Biomedical Applications of Perchloric Acid in Drug Development
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in Drug Development: Background and Objectives
Perchloric acid, a powerful oxidizing agent, has emerged as a significant compound in the field of drug development and biomedical applications. Its unique chemical properties and versatility have attracted considerable attention from researchers and pharmaceutical companies alike. The evolution of perchloric acid's role in this domain can be traced back to the mid-20th century when its potential in analytical chemistry was first recognized.
As the pharmaceutical industry progressed, the need for more efficient and precise analytical methods grew, leading to the exploration of perchloric acid's capabilities in drug analysis and synthesis. Its strong oxidizing properties made it particularly useful in the breakdown of complex organic compounds, enabling more accurate quantification and characterization of drug molecules. This application laid the foundation for its expanded use in various stages of drug development.
The objectives of utilizing perchloric acid in drug development are multifaceted. Primarily, it aims to enhance the efficiency and accuracy of drug analysis, enabling researchers to better understand the chemical composition and behavior of potential pharmaceutical compounds. Additionally, perchloric acid's role extends to improving the synthesis of certain drug molecules, where its oxidizing properties can facilitate specific chemical reactions crucial to the production process.
Another key objective is to leverage perchloric acid's unique characteristics in the development of novel drug delivery systems. Its ability to form stable perchlorate salts with various organic compounds has opened up new possibilities for creating more effective and targeted drug formulations. This aspect of perchloric acid's application aligns with the growing trend towards personalized medicine and advanced drug delivery technologies.
Furthermore, the use of perchloric acid in biomedical applications aims to advance diagnostic techniques. Its role in sample preparation and analysis has proven valuable in developing more sensitive and specific diagnostic tools, particularly in the detection of trace elements and biomarkers associated with various diseases. This objective underscores the compound's potential to contribute to early disease detection and more accurate monitoring of treatment efficacy.
As research in this field progresses, the overarching goal is to fully exploit the capabilities of perchloric acid while addressing the challenges associated with its use, such as safety concerns and environmental impact. The ongoing efforts focus on optimizing its application in drug development processes, exploring new methodologies that maximize its benefits while minimizing potential risks. This balanced approach is crucial for the sustainable integration of perchloric acid in the biomedical and pharmaceutical industries.
As the pharmaceutical industry progressed, the need for more efficient and precise analytical methods grew, leading to the exploration of perchloric acid's capabilities in drug analysis and synthesis. Its strong oxidizing properties made it particularly useful in the breakdown of complex organic compounds, enabling more accurate quantification and characterization of drug molecules. This application laid the foundation for its expanded use in various stages of drug development.
The objectives of utilizing perchloric acid in drug development are multifaceted. Primarily, it aims to enhance the efficiency and accuracy of drug analysis, enabling researchers to better understand the chemical composition and behavior of potential pharmaceutical compounds. Additionally, perchloric acid's role extends to improving the synthesis of certain drug molecules, where its oxidizing properties can facilitate specific chemical reactions crucial to the production process.
Another key objective is to leverage perchloric acid's unique characteristics in the development of novel drug delivery systems. Its ability to form stable perchlorate salts with various organic compounds has opened up new possibilities for creating more effective and targeted drug formulations. This aspect of perchloric acid's application aligns with the growing trend towards personalized medicine and advanced drug delivery technologies.
Furthermore, the use of perchloric acid in biomedical applications aims to advance diagnostic techniques. Its role in sample preparation and analysis has proven valuable in developing more sensitive and specific diagnostic tools, particularly in the detection of trace elements and biomarkers associated with various diseases. This objective underscores the compound's potential to contribute to early disease detection and more accurate monitoring of treatment efficacy.
As research in this field progresses, the overarching goal is to fully exploit the capabilities of perchloric acid while addressing the challenges associated with its use, such as safety concerns and environmental impact. The ongoing efforts focus on optimizing its application in drug development processes, exploring new methodologies that maximize its benefits while minimizing potential risks. This balanced approach is crucial for the sustainable integration of perchloric acid in the biomedical and pharmaceutical industries.
Market Analysis for Perchloric Acid in Pharmaceuticals
The market for perchloric acid in pharmaceutical applications has been experiencing steady growth, driven by its unique properties and versatility in drug development processes. As a strong oxidizing agent, perchloric acid plays a crucial role in various stages of pharmaceutical research and production, particularly in analytical chemistry and synthesis of active pharmaceutical ingredients (APIs).
In recent years, the global pharmaceutical industry has witnessed a surge in research and development activities, with a focus on novel drug formulations and improved manufacturing processes. This trend has significantly boosted the demand for high-purity reagents like perchloric acid. The market for perchloric acid in pharmaceuticals is closely tied to the overall growth of the drug development sector, which has been expanding at a robust rate due to increasing healthcare needs worldwide.
One of the key factors driving the market is the rising prevalence of chronic diseases and the subsequent need for innovative drug therapies. Perchloric acid's applications in drug analysis, quality control, and purification processes make it an essential component in the development of new medications targeting these conditions. Additionally, the growing emphasis on personalized medicine and targeted therapies has further amplified the demand for precise analytical tools, where perchloric acid excels.
