Lithium Acetate Use in Carbon Capture: Efficiency Metrics
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Acetate Carbon Capture Background and Objectives
Carbon capture technology has evolved significantly over the past decades as a critical solution to mitigate greenhouse gas emissions. The journey began with conventional amine-based solvents in the 1970s, which, while effective, presented challenges including high energy requirements for regeneration and corrosion issues. The technological landscape has since expanded to include various approaches such as physical adsorption, membrane separation, and chemical looping. Within this evolution, lithium-based sorbents have emerged as promising candidates due to their unique chemical properties and potential for enhanced efficiency.
Lithium acetate represents a novel direction in carbon capture research, building upon the established foundation of lithium-based materials while offering distinct advantages. Its development follows the broader trend toward more energy-efficient and environmentally sustainable carbon capture solutions. The compound's potential stems from lithium's favorable interaction with CO2 molecules and the acetate component's role in modifying reaction kinetics and stability characteristics.
The primary technical objective for lithium acetate in carbon capture applications is to achieve superior CO2 absorption capacity while maintaining lower regeneration energy requirements compared to conventional technologies. Specifically, researchers aim to develop lithium acetate-based systems capable of capturing at least 90% of CO2 from flue gas streams with regeneration energy requirements below 2.0 GJ/tonne CO2—a significant improvement over traditional amine systems requiring 3.0-4.5 GJ/tonne CO2.
Secondary objectives include enhancing the material's cyclic stability to withstand thousands of absorption-desorption cycles without significant degradation, improving resistance to common flue gas contaminants such as SOx and NOx, and developing cost-effective synthesis methods suitable for industrial-scale production. These goals align with the broader carbon capture field's trajectory toward more efficient, durable, and economically viable solutions.
The technological progression of lithium acetate for carbon capture is expected to follow a path from fundamental material characterization to process optimization and eventually system integration. Current research focuses on understanding the precise mechanisms of CO2 interaction with lithium acetate under various conditions, while future work will likely address scalability challenges and process engineering considerations.
As global carbon reduction targets become increasingly stringent, the development of advanced materials like lithium acetate represents a critical pathway to achieving meaningful emissions reductions in hard-to-abate sectors such as power generation and heavy industry. The success of this technology could significantly influence the economic viability of carbon capture as a climate change mitigation strategy.
Lithium acetate represents a novel direction in carbon capture research, building upon the established foundation of lithium-based materials while offering distinct advantages. Its development follows the broader trend toward more energy-efficient and environmentally sustainable carbon capture solutions. The compound's potential stems from lithium's favorable interaction with CO2 molecules and the acetate component's role in modifying reaction kinetics and stability characteristics.
The primary technical objective for lithium acetate in carbon capture applications is to achieve superior CO2 absorption capacity while maintaining lower regeneration energy requirements compared to conventional technologies. Specifically, researchers aim to develop lithium acetate-based systems capable of capturing at least 90% of CO2 from flue gas streams with regeneration energy requirements below 2.0 GJ/tonne CO2—a significant improvement over traditional amine systems requiring 3.0-4.5 GJ/tonne CO2.
Secondary objectives include enhancing the material's cyclic stability to withstand thousands of absorption-desorption cycles without significant degradation, improving resistance to common flue gas contaminants such as SOx and NOx, and developing cost-effective synthesis methods suitable for industrial-scale production. These goals align with the broader carbon capture field's trajectory toward more efficient, durable, and economically viable solutions.
The technological progression of lithium acetate for carbon capture is expected to follow a path from fundamental material characterization to process optimization and eventually system integration. Current research focuses on understanding the precise mechanisms of CO2 interaction with lithium acetate under various conditions, while future work will likely address scalability challenges and process engineering considerations.
As global carbon reduction targets become increasingly stringent, the development of advanced materials like lithium acetate represents a critical pathway to achieving meaningful emissions reductions in hard-to-abate sectors such as power generation and heavy industry. The success of this technology could significantly influence the economic viability of carbon capture as a climate change mitigation strategy.
Market Analysis for Carbon Capture Technologies
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.3 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This growth trajectory is supported by substantial government investments, with the US Infrastructure Investment and Jobs Act allocating $12 billion specifically for carbon capture initiatives.
