How to Optimize Purification of Lithium Acetate in Lab Settings
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Acetate Purification Background and Objectives
Lithium acetate has emerged as a critical compound in various laboratory applications, particularly in molecular biology, biochemistry, and materials science. The history of lithium acetate utilization can be traced back to the 1980s when it was first recognized for its effectiveness in transformation protocols for yeast cells. Since then, its applications have expanded significantly across multiple scientific disciplines.
The evolution of lithium acetate purification techniques has progressed from basic precipitation and recrystallization methods to more sophisticated approaches involving ion exchange, membrane filtration, and chromatographic separation. This technological progression has been driven by increasing demands for higher purity grades required in advanced research applications and industrial processes.
Current purification methodologies face several limitations, including inconsistent yield rates, presence of trace metal contaminants, and energy-intensive processes that reduce cost-effectiveness. These challenges have spurred ongoing research into optimization strategies that balance purity requirements with practical laboratory constraints.
The primary objective of lithium acetate purification optimization is to develop reproducible, scalable protocols that consistently yield high-purity product (>99.9%) while minimizing resource consumption and environmental impact. Secondary goals include reducing purification time, decreasing solvent usage, and establishing standardized quality control parameters.
Recent technological advancements in analytical instrumentation have significantly enhanced our ability to detect impurities at increasingly lower concentrations, revealing previously unidentified contaminants in supposedly pure lithium acetate samples. This improved detection capability has raised the bar for purification standards while simultaneously providing better tools for process validation.
Global research trends indicate growing interest in green chemistry approaches to lithium acetate purification, with particular emphasis on solvent recycling, energy efficiency, and waste reduction. These sustainable practices align with broader scientific community goals of developing environmentally responsible laboratory protocols.
The optimization of lithium acetate purification represents a convergence of multiple scientific disciplines, including analytical chemistry, process engineering, and materials science. This interdisciplinary nature necessitates a comprehensive approach that considers both fundamental chemical principles and practical laboratory implementation constraints.
As research applications for lithium acetate continue to diversify, particularly in emerging fields such as advanced battery technologies and pharmaceutical development, the demand for tailored purification protocols optimized for specific end-use requirements is expected to increase substantially in the coming years.
The evolution of lithium acetate purification techniques has progressed from basic precipitation and recrystallization methods to more sophisticated approaches involving ion exchange, membrane filtration, and chromatographic separation. This technological progression has been driven by increasing demands for higher purity grades required in advanced research applications and industrial processes.
Current purification methodologies face several limitations, including inconsistent yield rates, presence of trace metal contaminants, and energy-intensive processes that reduce cost-effectiveness. These challenges have spurred ongoing research into optimization strategies that balance purity requirements with practical laboratory constraints.
The primary objective of lithium acetate purification optimization is to develop reproducible, scalable protocols that consistently yield high-purity product (>99.9%) while minimizing resource consumption and environmental impact. Secondary goals include reducing purification time, decreasing solvent usage, and establishing standardized quality control parameters.
Recent technological advancements in analytical instrumentation have significantly enhanced our ability to detect impurities at increasingly lower concentrations, revealing previously unidentified contaminants in supposedly pure lithium acetate samples. This improved detection capability has raised the bar for purification standards while simultaneously providing better tools for process validation.
Global research trends indicate growing interest in green chemistry approaches to lithium acetate purification, with particular emphasis on solvent recycling, energy efficiency, and waste reduction. These sustainable practices align with broader scientific community goals of developing environmentally responsible laboratory protocols.
The optimization of lithium acetate purification represents a convergence of multiple scientific disciplines, including analytical chemistry, process engineering, and materials science. This interdisciplinary nature necessitates a comprehensive approach that considers both fundamental chemical principles and practical laboratory implementation constraints.
As research applications for lithium acetate continue to diversify, particularly in emerging fields such as advanced battery technologies and pharmaceutical development, the demand for tailored purification protocols optimized for specific end-use requirements is expected to increase substantially in the coming years.
Market Analysis for High-Purity Lithium Acetate
The global market for high-purity lithium acetate has experienced significant growth in recent years, driven primarily by increasing demand from pharmaceutical, battery, and specialty chemical industries. Market research indicates that the high-purity lithium acetate segment is growing at a compound annual growth rate (CAGR) of approximately 6.8% between 2020 and 2025, outpacing the broader lithium compounds market.
Laboratory-grade lithium acetate, particularly at purities exceeding 99.5%, commands premium pricing in the market, with current values ranging from $400 to $700 per kilogram depending on purity levels and packaging specifications. This represents a substantial price differential compared to industrial-grade lithium acetate, which typically sells for $150 to $250 per kilogram.
The pharmaceutical sector remains the largest consumer of high-purity lithium acetate, accounting for roughly 42% of total market demand. This is primarily due to its applications in psychiatric medications and as a reagent in drug development processes. The biotechnology research segment follows closely, representing approximately 31% of market share, where lithium acetate is essential for DNA transformation protocols and molecular biology applications.