The market landscape is characterized by a mix of established pharmaceutical companies and emerging biotech firms, all contributing to the increased consumption of perchloric acid. Geographically, North America and Europe dominate the market due to their advanced pharmaceutical research infrastructure and stringent quality control regulations. However, Asia-Pacific is emerging as a rapidly growing market, driven by the expansion of the pharmaceutical industry in countries like China and India.
Despite its growth potential, the market faces certain challenges. Stringent regulations regarding the handling and disposal of perchloric acid, due to its hazardous nature, can impact its adoption in some regions. Moreover, the search for safer alternatives in certain applications may pose a threat to market growth. However, ongoing research into safer handling methods and the development of specialized perchloric acid formulations for pharmaceutical use are expected to mitigate these concerns.
Looking ahead, the market for perchloric acid in pharmaceuticals is projected to continue its growth trajectory. The increasing focus on biopharmaceuticals and the advent of advanced drug delivery systems are likely to create new opportunities for perchloric acid applications. Furthermore, the trend towards continuous manufacturing in the pharmaceutical industry may lead to increased demand for high-purity reagents like perchloric acid, which are essential for maintaining consistent quality in production processes.
In recent years, the global pharmaceutical industry has witnessed a surge in research and development activities, with a focus on novel drug formulations and improved manufacturing processes. This trend has significantly boosted the demand for high-purity reagents like perchloric acid. The market for perchloric acid in pharmaceuticals is closely tied to the overall growth of the drug development sector, which has been expanding at a robust rate due to increasing healthcare needs worldwide.
One of the key factors driving the market is the rising prevalence of chronic diseases and the subsequent need for innovative drug therapies. Perchloric acid's applications in drug analysis, quality control, and purification processes make it an essential component in the development of new medications targeting these conditions. Additionally, the growing emphasis on personalized medicine and targeted therapies has further amplified the demand for precise analytical tools, where perchloric acid excels.
The market landscape is characterized by a mix of established pharmaceutical companies and emerging biotech firms, all contributing to the increased consumption of perchloric acid. Geographically, North America and Europe dominate the market due to their advanced pharmaceutical research infrastructure and stringent quality control regulations. However, Asia-Pacific is emerging as a rapidly growing market, driven by the expansion of the pharmaceutical industry in countries like China and India.
Despite its growth potential, the market faces certain challenges. Stringent regulations regarding the handling and disposal of perchloric acid, due to its hazardous nature, can impact its adoption in some regions. Moreover, the search for safer alternatives in certain applications may pose a threat to market growth. However, ongoing research into safer handling methods and the development of specialized perchloric acid formulations for pharmaceutical use are expected to mitigate these concerns.
Looking ahead, the market for perchloric acid in pharmaceuticals is projected to continue its growth trajectory. The increasing focus on biopharmaceuticals and the advent of advanced drug delivery systems are likely to create new opportunities for perchloric acid applications. Furthermore, the trend towards continuous manufacturing in the pharmaceutical industry may lead to increased demand for high-purity reagents like perchloric acid, which are essential for maintaining consistent quality in production processes.
Current Challenges in Biomedical Applications of Perchloric Acid
The biomedical applications of perchloric acid in drug development face several significant challenges that hinder its widespread adoption and efficacy. One of the primary obstacles is the inherent instability and reactivity of perchloric acid, which poses safety concerns during handling and storage. This high reactivity can lead to unwanted side reactions in complex biological systems, potentially altering the intended drug compounds or producing harmful byproducts.
Another major challenge lies in the precise control of perchloric acid concentration and pH in biological environments. The strong oxidizing properties of perchloric acid can disrupt cellular processes and damage sensitive biomolecules, making it difficult to maintain the delicate balance required for drug efficacy without causing unintended harm to surrounding tissues.
The potential for perchlorate contamination in drug formulations presents a significant regulatory hurdle. Stringent guidelines set by regulatory bodies such as the FDA and EMA require extensive testing and validation to ensure the absence of perchlorate residues in final drug products. This adds complexity and cost to the drug development process, potentially deterring pharmaceutical companies from exploring perchloric acid-based methodologies.
Furthermore, the limited understanding of perchloric acid's long-term effects on biological systems poses a challenge in assessing the safety profile of drugs developed using this compound. Chronic exposure to even trace amounts of perchlorate has been linked to thyroid dysfunction, raising concerns about the potential risks associated with perchloric acid-derived pharmaceuticals.
The scalability of perchloric acid-based processes in drug manufacturing presents technical challenges. Ensuring consistent quality and safety at industrial scales requires specialized equipment and rigorous process controls, which can be cost-prohibitive for many pharmaceutical companies.
Lastly, the environmental impact of perchloric acid usage in drug development is a growing concern. The potential release of perchlorate into water systems during manufacturing or disposal processes necessitates the development of effective treatment and containment strategies, adding another layer of complexity to the already challenging landscape of pharmaceutical research and development.
Another major challenge lies in the precise control of perchloric acid concentration and pH in biological environments. The strong oxidizing properties of perchloric acid can disrupt cellular processes and damage sensitive biomolecules, making it difficult to maintain the delicate balance required for drug efficacy without causing unintended harm to surrounding tissues.