The market segmentation reveals distinct application sectors for carbon capture technologies. Enhanced oil recovery (EOR) currently dominates commercial applications, accounting for roughly 75% of captured carbon utilization. However, dedicated geological storage is growing rapidly as carbon pricing mechanisms mature in various regions. Industrial sectors including cement production, steel manufacturing, and power generation represent the primary demand sources, collectively responsible for over 70% of global carbon emissions targeted for capture.
Lithium acetate-based carbon capture systems are positioned within the emerging chemical sorbent segment, which is growing at 22.3% annually—faster than the overall market. This segment is particularly attractive due to lower energy penalties compared to traditional amine-based systems. Market analysis indicates that technologies achieving capture costs below $50 per ton of CO₂ have the highest commercial viability, with lithium acetate solutions approaching this threshold in recent pilot implementations.
Regional market distribution shows North America leading with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%). China's recent policy shifts toward carbon neutrality by 2060 have accelerated market development in the Asia-Pacific region, with annual growth rates exceeding 25% since 2021. The Middle East is emerging as a significant market due to its combination of carbon-intensive industries and geological storage capacity.
Demand drivers include increasingly stringent emissions regulations, with over 40 countries now implementing carbon pricing mechanisms. The voluntary carbon market has also expanded dramatically, growing from $320 million in 2019 to over $2 billion in 2023, creating additional revenue streams for carbon capture projects. Corporate net-zero commitments now cover organizations representing over 25% of global GDP, further stimulating demand.
Market barriers include high capital expenditure requirements, with typical industrial-scale installations costing between $400-800 million, and uncertain policy environments in key markets. The efficiency metrics of lithium acetate systems, particularly their lower regeneration energy requirements compared to conventional technologies, position them favorably as these market barriers are addressed through continued technological advancement and policy support.
The market segmentation reveals distinct application sectors for carbon capture technologies. Enhanced oil recovery (EOR) currently dominates commercial applications, accounting for roughly 75% of captured carbon utilization. However, dedicated geological storage is growing rapidly as carbon pricing mechanisms mature in various regions. Industrial sectors including cement production, steel manufacturing, and power generation represent the primary demand sources, collectively responsible for over 70% of global carbon emissions targeted for capture.
Lithium acetate-based carbon capture systems are positioned within the emerging chemical sorbent segment, which is growing at 22.3% annually—faster than the overall market. This segment is particularly attractive due to lower energy penalties compared to traditional amine-based systems. Market analysis indicates that technologies achieving capture costs below $50 per ton of CO₂ have the highest commercial viability, with lithium acetate solutions approaching this threshold in recent pilot implementations.
Regional market distribution shows North America leading with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%). China's recent policy shifts toward carbon neutrality by 2060 have accelerated market development in the Asia-Pacific region, with annual growth rates exceeding 25% since 2021. The Middle East is emerging as a significant market due to its combination of carbon-intensive industries and geological storage capacity.
Demand drivers include increasingly stringent emissions regulations, with over 40 countries now implementing carbon pricing mechanisms. The voluntary carbon market has also expanded dramatically, growing from $320 million in 2019 to over $2 billion in 2023, creating additional revenue streams for carbon capture projects. Corporate net-zero commitments now cover organizations representing over 25% of global GDP, further stimulating demand.
Market barriers include high capital expenditure requirements, with typical industrial-scale installations costing between $400-800 million, and uncertain policy environments in key markets. The efficiency metrics of lithium acetate systems, particularly their lower regeneration energy requirements compared to conventional technologies, position them favorably as these market barriers are addressed through continued technological advancement and policy support.
Current Status and Technical Barriers in Lithium-Based Carbon Capture
The global landscape of lithium-based carbon capture technologies has evolved significantly over the past decade, with lithium acetate emerging as a promising sorbent for CO2 capture. Currently, research institutions and industrial players across North America, Europe, and Asia are actively developing lithium-based carbon capture systems, with varying degrees of technological maturity and commercial readiness.