Regional analysis reveals that North America and Europe currently dominate the high-purity lithium acetate market, collectively accounting for over 60% of global consumption. However, the Asia-Pacific region, particularly China, South Korea, and Japan, is demonstrating the fastest growth trajectory, with demand increasing at nearly 9% annually due to expanding pharmaceutical manufacturing and research facilities.
Supply chain analysis indicates potential vulnerabilities in the high-purity lithium acetate market. Currently, production is concentrated among a relatively small number of specialty chemical manufacturers, with five companies controlling approximately 73% of global production capacity. This concentration creates potential supply risks and price volatility, particularly as demand continues to increase.
Market forecasts suggest that laboratory optimization of lithium acetate purification processes could significantly impact the economics of high-purity production. Innovations that reduce purification costs by even 15-20% could expand market accessibility and drive increased adoption in cost-sensitive applications such as academic research and smaller biotechnology startups.
Customer segmentation analysis reveals distinct requirements across different end-users. While pharmaceutical manufacturers prioritize consistent purity and regulatory documentation, research laboratories place greater emphasis on specific impurity profiles and batch-to-batch consistency. Understanding these differentiated needs is crucial for developing optimized purification protocols that align with market requirements.
Laboratory-grade lithium acetate, particularly at purities exceeding 99.5%, commands premium pricing in the market, with current values ranging from $400 to $700 per kilogram depending on purity levels and packaging specifications. This represents a substantial price differential compared to industrial-grade lithium acetate, which typically sells for $150 to $250 per kilogram.
The pharmaceutical sector remains the largest consumer of high-purity lithium acetate, accounting for roughly 42% of total market demand. This is primarily due to its applications in psychiatric medications and as a reagent in drug development processes. The biotechnology research segment follows closely, representing approximately 31% of market share, where lithium acetate is essential for DNA transformation protocols and molecular biology applications.
Regional analysis reveals that North America and Europe currently dominate the high-purity lithium acetate market, collectively accounting for over 60% of global consumption. However, the Asia-Pacific region, particularly China, South Korea, and Japan, is demonstrating the fastest growth trajectory, with demand increasing at nearly 9% annually due to expanding pharmaceutical manufacturing and research facilities.
Supply chain analysis indicates potential vulnerabilities in the high-purity lithium acetate market. Currently, production is concentrated among a relatively small number of specialty chemical manufacturers, with five companies controlling approximately 73% of global production capacity. This concentration creates potential supply risks and price volatility, particularly as demand continues to increase.
Market forecasts suggest that laboratory optimization of lithium acetate purification processes could significantly impact the economics of high-purity production. Innovations that reduce purification costs by even 15-20% could expand market accessibility and drive increased adoption in cost-sensitive applications such as academic research and smaller biotechnology startups.
Customer segmentation analysis reveals distinct requirements across different end-users. While pharmaceutical manufacturers prioritize consistent purity and regulatory documentation, research laboratories place greater emphasis on specific impurity profiles and batch-to-batch consistency. Understanding these differentiated needs is crucial for developing optimized purification protocols that align with market requirements.
Current Purification Techniques and Limitations
The purification of lithium acetate in laboratory settings currently employs several established techniques, each with specific advantages and limitations. Recrystallization remains the most common method, involving dissolution of crude lithium acetate in an appropriate solvent (typically water or ethanol-water mixtures) followed by controlled cooling to form pure crystals. While this technique offers good purity levels of 98-99%, it suffers from significant yield losses, often recovering only 70-85% of the initial material, and requires substantial solvent volumes.
Precipitation techniques represent another widely used approach, where lithium acetate solutions are treated with anti-solvents like acetone or ethanol to reduce solubility and induce crystallization. This method offers faster processing times compared to recrystallization but frequently results in co-precipitation of impurities, limiting achievable purity to 95-98% without multiple repetitions.
Ion-exchange chromatography has emerged as a more sophisticated purification method, particularly effective for removing metal ion contaminants. However, this technique requires specialized equipment, significant expertise, and faces scalability challenges beyond small-batch production. The high cost of ion-exchange resins and their limited operational lifespan further constrain widespread adoption in standard laboratory settings.
Membrane filtration technologies, including nanofiltration and reverse osmosis, have been adapted for lithium acetate purification with promising results. These methods offer continuous processing capabilities and reduced solvent consumption but struggle with membrane fouling issues and require precise pressure control systems that may be unavailable in typical laboratories.
A critical limitation across all current purification methods is energy intensity. Traditional heating and cooling cycles in recrystallization processes consume significant energy, while vacuum filtration and drying steps further increase the energy footprint. Most laboratories lack energy-efficient equipment optimized specifically for lithium salt purification.
Analytical challenges also persist throughout purification workflows. Real-time monitoring of purity levels remains difficult, with most laboratories relying on post-process testing rather than in-process analytical techniques. This reactive approach extends development timelines and increases material waste through trial-and-error optimization.
Water management represents another significant limitation, as current purification methods generate substantial volumes of contaminated wastewater containing trace lithium compounds and processing chemicals. Environmental regulations increasingly restrict disposal options, while recovery systems for lithium from waste streams remain prohibitively expensive for most laboratory settings.
