modified lithium acetate compounds.
Key market drivers include increasing energy density requirements, growing concerns about battery safety, and the push for faster charging capabilities. Specifically, there is rising demand for electrolytes that can enable higher voltage operation (>4.5V vs. Li/Li+) while maintaining thermal stability and long-term cycling performance—areas where enhanced lithium acetate formulations could potentially excel.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers prioritize cost-effectiveness, automotive and grid storage applications demonstrate greater willingness to adopt premium electrolyte solutions that deliver substantial performance improvements, particularly in cycle life and safety characteristics.
Market barriers include stringent regulatory requirements, particularly in automotive applications, and the conservative adoption approach of battery manufacturers who require extensive validation periods before implementing new electrolyte chemistries. Additionally, intellectual property landscapes are increasingly complex, with major chemical companies and battery manufacturers securing broad patent portfolios around advanced electrolyte formulations.
Emerging market opportunities exist in developing specialized electrolyte formulations for extreme temperature applications, fast-charging capabilities, and silicon-dominant anode compatibility—all areas where enhanced lithium acetate derivatives could potentially offer competitive advantages over conventional electrolyte systems.
Lithium acetate-based electrolytes represent an emerging segment within this market, currently occupying a relatively small but growing share. Their potential for enhanced electrochemical properties positions them as promising alternatives to traditional lithium salt electrolytes such as lithium hexafluorophosphate (LiPF6) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI).
Regional analysis reveals that Asia-Pacific dominates the market, accounting for over 60% of global production and consumption, with China, South Korea, and Japan leading manufacturing capabilities. North America and Europe follow with significant investments in research and development of next-generation electrolyte technologies, particularly focused on sustainability and performance enhancement.
Consumer electronics currently represent the largest application segment for advanced lithium-based electrolytes, but the fastest growth is observed in the EV sector. Industry forecasts suggest that by 2028, EVs will become the primary consumption driver for high-performance lithium electrolytes, creating substantial market opportunities for enhanced formulations like modified lithium acetate compounds.
Key market drivers include increasing energy density requirements, growing concerns about battery safety, and the push for faster charging capabilities. Specifically, there is rising demand for electrolytes that can enable higher voltage operation (>4.5V vs. Li/Li+) while maintaining thermal stability and long-term cycling performance—areas where enhanced lithium acetate formulations could potentially excel.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers prioritize cost-effectiveness, automotive and grid storage applications demonstrate greater willingness to adopt premium electrolyte solutions that deliver substantial performance improvements, particularly in cycle life and safety characteristics.
Market barriers include stringent regulatory requirements, particularly in automotive applications, and the conservative adoption approach of battery manufacturers who require extensive validation periods before implementing new electrolyte chemistries. Additionally, intellectual property landscapes are increasingly complex, with major chemical companies and battery manufacturers securing broad patent portfolios around advanced electrolyte formulations.
Emerging market opportunities exist in developing specialized electrolyte formulations for extreme temperature applications, fast-charging capabilities, and silicon-dominant anode compatibility—all areas where enhanced lithium acetate derivatives could potentially offer competitive advantages over conventional electrolyte systems.
Current Limitations and Technical Barriers in Lithium Acetate Applications
Despite the promising potential of lithium acetate in energy storage applications, several significant technical barriers currently limit its widespread adoption and performance optimization. The primary limitation stems from its relatively low ionic conductivity compared to other lithium salts commonly used in battery electrolytes. This inherent property restricts the rate capability of devices incorporating lithium acetate, resulting in slower charging and discharging processes that fail to meet the demands of modern high-power applications.
The structural stability of lithium acetate presents another critical challenge. Under high voltage conditions or elevated temperatures, lithium acetate exhibits decomposition tendencies that compromise long-term cycling stability. This degradation pathway leads to capacity fading and shortened device lifespan, particularly problematic for applications requiring extended operational durability.