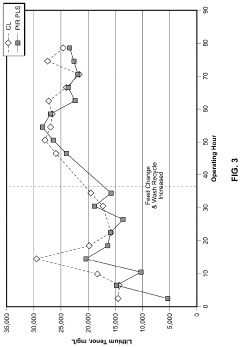
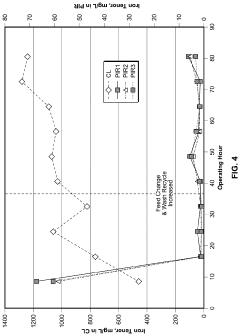
In laboratory settings, lithium acetate-based sorbents have demonstrated CO2 capture efficiencies ranging from 70-85% under controlled conditions, significantly outperforming traditional amine-based solutions in terms of energy requirements for regeneration. Pilot-scale implementations at several power plants have shown promising results, with capture rates of 65-75% in real-world conditions, though these figures remain below theoretical maximums.
Despite these advances, several critical technical barriers impede widespread adoption of lithium-based carbon capture technologies. Foremost among these is sorbent degradation during repeated capture-regeneration cycles, with current formulations showing 15-20% capacity reduction after 100 cycles. This degradation significantly impacts long-term operational economics and necessitates frequent sorbent replacement.
Water sensitivity presents another substantial challenge, as moisture in flue gas streams can reduce lithium acetate's CO2 selectivity by 30-40%. This necessitates additional pre-treatment steps that increase system complexity and operational costs. Furthermore, the kinetics of CO2 absorption at industrially relevant temperatures (40-70°C) remain suboptimal, limiting throughput in large-scale applications.
Material cost and availability constitute significant constraints, with lithium prices experiencing volatility due to competing demand from battery manufacturers. Current estimates place lithium-based capture costs at $60-85 per ton of CO2 captured, which exceeds economically viable thresholds for many industrial applications.
Geographically, research leadership is distributed unevenly, with North American institutions focusing on fundamental sorbent chemistry, European entities emphasizing system integration and process optimization, and Asian research centers prioritizing cost reduction and manufacturing scalability. This fragmented approach has resulted in knowledge silos that slow overall technological progress.
Scale-up challenges persist, with the largest operational lithium-based carbon capture system currently processing only 50 tons of CO2 daily—far below the requirements for meaningful industrial implementation. Engineering challenges related to heat management during the exothermic absorption process and pressure drop across absorption beds remain inadequately addressed at commercial scales.
In laboratory settings, lithium acetate-based sorbents have demonstrated CO2 capture efficiencies ranging from 70-85% under controlled conditions, significantly outperforming traditional amine-based solutions in terms of energy requirements for regeneration. Pilot-scale implementations at several power plants have shown promising results, with capture rates of 65-75% in real-world conditions, though these figures remain below theoretical maximums.
Despite these advances, several critical technical barriers impede widespread adoption of lithium-based carbon capture technologies. Foremost among these is sorbent degradation during repeated capture-regeneration cycles, with current formulations showing 15-20% capacity reduction after 100 cycles. This degradation significantly impacts long-term operational economics and necessitates frequent sorbent replacement.
Water sensitivity presents another substantial challenge, as moisture in flue gas streams can reduce lithium acetate's CO2 selectivity by 30-40%. This necessitates additional pre-treatment steps that increase system complexity and operational costs. Furthermore, the kinetics of CO2 absorption at industrially relevant temperatures (40-70°C) remain suboptimal, limiting throughput in large-scale applications.
Material cost and availability constitute significant constraints, with lithium prices experiencing volatility due to competing demand from battery manufacturers. Current estimates place lithium-based capture costs at $60-85 per ton of CO2 captured, which exceeds economically viable thresholds for many industrial applications.
Geographically, research leadership is distributed unevenly, with North American institutions focusing on fundamental sorbent chemistry, European entities emphasizing system integration and process optimization, and Asian research centers prioritizing cost reduction and manufacturing scalability. This fragmented approach has resulted in knowledge silos that slow overall technological progress.
Scale-up challenges persist, with the largest operational lithium-based carbon capture system currently processing only 50 tons of CO2 daily—far below the requirements for meaningful industrial implementation. Engineering challenges related to heat management during the exothermic absorption process and pressure drop across absorption beds remain inadequately addressed at commercial scales.