Precipitation techniques represent another widely used approach, where lithium acetate solutions are treated with anti-solvents like acetone or ethanol to reduce solubility and induce crystallization. This method offers faster processing times compared to recrystallization but frequently results in co-precipitation of impurities, limiting achievable purity to 95-98% without multiple repetitions.
Ion-exchange chromatography has emerged as a more sophisticated purification method, particularly effective for removing metal ion contaminants. However, this technique requires specialized equipment, significant expertise, and faces scalability challenges beyond small-batch production. The high cost of ion-exchange resins and their limited operational lifespan further constrain widespread adoption in standard laboratory settings.
Membrane filtration technologies, including nanofiltration and reverse osmosis, have been adapted for lithium acetate purification with promising results. These methods offer continuous processing capabilities and reduced solvent consumption but struggle with membrane fouling issues and require precise pressure control systems that may be unavailable in typical laboratories.
A critical limitation across all current purification methods is energy intensity. Traditional heating and cooling cycles in recrystallization processes consume significant energy, while vacuum filtration and drying steps further increase the energy footprint. Most laboratories lack energy-efficient equipment optimized specifically for lithium salt purification.
Analytical challenges also persist throughout purification workflows. Real-time monitoring of purity levels remains difficult, with most laboratories relying on post-process testing rather than in-process analytical techniques. This reactive approach extends development timelines and increases material waste through trial-and-error optimization.
Water management represents another significant limitation, as current purification methods generate substantial volumes of contaminated wastewater containing trace lithium compounds and processing chemicals. Environmental regulations increasingly restrict disposal options, while recovery systems for lithium from waste streams remain prohibitively expensive for most laboratory settings.
Standard Laboratory Purification Protocols
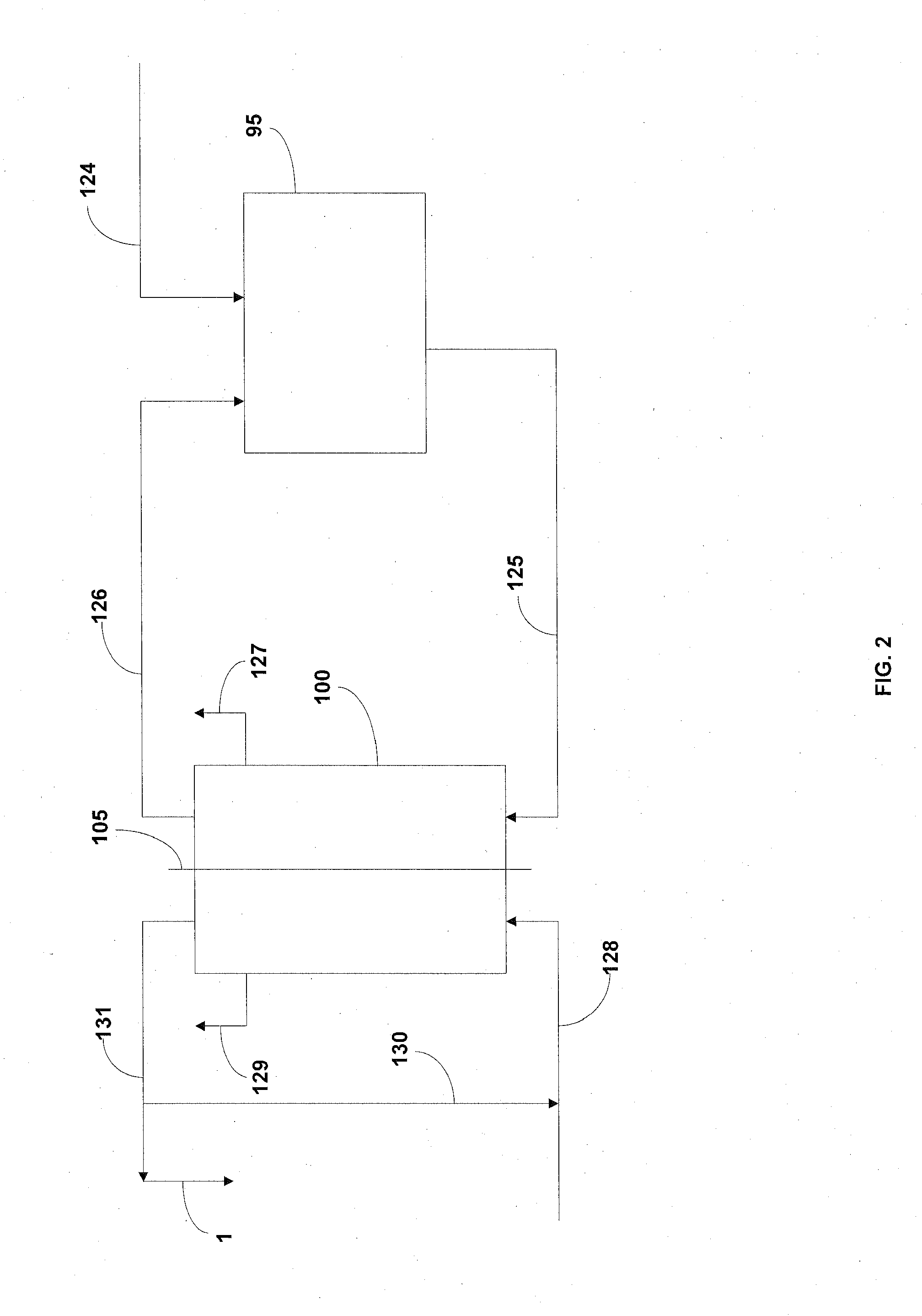
01 Crystallization and recrystallization methods
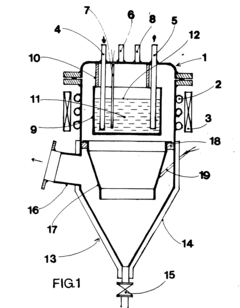
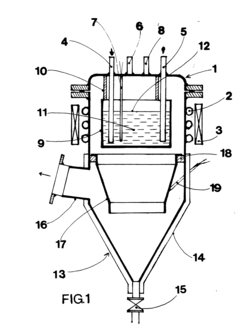
Lithium acetate can be purified through crystallization and recrystallization processes. These methods involve dissolving the impure lithium acetate in a suitable solvent, followed by controlled cooling or addition of an anti-solvent to induce crystallization. The crystals formed are then separated from the mother liquor, which contains most of the impurities. Multiple recrystallization steps may be performed to achieve higher purity levels. This approach is effective for removing metal impurities and other soluble contaminants.