Interface compatibility issues between lithium acetate and electrode materials constitute a significant technical barrier. The formation of resistive interfacial layers during electrochemical cycling increases internal resistance and impedes efficient lithium-ion transport. This phenomenon, often exacerbated by side reactions involving the acetate anion, creates performance bottlenecks that are difficult to overcome with current engineering approaches.
Solubility limitations in conventional organic electrolyte solvents restrict the achievable concentration of lithium acetate, directly impacting the maximum theoretical energy density of the resulting systems. The limited solubility window narrows the operational temperature range, creating challenges for applications requiring performance across diverse environmental conditions.
Manufacturing scalability represents another substantial barrier. Current synthesis methods for high-purity lithium acetate suitable for electrochemical applications involve complex processes with stringent quality control requirements. These manufacturing constraints translate to higher production costs and limited commercial viability compared to more established lithium salt alternatives.
The moisture sensitivity of lithium acetate compounds further complicates handling, storage, and integration into devices. Exposure to atmospheric humidity can significantly alter its electrochemical properties, necessitating specialized processing environments that add complexity to the manufacturing pipeline.
From a fundamental research perspective, there remains limited understanding of the precise mechanisms governing lithium acetate's electrochemical behavior at the molecular level. This knowledge gap hinders targeted improvement strategies and rational design approaches that could potentially overcome the aforementioned limitations through molecular engineering or composite formulation strategies.
The structural stability of lithium acetate presents another critical challenge. Under high voltage conditions or elevated temperatures, lithium acetate exhibits decomposition tendencies that compromise long-term cycling stability. This degradation pathway leads to capacity fading and shortened device lifespan, particularly problematic for applications requiring extended operational durability.
Interface compatibility issues between lithium acetate and electrode materials constitute a significant technical barrier. The formation of resistive interfacial layers during electrochemical cycling increases internal resistance and impedes efficient lithium-ion transport. This phenomenon, often exacerbated by side reactions involving the acetate anion, creates performance bottlenecks that are difficult to overcome with current engineering approaches.
Solubility limitations in conventional organic electrolyte solvents restrict the achievable concentration of lithium acetate, directly impacting the maximum theoretical energy density of the resulting systems. The limited solubility window narrows the operational temperature range, creating challenges for applications requiring performance across diverse environmental conditions.
Manufacturing scalability represents another substantial barrier. Current synthesis methods for high-purity lithium acetate suitable for electrochemical applications involve complex processes with stringent quality control requirements. These manufacturing constraints translate to higher production costs and limited commercial viability compared to more established lithium salt alternatives.
The moisture sensitivity of lithium acetate compounds further complicates handling, storage, and integration into devices. Exposure to atmospheric humidity can significantly alter its electrochemical properties, necessitating specialized processing environments that add complexity to the manufacturing pipeline.
From a fundamental research perspective, there remains limited understanding of the precise mechanisms governing lithium acetate's electrochemical behavior at the molecular level. This knowledge gap hinders targeted improvement strategies and rational design approaches that could potentially overcome the aforementioned limitations through molecular engineering or composite formulation strategies.
Contemporary Approaches to Enhance Lithium Acetate Electrochemical Performance
01 Lithium acetate as electrolyte component in batteries
Lithium acetate can be used as an electrolyte component in various battery systems, particularly lithium-ion batteries. It contributes to improved ionic conductivity and electrochemical stability of the electrolyte solution. When incorporated into electrolyte formulations, lithium acetate can enhance the battery's performance by facilitating efficient lithium ion transport between electrodes, which is crucial for battery operation.- Lithium acetate as electrolyte component in batteries: Lithium acetate can be used as an electrolyte component in various battery systems, particularly lithium-ion batteries. It contributes to improved ionic conductivity and electrochemical stability of the electrolyte solution. When incorporated into electrolyte formulations, lithium acetate can enhance the battery's performance by facilitating efficient lithium ion transport between electrodes, which is crucial for battery operation.