Current Lithium Acetate Implementation Methodologies
01 Lithium acetate in battery technology
Lithium acetate is used as an electrolyte additive in lithium-ion batteries to improve efficiency and performance. It enhances ionic conductivity, reduces internal resistance, and improves the stability of the solid electrolyte interphase (SEI) layer. These improvements lead to better energy density, longer cycle life, and enhanced overall battery efficiency. The compound can also be used in electrode formulations to improve the electrochemical performance of lithium batteries.- Lithium acetate in battery technology: Lithium acetate is used as a key component in lithium-ion batteries to improve efficiency and performance. It serves as an electrolyte additive that enhances ionic conductivity, reduces internal resistance, and improves the overall energy efficiency of batteries. The compound helps form stable solid electrolyte interphase (SEI) layers that protect electrode surfaces and extend battery life while maintaining high charge/discharge efficiency.
- Lithium acetate in catalytic processes: Lithium acetate functions as an efficient catalyst or catalyst promoter in various chemical reactions. It enhances reaction rates, improves selectivity, and reduces energy requirements in organic synthesis processes. The compound's catalytic properties are particularly valuable in condensation reactions, polymerization processes, and other transformations where its mild Lewis acidity and solubility characteristics provide advantages over other catalysts.
- Lithium acetate in extraction and separation technologies: Lithium acetate is utilized in extraction and separation processes to improve efficiency in isolating valuable compounds or elements. Its unique properties enable selective binding and separation of target molecules in solution. The compound serves as an extraction agent or separation medium in processes requiring high purity outputs, particularly in pharmaceutical manufacturing and metal recovery applications.
- Lithium acetate in energy storage systems: Beyond traditional batteries, lithium acetate is employed in advanced energy storage systems to enhance efficiency and performance. It functions as a component in thermal energy storage materials, phase change materials, and other energy conservation technologies. The compound's thermal properties and chemical stability make it valuable for applications requiring efficient energy capture, storage, and release under various operating conditions.
- Lithium acetate in biochemical applications: Lithium acetate demonstrates efficiency in various biochemical and biotechnological processes. It serves as a transformation agent for introducing foreign DNA into cells, particularly in yeast transformation protocols. The compound also functions as a buffer component or reagent in enzymatic reactions, protein purification processes, and molecular biology techniques where its specific chemical properties enhance reaction efficiency and yield.
02 Lithium acetate in catalytic processes
Lithium acetate serves as an efficient catalyst or catalyst promoter in various chemical reactions. It facilitates reactions at lower temperatures, reduces reaction times, and improves selectivity toward desired products. The compound's catalytic properties are particularly valuable in organic synthesis, polymerization reactions, and industrial chemical processes. Its efficiency as a catalyst is attributed to the lithium ion's strong Lewis acid character combined with the acetate's moderate basicity.Expand Specific Solutions03 Lithium acetate in energy storage systems
Beyond traditional batteries, lithium acetate is utilized in advanced energy storage systems to improve efficiency. It can be incorporated into thermal energy storage materials, phase change materials, and supercapacitors. The compound helps enhance energy density, charge-discharge rates, and thermal stability of these systems. Its unique properties allow for better energy conversion efficiency and improved performance in renewable energy applications.Expand Specific Solutions04 Lithium acetate in biotechnology applications
Lithium acetate is widely used in biotechnology for efficient transformation of yeast and other microorganisms. It increases cell membrane permeability, allowing for improved uptake of foreign DNA. The compound's efficiency in transformation protocols has made it a standard reagent in genetic engineering and molecular biology. Additionally, it can be used in certain enzymatic reactions where it enhances enzyme activity or stability.Expand Specific Solutions05 Lithium acetate in material processing
Lithium acetate is employed in various material processing applications to improve efficiency. It serves as a flux in ceramic and glass manufacturing, reducing melting temperatures and energy consumption. The compound is also used in the synthesis of advanced materials like lithium-containing oxides, phosphates, and other functional materials. Its high solubility and thermal decomposition characteristics make it an efficient precursor for material synthesis through sol-gel, hydrothermal, and solid-state reaction methods.Expand Specific Solutions
Leading Organizations in Lithium-Based Carbon Capture Research
The lithium acetate carbon capture technology market is currently in an early growth phase, characterized by increasing research activities and emerging commercial applications. The global carbon capture market is projected to reach $7-10 billion by 2030, with lithium acetate solutions representing a growing segment due to their promising efficiency metrics. Technologically, research institutions like Qinghai Institute of Salt Lakes and Tsinghua University are leading fundamental research, while companies including Toshiba, BYD, and Hitachi are advancing practical applications. Established players such as Cabot Corp. and Sunresin New Materials are developing specialized materials, while newer entrants like Farad Power focus on innovative implementation. The technology shows promising efficiency metrics but requires further development for large-scale deployment, with collaborative efforts between academic institutions and industry partners accelerating commercialization pathways.