- Crystallization and recrystallization methods: Purification of lithium acetate can be achieved through crystallization and recrystallization processes. These methods involve dissolving the impure lithium acetate in a suitable solvent, followed by controlled crystallization to separate the pure compound from impurities. The process may include specific temperature control, solvent selection, and multiple recrystallization steps to achieve high purity levels. This approach is effective for removing various impurities and obtaining high-quality lithium acetate crystals.
- Solvent extraction techniques: Solvent extraction is an effective method for purifying lithium acetate by utilizing the differential solubility of lithium acetate and its impurities in various solvents. This process typically involves selecting appropriate organic solvents that preferentially dissolve either the lithium acetate or the impurities. Multiple extraction stages may be employed to enhance purification efficiency. The method is particularly useful for removing metal impurities and organic contaminants from lithium acetate solutions.
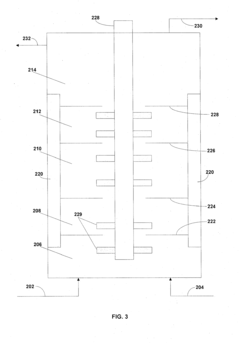
- Ion exchange and membrane filtration: Ion exchange resins and membrane filtration technologies can be used to purify lithium acetate solutions by selectively removing unwanted ions and impurities. Ion exchange columns containing specific resins can capture metal impurities while allowing lithium ions to pass through. Membrane filtration processes, including nanofiltration and ultrafiltration, can separate lithium acetate from larger molecular weight impurities. These methods are particularly effective for obtaining high-purity lithium acetate for pharmaceutical and electronic applications.
- Chemical precipitation methods: Chemical precipitation techniques involve adding specific reagents to lithium acetate solutions to selectively precipitate impurities while keeping lithium acetate in solution, or vice versa. This approach can target specific metal contaminants by using reagents that form insoluble compounds with the impurities. The precipitates can then be removed by filtration, leaving purified lithium acetate in solution. This method is particularly useful for removing heavy metal impurities and can be combined with other purification steps for enhanced results.
- Continuous flow purification systems: Continuous flow purification systems offer an efficient approach to lithium acetate purification at industrial scale. These systems integrate multiple purification steps such as filtration, crystallization, and extraction in a continuous process flow. The advantage of continuous systems is higher throughput, consistent product quality, and reduced labor costs compared to batch processes. Advanced monitoring and control systems ensure optimal operating conditions throughout the purification process, resulting in high-purity lithium acetate with minimal impurities.