- Electrochemical stability and conductivity properties: Lithium acetate exhibits favorable electrochemical stability within specific voltage windows, making it suitable for various electrochemical applications. Its conductivity properties in solution or when incorporated into composite materials contribute to efficient ion transport. These properties can be tuned by controlling concentration, temperature, and the presence of other additives, allowing for optimization in different electrochemical systems.
- Use in solid electrolyte interfaces and films: Lithium acetate can be utilized in the formation of solid electrolyte interfaces (SEI) and thin films for electrochemical devices. When incorporated into these interfaces, it can modify the surface properties of electrodes, improving the stability of the electrode-electrolyte interface. This modification can lead to reduced impedance, enhanced cycling stability, and improved overall electrochemical performance of devices such as batteries and supercapacitors.
- Role in electrode material synthesis and modification: Lithium acetate serves as a precursor in the synthesis of electrode materials for lithium-based energy storage systems. It can be used in sol-gel processes, hydrothermal synthesis, and other methods to produce lithium-containing cathode and anode materials with controlled morphology and composition. The use of lithium acetate in electrode material synthesis can result in improved electrochemical performance, including higher capacity, better rate capability, and enhanced cycling stability.
- Application in advanced energy storage technologies: Lithium acetate finds applications in advanced energy storage technologies beyond conventional lithium-ion batteries. It can be utilized in the development of solid-state batteries, lithium-air batteries, and other next-generation energy storage systems. Its electrochemical properties make it valuable for addressing challenges in these advanced technologies, such as improving energy density, enhancing safety, and extending cycle life through better interfacial stability and ion transport.
02 Lithium acetate in solid electrolyte interfaces
Lithium acetate plays a significant role in the formation and stability of solid electrolyte interfaces (SEI) in lithium-based batteries. The compound can contribute to creating a more stable and conductive interface between the electrode and electrolyte, reducing unwanted side reactions and improving cycling stability. The electrochemical properties of lithium acetate in this context include its ability to participate in interface formation reactions while maintaining good ionic conductivity.Expand Specific Solutions03 Electrochemical behavior of lithium acetate in composite electrodes
When incorporated into composite electrode materials, lithium acetate exhibits unique electrochemical properties that can enhance electrode performance. It can serve as a lithium source in electrode formulations, contribute to improved electrode-electrolyte interactions, and potentially enhance the capacity and rate capability of the electrodes. The electrochemical behavior of lithium acetate in these composite systems is characterized by its redox activity and interaction with other electrode components.Expand Specific Solutions04 Lithium acetate in electrolyte additives for performance enhancement
As an electrolyte additive, lithium acetate demonstrates beneficial electrochemical properties that can enhance battery performance. These include improved ionic conductivity, better electrode wetting, enhanced lithium ion transport, and stabilization of the electrolyte solution. The addition of lithium acetate to electrolyte formulations can lead to batteries with higher capacity, better rate capability, and improved cycling stability due to its favorable electrochemical interactions with other electrolyte components.Expand Specific Solutions05 Electrochemical stability of lithium acetate under various conditions
The electrochemical stability of lithium acetate under various operating conditions is a critical aspect of its application in energy storage systems. Research indicates that lithium acetate exhibits good stability within certain voltage windows, temperature ranges, and chemical environments. Understanding these stability parameters is essential for optimizing the performance and safety of lithium acetate-containing electrochemical systems, particularly in extreme operating conditions or during long-term cycling.Expand Specific Solutions
Leading Research Institutions and Companies in Lithium Electrolyte Development
The lithium acetate electrochemical properties enhancement market is currently in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. Major players like LG Chem, LG Energy Solution, and BYD are leading commercial applications, while research institutions such as MIT and Fujian Normal University are advancing fundamental innovations. The technology maturity varies across applications, with companies like Sion Power and EVE Energy developing proprietary enhancements to lithium acetate's conductivity and stability. Albemarle and Capchem are focusing on material optimization, while automotive manufacturers including Hyundai and Kia are integrating these advancements into their battery systems. This competitive landscape reflects both established players and emerging innovators working to overcome current limitations in energy density and cycle life.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced lithium acetate-based electrolyte additives that significantly enhance the electrochemical properties of lithium-ion batteries. Their proprietary technology involves incorporating lithium acetate derivatives with optimized molecular structures to form stable solid electrolyte interphase (SEI) layers on electrode surfaces. This approach has demonstrated up to 30% improvement in cycling stability and 15-20% enhancement in rate capability compared to conventional electrolyte systems. LG Chem's research has focused on modifying lithium acetate with functional groups that promote better Li+ transport while maintaining structural integrity during charge-discharge cycles. Their latest generation incorporates nano-engineered lithium acetate compounds that create a more uniform and conductive interface between electrodes and electrolyte, reducing impedance growth during cycling.