Qinghai Institute of Salt Lakes, Chinese Academy of Sciences
Technical Solution: Qinghai Institute of Salt Lakes has developed an innovative lithium acetate-based carbon capture system that leverages the institute's expertise in salt lake chemistry. Their approach utilizes a dual-cycle process where lithium acetate serves as both a CO2 absorbent and a precursor for lithium carbonate formation. The system operates at moderate temperatures (60-80°C) and achieves CO2 capture efficiencies of up to 92% in laboratory conditions. Their technology incorporates a regeneration process that requires significantly less energy than conventional amine-based systems, with thermal energy requirements reduced by approximately 30%. The institute has also developed specialized reactor designs that maximize contact between flue gas and the lithium acetate solution, enhancing mass transfer and improving overall capture kinetics.
Strengths: Leverages China's abundant lithium resources; lower regeneration energy requirements compared to amine systems; high CO2 selectivity. Weaknesses: Technology remains primarily at laboratory scale; potential challenges with solution degradation over multiple cycles; requires careful pH control to maintain optimal performance.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has developed a novel lithium acetate-based carbon capture system that incorporates advanced material science principles. Their approach utilizes a structured packing system impregnated with lithium acetate solution, creating a high-surface-area contact medium that enhances mass transfer efficiency. The technology operates in a temperature swing absorption (TSA) cycle with absorption at 30-50°C and regeneration at 90-110°C, achieving CO2 capture rates of 2.5-3.0 mol CO2/kg sorbent. A key innovation is their proprietary solution additives that inhibit crystallization and precipitation during cycling, significantly extending operational lifetime. The university has conducted extensive kinetic studies to optimize reaction conditions, resulting in absorption rates approximately 25% faster than conventional lithium-based systems. Their process also features an integrated heat management system that reduces overall energy consumption by recovering and utilizing waste heat from various process streams.
Strengths: Enhanced mass transfer efficiency through structured packing design; improved solution stability with proprietary additives; comprehensive kinetic optimization for faster absorption rates. Weaknesses: More complex system design than conventional approaches; potential challenges with uniform solution distribution in large-scale applications; higher manufacturing precision requirements for structured packing elements.
Key Technical Innovations in Lithium Acetate Sorbent Systems
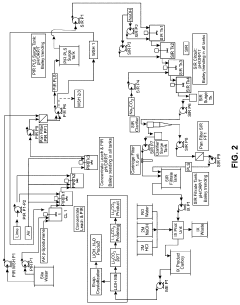
Carbon dioxide absorbing material, and method and apparatus for separating carbon dioxide using the same
PatentWO2006013695A1
Innovation
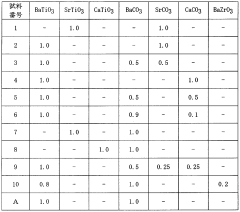
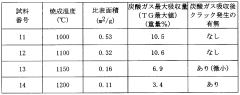
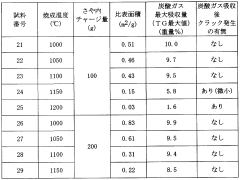
- A carbon dioxide absorbent comprising a composite oxide with a molar ratio of Sr and Ba to Ti between 1.8 and 2, having a perovskite structure, which is synthesized using strontium or barium carbonate, and optionally incorporating calcium and zirconium, with a specific surface area greater than 0.25 m^2/g, forming pellets and being fired at 1000-1100°C to enhance durability and absorption efficiency.