02 Solvent extraction techniques
Solvent extraction is used to purify lithium acetate by exploiting the differential solubility of lithium acetate and its impurities in various solvents. The process typically involves dissolving the crude lithium acetate in one solvent and then extracting impurities using another immiscible solvent. This technique is particularly effective for removing organic impurities and certain metal ions. Sequential extraction steps with different solvents can be employed to target specific impurities, resulting in high-purity lithium acetate.Expand Specific Solutions03 Ion exchange purification
Ion exchange resins can be used to purify lithium acetate solutions by selectively removing impurity ions. The process involves passing the lithium acetate solution through columns containing cation and/or anion exchange resins that capture impurity ions while allowing lithium acetate to pass through. This method is particularly effective for removing trace metal impurities such as sodium, potassium, calcium, and magnesium ions. The purified lithium acetate solution can then be concentrated and crystallized to obtain the solid product.Expand Specific Solutions04 Membrane filtration and ultrafiltration
Membrane-based separation techniques, including ultrafiltration, nanofiltration, and reverse osmosis, can be employed to purify lithium acetate solutions. These processes use semi-permeable membranes with specific pore sizes to separate lithium acetate from impurities based on molecular size differences. Ultrafiltration is particularly useful for removing large molecular weight impurities and particulates, while nanofiltration and reverse osmosis can separate smaller ionic impurities. These techniques can be combined with other purification methods to achieve high-purity lithium acetate.Expand Specific Solutions05 Chemical precipitation of impurities
Chemical precipitation methods involve adding specific reagents to lithium acetate solutions to selectively precipitate impurities while keeping lithium acetate in solution. For example, certain reagents can be added to precipitate heavy metal impurities as insoluble compounds, which can then be removed by filtration. pH adjustment can also be used to selectively precipitate impurities. This approach is often used as a pre-treatment step before other purification methods to remove major impurities and improve the efficiency of subsequent purification steps.Expand Specific Solutions