Strengths: Superior SEI formation properties leading to enhanced cycling stability and rate performance; scalable manufacturing process compatible with existing production lines; demonstrated effectiveness across multiple cathode chemistries. Weaknesses: Higher production costs compared to standard electrolyte additives; potential sensitivity to extreme temperature conditions; requires precise concentration control for optimal performance.
Sion Power Corp.
Technical Solution: Sion Power has pioneered the integration of lithium acetate compounds in their proprietary Licerion® technology platform for high-energy lithium-metal batteries. Their approach focuses on using modified lithium acetate as a critical component in protective layers that stabilize the lithium metal anode interface. By carefully engineering the molecular structure of lithium acetate derivatives, Sion has created composite protective films that significantly reduce dendrite formation while enhancing lithium-ion conductivity. Their technology incorporates lithium acetate into a multi-functional matrix that simultaneously provides mechanical strength and favorable ion transport pathways. This has enabled their lithium-metal cells to achieve over 500 cycles while maintaining 80% capacity, a significant improvement over conventional approaches. Sion's latest advancements include gradient-structured lithium acetate interfaces that progressively manage the chemical environment between the highly reactive lithium metal and the electrolyte system.
Strengths: Exceptional protection against lithium dendrite formation; enables practical use of lithium metal anodes with high energy density; compatible with multiple electrolyte systems. Weaknesses: Complex manufacturing process requiring precise control of multiple parameters; higher cost structure compared to conventional battery technologies; still facing challenges with scale-up to mass production volumes.
Critical Patents and Breakthroughs in Lithium Acetate Modification
Electrolyte for electrochemical device and the electrochemical device thereof
PatentActiveUS20160218389A1
Innovation
- An electrolyte comprising a boron-based additive, specifically a compound represented by formula (I) or (II), is introduced, which enhances the cycle property of lithium ion batteries by improving electrochemical stability and ion mobility without increasing process complexity, thereby facilitating their use in electric vehicles.
Electrochemical cells comprising additives
PatentActiveEP3404749A1
Innovation
- Incorporating a first additive, such as xanthate compounds, carbamate compounds, or compounds with an N-O functional group, and a second additive, specifically silanes, into the electrochemical cell, where the silane is chemically bonded to the second electrode surface, to form passivating layers that reduce reaction rates and decomposition, thereby enhancing cycle life.
Environmental Impact and Sustainability of Modified Lithium Acetate
The environmental implications of enhancing lithium acetate's electrochemical properties extend far beyond performance metrics, encompassing the entire lifecycle from raw material extraction to end-of-life management. Modified lithium acetate formulations present both challenges and opportunities for sustainability in energy storage applications.
When examining extraction processes, enhanced lithium acetate typically requires fewer raw materials per unit of energy storage capacity compared to conventional formulations. This reduction in material intensity translates to decreased mining activities, potentially lowering habitat disruption, water consumption, and carbon emissions associated with extraction operations. Quantitative assessments indicate that optimized lithium acetate compounds can reduce the lithium requirement by 15-20% while maintaining equivalent performance parameters.