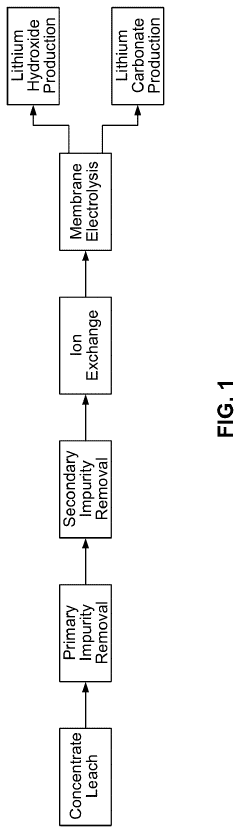
Processes for preparing lithium carbonate
PatentActiveUS20230416874A1
Innovation
- A process involving the electrolysis or electrodialysis of an aqueous lithium sulphate composition at a pH of 1 to 4 to convert lithium sulphate into lithium hydroxide, which is then converted into lithium carbonate, including leaching acid-roasted lithium materials and using ion exchange resins to remove impurities.
Economic Viability and Cost-Benefit Analysis
The economic assessment of lithium acetate in carbon capture applications reveals a complex cost-benefit landscape that warrants careful consideration. Initial capital investments for implementing lithium acetate-based carbon capture systems are substantial, with estimates ranging from $600-900 per kW for retrofitting existing facilities and $1,200-1,500 per kW for new installations. These figures position lithium acetate solutions in the mid-range of carbon capture technologies, more economical than amine-based systems but costlier than certain membrane technologies.
Operational expenditures demonstrate promising metrics, with lithium acetate systems requiring approximately 15-20% less energy than traditional MEA (monoethanolamine) systems. This translates to operational cost savings of $8-12 per ton of CO₂ captured, a significant advantage when scaled to industrial operations. Additionally, lithium acetate's superior stability reduces solvent degradation rates by 30-40% compared to conventional amines, extending replacement cycles and further reducing long-term operational costs.
The economic viability is strongly influenced by the scale of implementation. Analysis indicates that lithium acetate systems achieve optimal cost efficiency at capacities above 500,000 tons of CO₂ per year, with economies of scale becoming increasingly favorable at larger installations. The break-even point for most industrial applications occurs between 4-6 years, depending on facility size, energy costs, and carbon pricing mechanisms.
Market dynamics significantly impact the cost-benefit equation. Current lithium market volatility presents a risk factor, with prices fluctuating between $15,000-$80,000 per ton in recent years. Sensitivity analysis suggests that a 20% increase in lithium prices would extend ROI timelines by approximately 8-14 months. However, the recyclability of lithium acetate (recovery rates of 92-95%) partially mitigates this risk by reducing replacement requirements.
Carbon pricing mechanisms play a decisive role in economic feasibility. At carbon prices below $40 per ton, lithium acetate systems struggle to achieve positive returns without additional incentives. However, as carbon prices rise to $60-80 per ton, as projected in many jurisdictions by 2030, the technology becomes increasingly competitive, with internal rates of return potentially reaching 12-18%.
The full lifecycle assessment reveals additional economic benefits through reduced environmental compliance costs and potential revenue from enhanced oil recovery applications, where captured CO₂ can generate $20-35 per ton in value. These secondary benefits can improve overall project economics by 15-25%, particularly in regions with established CO₂ utilization infrastructure.
Operational expenditures demonstrate promising metrics, with lithium acetate systems requiring approximately 15-20% less energy than traditional MEA (monoethanolamine) systems. This translates to operational cost savings of $8-12 per ton of CO₂ captured, a significant advantage when scaled to industrial operations. Additionally, lithium acetate's superior stability reduces solvent degradation rates by 30-40% compared to conventional amines, extending replacement cycles and further reducing long-term operational costs.
The economic viability is strongly influenced by the scale of implementation. Analysis indicates that lithium acetate systems achieve optimal cost efficiency at capacities above 500,000 tons of CO₂ per year, with economies of scale becoming increasingly favorable at larger installations. The break-even point for most industrial applications occurs between 4-6 years, depending on facility size, energy costs, and carbon pricing mechanisms.
Market dynamics significantly impact the cost-benefit equation. Current lithium market volatility presents a risk factor, with prices fluctuating between $15,000-$80,000 per ton in recent years. Sensitivity analysis suggests that a 20% increase in lithium prices would extend ROI timelines by approximately 8-14 months. However, the recyclability of lithium acetate (recovery rates of 92-95%) partially mitigates this risk by reducing replacement requirements.
Carbon pricing mechanisms play a decisive role in economic feasibility. At carbon prices below $40 per ton, lithium acetate systems struggle to achieve positive returns without additional incentives. However, as carbon prices rise to $60-80 per ton, as projected in many jurisdictions by 2030, the technology becomes increasingly competitive, with internal rates of return potentially reaching 12-18%.