Leading Research Institutions and Chemical Suppliers
The lithium acetate purification technology landscape is currently in a growth phase, with an estimated market size of $300-400 million annually. The industry is characterized by a mix of established chemical companies and emerging specialists. Technical maturity varies significantly across players: Kaneka Corp., Sumitomo Metal Mining, and Arkema France represent mature industrial-scale purification technologies, while innovative approaches are being developed by specialized entities like Lilac Solutions and Pure Lithium Corp. Academic institutions including Central South University and The University of Sydney are advancing fundamental research. Companies like Tianqi Lithium and General Lithium are scaling commercial applications, particularly for battery-grade materials, reflecting the technology's critical role in the expanding lithium-ion battery supply chain.
Central South University
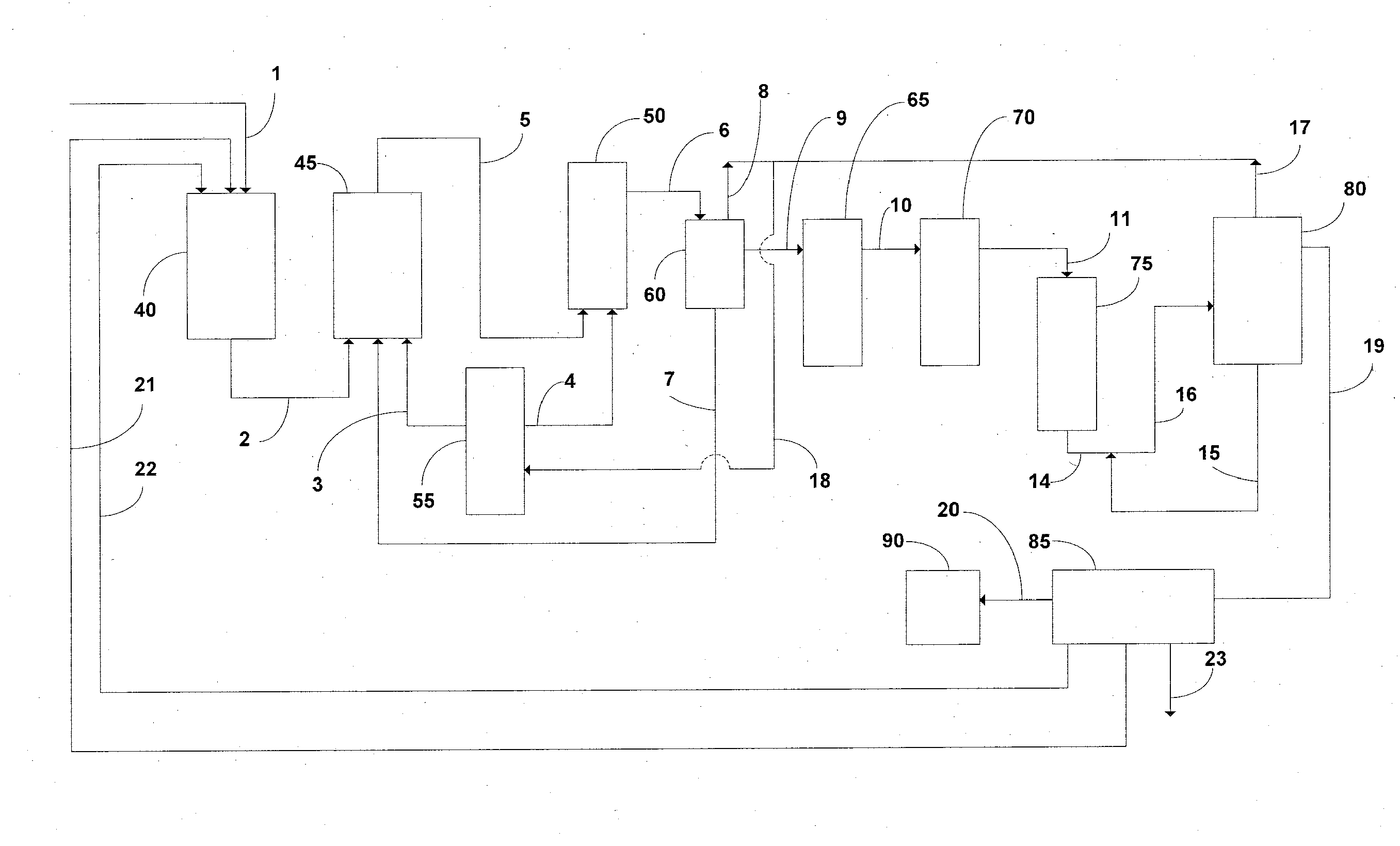
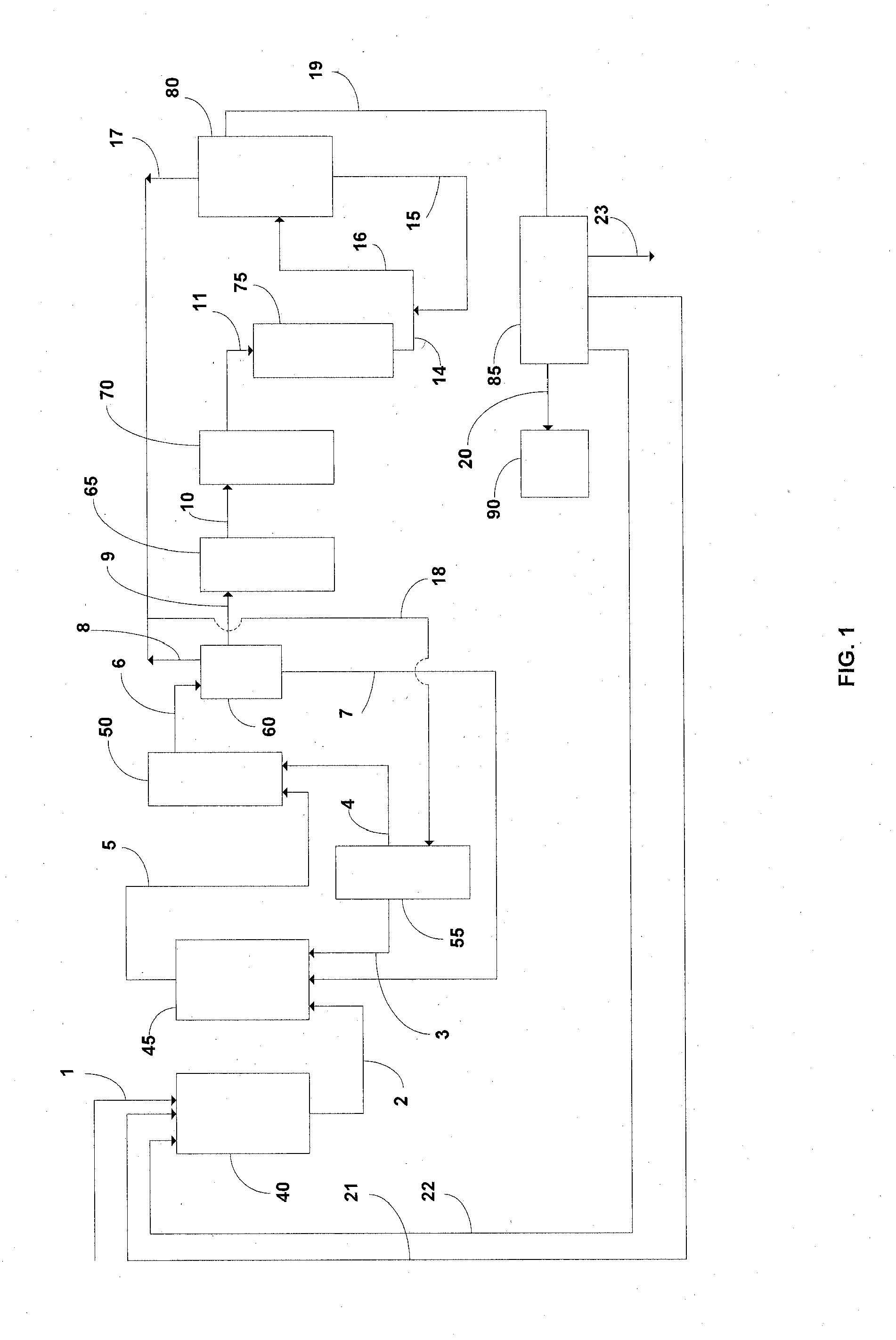
Technical Solution: Central South University has developed a green chemistry approach to lithium acetate purification focusing on sustainability in laboratory settings. Their method employs a combination of bio-derived solvents and chelating agents to achieve high purity. The process begins with crude lithium acetate that undergoes treatment with biodegradable chelating agents derived from modified citric acid compounds that selectively bind to metal impurities. This is followed by liquid-liquid extraction using bio-derived solvents such as cyrene or 2-methyltetrahydrofuran that have favorable environmental profiles compared to traditional organic solvents. Their research demonstrates that this approach can reduce solvent waste by approximately 60% compared to conventional methods while still achieving 99.9% purity. The final purification step involves a controlled anti-solvent crystallization technique that produces well-defined lithium acetate crystals with consistent morphology and minimal inclusions of impurities.