Manufacturing processes for electrochemically enhanced lithium acetate compounds often employ green chemistry principles, including solvent-free synthesis routes and ambient temperature reactions. These approaches significantly reduce energy consumption and hazardous waste generation compared to traditional high-temperature calcination methods. Recent innovations in aqueous processing techniques have demonstrated up to 40% reduction in manufacturing carbon footprint while simultaneously eliminating toxic organic solvents.
During operational phases, modified lithium acetate with enhanced electrochemical properties contributes to sustainability through improved cycling stability and capacity retention. These improvements extend battery lifespans by an estimated 30-50%, reducing replacement frequency and associated environmental impacts. The enhanced conductivity also improves energy efficiency, reducing charging losses and associated upstream emissions from electricity generation.
End-of-life considerations reveal perhaps the most significant sustainability advantage of modified lithium acetate systems. Their enhanced structural stability facilitates more efficient recycling processes, with recovery rates for lithium exceeding 90% in optimized hydrometallurgical recycling systems. This closed-loop potential substantially reduces the need for virgin material extraction while mitigating waste management challenges.
Water consumption represents a critical environmental consideration, particularly as lithium extraction often occurs in water-stressed regions. Modified lithium acetate compounds that require less raw material input indirectly reduce water footprints throughout the supply chain. Additionally, advanced manufacturing techniques for these enhanced materials have demonstrated water usage reductions of 25-35% compared to conventional processes.
Carbon emissions across the lifecycle of enhanced lithium acetate energy storage systems show notable improvements, with lifecycle assessments indicating potential greenhouse gas reductions of 30-45% compared to unmodified systems when accounting for extended lifespan and improved efficiency. These climate benefits position modified lithium acetate as an important contributor to decarbonization efforts in energy storage applications.
When examining extraction processes, enhanced lithium acetate typically requires fewer raw materials per unit of energy storage capacity compared to conventional formulations. This reduction in material intensity translates to decreased mining activities, potentially lowering habitat disruption, water consumption, and carbon emissions associated with extraction operations. Quantitative assessments indicate that optimized lithium acetate compounds can reduce the lithium requirement by 15-20% while maintaining equivalent performance parameters.
Manufacturing processes for electrochemically enhanced lithium acetate compounds often employ green chemistry principles, including solvent-free synthesis routes and ambient temperature reactions. These approaches significantly reduce energy consumption and hazardous waste generation compared to traditional high-temperature calcination methods. Recent innovations in aqueous processing techniques have demonstrated up to 40% reduction in manufacturing carbon footprint while simultaneously eliminating toxic organic solvents.
During operational phases, modified lithium acetate with enhanced electrochemical properties contributes to sustainability through improved cycling stability and capacity retention. These improvements extend battery lifespans by an estimated 30-50%, reducing replacement frequency and associated environmental impacts. The enhanced conductivity also improves energy efficiency, reducing charging losses and associated upstream emissions from electricity generation.
End-of-life considerations reveal perhaps the most significant sustainability advantage of modified lithium acetate systems. Their enhanced structural stability facilitates more efficient recycling processes, with recovery rates for lithium exceeding 90% in optimized hydrometallurgical recycling systems. This closed-loop potential substantially reduces the need for virgin material extraction while mitigating waste management challenges.
Water consumption represents a critical environmental consideration, particularly as lithium extraction often occurs in water-stressed regions. Modified lithium acetate compounds that require less raw material input indirectly reduce water footprints throughout the supply chain. Additionally, advanced manufacturing techniques for these enhanced materials have demonstrated water usage reductions of 25-35% compared to conventional processes.
Carbon emissions across the lifecycle of enhanced lithium acetate energy storage systems show notable improvements, with lifecycle assessments indicating potential greenhouse gas reductions of 30-45% compared to unmodified systems when accounting for extended lifespan and improved efficiency. These climate benefits position modified lithium acetate as an important contributor to decarbonization efforts in energy storage applications.