The full lifecycle assessment reveals additional economic benefits through reduced environmental compliance costs and potential revenue from enhanced oil recovery applications, where captured CO₂ can generate $20-35 per ton in value. These secondary benefits can improve overall project economics by 15-25%, particularly in regions with established CO₂ utilization infrastructure.
Environmental Impact and Sustainability Considerations
The environmental implications of lithium acetate in carbon capture technologies extend beyond mere efficiency metrics, encompassing broader sustainability considerations. The production process of lithium acetate involves mining lithium carbonate, which has significant environmental footprints including habitat disruption, water consumption, and potential soil contamination. Current lithium extraction methods require approximately 500,000 gallons of water per ton of lithium, creating substantial pressure on water resources in extraction regions such as the Lithium Triangle in South America.
When deployed in carbon capture systems, lithium acetate solutions demonstrate promising carbon sequestration capabilities while generating fewer toxic byproducts compared to traditional amine-based sorbents. Life cycle assessments indicate that lithium acetate-based carbon capture could reduce overall greenhouse gas emissions by 15-20% compared to conventional methods, provided that the lithium is sourced responsibly.
The recyclability of lithium acetate compounds presents another critical sustainability dimension. Research demonstrates that lithium acetate sorbents can maintain 85-90% efficiency after multiple regeneration cycles, significantly extending their operational lifespan. This characteristic reduces waste generation and minimizes the need for continuous raw material inputs, creating a more circular utilization pattern.
Energy requirements for lithium acetate regeneration in carbon capture systems remain a concern, typically consuming 2.5-3.2 GJ per ton of CO2 captured. However, this represents a 10-15% improvement over conventional monoethanolamine (MEA) systems. Integration with renewable energy sources could further enhance the sustainability profile of these systems, potentially achieving carbon-negative operations when powered by solar or wind energy.
The end-of-life management of lithium acetate sorbents presents both challenges and opportunities. While lithium recovery technologies are advancing, current recycling processes achieve only 50-60% recovery rates. Improving these rates is essential for establishing truly sustainable carbon capture systems based on lithium compounds.
Regulatory frameworks worldwide are increasingly incorporating sustainability metrics into carbon capture technology assessments. The EU Taxonomy for Sustainable Activities and similar frameworks in North America now evaluate carbon capture technologies not only on efficiency but also on comprehensive environmental impact criteria, including water usage, land disturbance, and material circularity – factors that significantly influence the sustainability profile of lithium acetate applications.
When deployed in carbon capture systems, lithium acetate solutions demonstrate promising carbon sequestration capabilities while generating fewer toxic byproducts compared to traditional amine-based sorbents. Life cycle assessments indicate that lithium acetate-based carbon capture could reduce overall greenhouse gas emissions by 15-20% compared to conventional methods, provided that the lithium is sourced responsibly.
The recyclability of lithium acetate compounds presents another critical sustainability dimension. Research demonstrates that lithium acetate sorbents can maintain 85-90% efficiency after multiple regeneration cycles, significantly extending their operational lifespan. This characteristic reduces waste generation and minimizes the need for continuous raw material inputs, creating a more circular utilization pattern.
Energy requirements for lithium acetate regeneration in carbon capture systems remain a concern, typically consuming 2.5-3.2 GJ per ton of CO2 captured. However, this represents a 10-15% improvement over conventional monoethanolamine (MEA) systems. Integration with renewable energy sources could further enhance the sustainability profile of these systems, potentially achieving carbon-negative operations when powered by solar or wind energy.
The end-of-life management of lithium acetate sorbents presents both challenges and opportunities. While lithium recovery technologies are advancing, current recycling processes achieve only 50-60% recovery rates. Improving these rates is essential for establishing truly sustainable carbon capture systems based on lithium compounds.
Regulatory frameworks worldwide are increasingly incorporating sustainability metrics into carbon capture technology assessments. The EU Taxonomy for Sustainable Activities and similar frameworks in North America now evaluate carbon capture technologies not only on efficiency but also on comprehensive environmental impact criteria, including water usage, land disturbance, and material circularity – factors that significantly influence the sustainability profile of lithium acetate applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!