Strengths: Environmentally sustainable approach with reduced toxic solvent usage; process generates significantly less hazardous waste; final product has excellent batch-to-batch consistency. Weaknesses: Bio-derived solvents can be more expensive than conventional alternatives; process requires slightly longer processing times; some bio-derived reagents have limited shelf stability requiring fresh preparation.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed an advanced membrane-assisted purification technology for lithium compounds including lithium acetate. Their laboratory-scale system employs a cascade of specialized nanofiltration and electrodialysis membranes with precisely controlled pore sizes and surface chemistries. The process begins with pre-filtration through ceramic membranes to remove particulates, followed by nanofiltration through composite membranes with lithium-selective transport channels that retain larger impurity ions while allowing lithium ions to pass through. The solution then undergoes electrodialysis with bipolar membranes that enable simultaneous purification and concentration of lithium acetate. Their research demonstrates that this integrated membrane approach can achieve purification factors exceeding 1000 for common impurities such as sodium, calcium, and magnesium, while maintaining lithium recovery rates above 95%. The final product consistently meets USP/EP grade specifications with minimal chemical inputs.
Strengths: Highly efficient separation of lithium from chemically similar alkali metals; process operates continuously rather than batch-wise; significantly reduced chemical consumption compared to precipitation methods. Weaknesses: Membrane fouling can occur requiring periodic cleaning protocols; higher initial capital investment for specialized membrane systems; requires expertise in membrane technology for optimal operation.
Critical Analytical Methods for Purity Assessment
Process and apparatus for purifying lithium
PatentInactiveEP0202174A1
Innovation
- A process involving melting lithium under an inert atmosphere, stirring, and reducing pressure to selectively evaporate impurities between 400-700°C, followed by condensation at below 100°C, using a device with heating, stirring, and pumping systems to maintain optimal conditions for selective evaporation and minimize lithium loss.
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
PatentActiveUS20120100056A1
Innovation
- A method involving the reaction of technical grade lithium carbonate with CO2 to form lithium bicarbonate, followed by separation of insoluble compounds and purification using ion-selective media, achieving a purity of at least 99.99% lithium carbonate through multiple steps including reverse osmosis and precipitation.
Safety Considerations in Lithium Compound Handling
The handling of lithium compounds in laboratory settings requires stringent safety protocols due to their reactive nature and potential health hazards. When working with lithium acetate during purification processes, researchers must first understand that lithium compounds are alkali metals with high reactivity to moisture and air. Personal protective equipment (PPE) including chemical-resistant gloves, safety goggles, lab coats, and in some cases, face shields are mandatory when handling concentrated lithium solutions or dry compounds.
Proper ventilation systems are critical in laboratories where lithium acetate purification takes place. Fume hoods with adequate airflow should be utilized during all procedures involving volatile solvents or when there is potential for dust generation. This minimizes inhalation risks associated with lithium compounds, which can cause respiratory irritation and more severe health effects with prolonged exposure.
Fire safety represents another significant concern, as some lithium compounds can be flammable or react violently with water. Laboratories should be equipped with appropriate fire extinguishers (Class D for metal fires) and personnel should be trained in emergency response procedures. All lithium-containing waste must be segregated and disposed of according to established hazardous waste protocols, never discarded in regular waste streams or sinks.
Storage considerations for lithium compounds require particular attention. Purified lithium acetate and intermediates should be stored in tightly sealed containers in cool, dry locations away from incompatible materials. Hygroscopic lithium compounds must be protected from moisture to prevent degradation and potentially hazardous reactions. Proper labeling of all containers with complete hazard information is essential for maintaining laboratory safety.
Chemical spill management protocols specific to lithium compounds should be established and communicated to all laboratory personnel. Spill kits designed for alkali metal compounds should be readily accessible, and staff should receive training on containment and cleanup procedures. For larger spills, laboratory evacuation may be necessary until properly equipped personnel can address the situation.
Health monitoring may be advisable for researchers regularly working with lithium compounds, as chronic exposure can lead to systemic health effects. This is particularly important in settings where purification processes are conducted frequently or at scale. Documentation of all safety incidents, near-misses, and exposure events provides valuable information for continuous improvement of safety protocols.
Regular safety audits and refresher training sessions ensure that all personnel maintain awareness of proper handling techniques and emergency procedures when working with lithium acetate and related compounds. These proactive measures significantly reduce the risk of accidents and exposure incidents during laboratory purification processes.
Proper ventilation systems are critical in laboratories where lithium acetate purification takes place. Fume hoods with adequate airflow should be utilized during all procedures involving volatile solvents or when there is potential for dust generation. This minimizes inhalation risks associated with lithium compounds, which can cause respiratory irritation and more severe health effects with prolonged exposure.
Fire safety represents another significant concern, as some lithium compounds can be flammable or react violently with water. Laboratories should be equipped with appropriate fire extinguishers (Class D for metal fires) and personnel should be trained in emergency response procedures. All lithium-containing waste must be segregated and disposed of according to established hazardous waste protocols, never discarded in regular waste streams or sinks.