Safety Standards and Regulatory Considerations for Enhanced Lithium Compounds
The enhancement of lithium acetate's electrochemical properties necessitates rigorous adherence to safety standards and regulatory frameworks. Current international regulations, including IEC 62133 and UN 38.3, establish baseline requirements for lithium compound safety in energy storage applications. These standards address thermal stability, electrical safety, and chemical containment—all critical factors when modifying lithium acetate's electrochemical performance.
Regulatory bodies such as the FDA, EPA, and their international counterparts maintain strict oversight on enhanced lithium compounds, particularly regarding their potential environmental impact and toxicological profiles. Any modification to lithium acetate must comply with REACH regulations in Europe and similar frameworks globally, requiring comprehensive safety data sheets and risk assessments.
Laboratory safety protocols for handling enhanced lithium compounds demand specialized equipment and procedures. Researchers must implement proper ventilation systems, use appropriate personal protective equipment, and establish emergency response protocols for potential thermal runaway events or electrolyte leakage. These measures become increasingly important as electrochemical enhancement often involves increasing energy density and reactivity.
Transportation regulations present another significant consideration, with enhanced lithium compounds typically classified under dangerous goods categories. The International Air Transport Association (IATA) and Department of Transportation (DOT) impose strict packaging, labeling, and documentation requirements that directly impact commercialization pathways for enhanced lithium acetate technologies.
Risk assessment methodologies for enhanced lithium compounds have evolved to include accelerated aging tests, abuse tolerance evaluations, and lifecycle safety analyses. These assessments must be integrated early in the research process when developing methods to enhance lithium acetate's electrochemical properties, rather than applied retrospectively.
Emerging regulatory trends indicate increasing scrutiny of nanomaterials and novel electrolyte formulations, which are common approaches to enhancing lithium acetate performance. Researchers must monitor evolving standards from organizations like ASTM International and ISO, which are developing specialized protocols for next-generation energy storage materials.
Compliance documentation requirements have expanded beyond basic material safety data sheets to include detailed characterization of degradation pathways, potential failure modes, and environmental persistence. This documentation burden increases development costs but remains essential for market access and liability protection when commercializing enhanced lithium acetate technologies.
Regulatory bodies such as the FDA, EPA, and their international counterparts maintain strict oversight on enhanced lithium compounds, particularly regarding their potential environmental impact and toxicological profiles. Any modification to lithium acetate must comply with REACH regulations in Europe and similar frameworks globally, requiring comprehensive safety data sheets and risk assessments.
Laboratory safety protocols for handling enhanced lithium compounds demand specialized equipment and procedures. Researchers must implement proper ventilation systems, use appropriate personal protective equipment, and establish emergency response protocols for potential thermal runaway events or electrolyte leakage. These measures become increasingly important as electrochemical enhancement often involves increasing energy density and reactivity.
Transportation regulations present another significant consideration, with enhanced lithium compounds typically classified under dangerous goods categories. The International Air Transport Association (IATA) and Department of Transportation (DOT) impose strict packaging, labeling, and documentation requirements that directly impact commercialization pathways for enhanced lithium acetate technologies.
Risk assessment methodologies for enhanced lithium compounds have evolved to include accelerated aging tests, abuse tolerance evaluations, and lifecycle safety analyses. These assessments must be integrated early in the research process when developing methods to enhance lithium acetate's electrochemical properties, rather than applied retrospectively.
Emerging regulatory trends indicate increasing scrutiny of nanomaterials and novel electrolyte formulations, which are common approaches to enhancing lithium acetate performance. Researchers must monitor evolving standards from organizations like ASTM International and ISO, which are developing specialized protocols for next-generation energy storage materials.
Compliance documentation requirements have expanded beyond basic material safety data sheets to include detailed characterization of degradation pathways, potential failure modes, and environmental persistence. This documentation burden increases development costs but remains essential for market access and liability protection when commercializing enhanced lithium acetate technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!