Storage considerations for lithium compounds require particular attention. Purified lithium acetate and intermediates should be stored in tightly sealed containers in cool, dry locations away from incompatible materials. Hygroscopic lithium compounds must be protected from moisture to prevent degradation and potentially hazardous reactions. Proper labeling of all containers with complete hazard information is essential for maintaining laboratory safety.
Chemical spill management protocols specific to lithium compounds should be established and communicated to all laboratory personnel. Spill kits designed for alkali metal compounds should be readily accessible, and staff should receive training on containment and cleanup procedures. For larger spills, laboratory evacuation may be necessary until properly equipped personnel can address the situation.
Health monitoring may be advisable for researchers regularly working with lithium compounds, as chronic exposure can lead to systemic health effects. This is particularly important in settings where purification processes are conducted frequently or at scale. Documentation of all safety incidents, near-misses, and exposure events provides valuable information for continuous improvement of safety protocols.
Regular safety audits and refresher training sessions ensure that all personnel maintain awareness of proper handling techniques and emergency procedures when working with lithium acetate and related compounds. These proactive measures significantly reduce the risk of accidents and exposure incidents during laboratory purification processes.
Scalability from Lab to Industrial Production
The transition from laboratory-scale purification of lithium acetate to industrial production represents a critical challenge that requires systematic planning and technological adaptation. Laboratory methods typically focus on achieving high purity with less emphasis on cost efficiency or throughput, whereas industrial production demands optimization across multiple parameters simultaneously.
When scaling up lithium acetate purification processes, several key factors must be addressed. First, reactor design and materials must be reconsidered, as glass vessels common in laboratories are impractical for industrial volumes. Industrial-grade stainless steel or specialized polymer-lined reactors become necessary, requiring validation to ensure they don't introduce contamination or affect product quality.
Heat and mass transfer dynamics change dramatically with scale. Laboratory processes that rely on rapid temperature control or efficient mixing may encounter significant challenges when vessel size increases. Computational fluid dynamics modeling becomes essential to predict and optimize these parameters before physical implementation, potentially saving substantial resources during scale-up.
Continuous flow processing represents a promising alternative to batch production for lithium acetate purification. This approach allows for more consistent product quality and reduced energy consumption compared to traditional batch methods. Recent innovations in flow chemistry have demonstrated up to 30% improvement in energy efficiency and 25% reduction in solvent usage when applied to similar ionic compound purifications.
Waste management and environmental considerations gain prominence at industrial scale. Laboratory purification methods often generate significant waste relative to product volume. Industrial implementation necessitates closed-loop systems for solvent recovery and recycling of process water, with potential for recovering valuable by-products that may be economically negligible at laboratory scale.
Automation and process control systems become indispensable during scale-up. While manual monitoring might suffice for laboratory operations, industrial production requires sophisticated sensor networks and control algorithms to maintain optimal conditions. Implementation of PAT (Process Analytical Technology) enables real-time quality monitoring, reducing batch failures and ensuring consistent purity levels.
Economic viability ultimately determines successful scale-up. A comprehensive techno-economic analysis must balance capital expenditure against operational costs, considering factors such as energy consumption, labor requirements, and maintenance needs. Sensitivity analysis should identify which process parameters most significantly impact production costs, guiding optimization efforts toward maximum return on investment.
When scaling up lithium acetate purification processes, several key factors must be addressed. First, reactor design and materials must be reconsidered, as glass vessels common in laboratories are impractical for industrial volumes. Industrial-grade stainless steel or specialized polymer-lined reactors become necessary, requiring validation to ensure they don't introduce contamination or affect product quality.
Heat and mass transfer dynamics change dramatically with scale. Laboratory processes that rely on rapid temperature control or efficient mixing may encounter significant challenges when vessel size increases. Computational fluid dynamics modeling becomes essential to predict and optimize these parameters before physical implementation, potentially saving substantial resources during scale-up.
Continuous flow processing represents a promising alternative to batch production for lithium acetate purification. This approach allows for more consistent product quality and reduced energy consumption compared to traditional batch methods. Recent innovations in flow chemistry have demonstrated up to 30% improvement in energy efficiency and 25% reduction in solvent usage when applied to similar ionic compound purifications.
Waste management and environmental considerations gain prominence at industrial scale. Laboratory purification methods often generate significant waste relative to product volume. Industrial implementation necessitates closed-loop systems for solvent recovery and recycling of process water, with potential for recovering valuable by-products that may be economically negligible at laboratory scale.
Automation and process control systems become indispensable during scale-up. While manual monitoring might suffice for laboratory operations, industrial production requires sophisticated sensor networks and control algorithms to maintain optimal conditions. Implementation of PAT (Process Analytical Technology) enables real-time quality monitoring, reducing batch failures and ensuring consistent purity levels.
Economic viability ultimately determines successful scale-up. A comprehensive techno-economic analysis must balance capital expenditure against operational costs, considering factors such as energy consumption, labor requirements, and maintenance needs. Sensitivity analysis should identify which process parameters most significantly impact production costs, guiding optimization efforts toward maximum return on investment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!