The potential for perchlorate contamination in drug formulations presents a significant regulatory hurdle. Stringent guidelines set by regulatory bodies such as the FDA and EMA require extensive testing and validation to ensure the absence of perchlorate residues in final drug products. This adds complexity and cost to the drug development process, potentially deterring pharmaceutical companies from exploring perchloric acid-based methodologies.
Furthermore, the limited understanding of perchloric acid's long-term effects on biological systems poses a challenge in assessing the safety profile of drugs developed using this compound. Chronic exposure to even trace amounts of perchlorate has been linked to thyroid dysfunction, raising concerns about the potential risks associated with perchloric acid-derived pharmaceuticals.
The scalability of perchloric acid-based processes in drug manufacturing presents technical challenges. Ensuring consistent quality and safety at industrial scales requires specialized equipment and rigorous process controls, which can be cost-prohibitive for many pharmaceutical companies.
Lastly, the environmental impact of perchloric acid usage in drug development is a growing concern. The potential release of perchlorate into water systems during manufacturing or disposal processes necessitates the development of effective treatment and containment strategies, adding another layer of complexity to the already challenging landscape of pharmaceutical research and development.
Existing Methodologies for Perchloric Acid in Drug Development
01 Synthesis and production of perchloric acid
Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to efficiently generate perchloric acid while maintaining safety and quality standards.- Synthesis and production of perchloric acid: Various methods and processes for synthesizing and producing perchloric acid are described. These may include electrochemical processes, chemical reactions, or industrial-scale production techniques. The focus is on improving efficiency, yield, and purity of the final product.
- Applications of perchloric acid in chemical analysis: Perchloric acid is widely used in chemical analysis due to its strong oxidizing properties. It is employed in various analytical techniques, including spectroscopy, chromatography, and elemental analysis. The acid's ability to dissolve many substances makes it valuable in sample preparation and digestion processes.
- Safety measures and handling of perchloric acid: Due to its highly reactive and potentially explosive nature, special safety measures are required when handling perchloric acid. This includes the use of specialized equipment, protective gear, and storage facilities. Proper disposal methods and emergency procedures are also crucial to prevent accidents and environmental contamination.
- Perchloric acid in battery technology: Perchloric acid and its derivatives play a role in battery technology, particularly in the development of high-performance batteries. It may be used in electrolytes or as a component in electrode materials to enhance battery efficiency, capacity, or longevity.
- Purification and concentration of perchloric acid: Various methods for purifying and concentrating perchloric acid are described. These may include distillation techniques, membrane processes, or chemical treatments to remove impurities and achieve higher concentrations. The goal is to produce high-purity perchloric acid for specialized applications in research and industry.
02 Applications of perchloric acid in chemical analysis
Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in sample preparation, digestion of materials for elemental analysis, and as a component in specialized analytical reagents.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Specialized equipment, protocols, and safety measures for handling and storing perchloric acid due to its highly corrosive and potentially explosive nature. This includes the design of dedicated fume hoods, storage containers, and disposal methods to minimize risks associated with perchloric acid use.Expand Specific Solutions04 Perchloric acid in battery technology
Use of perchloric acid in the development and improvement of battery technologies. This may involve its application in electrolyte formulations, electrode materials, or other battery components to enhance performance, energy density, or longevity of various types of batteries.Expand Specific Solutions05 Perchloric acid in material processing
Applications of perchloric acid in various material processing techniques, such as etching, surface treatment, or purification of materials. This includes its use in semiconductor manufacturing, metal surface preparation, and other industrial processes where its strong oxidizing properties are beneficial.Expand Specific Solutions
Key Players in Perchloric Acid and Pharmaceutical Industries
The biomedical applications of perchloric acid in drug development represent a niche yet evolving field within the pharmaceutical industry. The market is in its early growth stage, with a relatively small but expanding market size. The technology's maturity is still developing, with key players like Arena Pharmaceuticals, Takeda Pharmaceutical, and Astex Therapeutics leading research efforts. These companies are investing in R&D to explore perchloric acid's potential in drug synthesis and formulation. The competitive landscape is characterized by a mix of established pharmaceutical giants and specialized biotech firms, each leveraging their unique expertise to advance the technology. As the field progresses, we can expect increased collaboration between academia and industry to drive innovation and market growth.
Arena Pharmaceuticals, Inc.
Technical Solution: Arena Pharmaceuticals has developed a novel approach using perchloric acid in drug development, particularly for small molecule therapeutics. Their method involves using perchloric acid as a catalyst in the synthesis of complex pharmaceutical compounds, enabling more efficient and cost-effective production processes. The company has implemented a proprietary platform that utilizes perchloric acid's strong oxidizing properties to facilitate challenging chemical transformations in drug candidates[1]. This technology has been applied to several of their pipeline products, including those targeting G protein-coupled receptors (GPCRs)[2]. Arena's approach also incorporates safety measures to handle the reactive nature of perchloric acid, such as specialized containment systems and neutralization protocols[3].
Strengths: Improved synthesis efficiency, cost-effective production, and ability to perform complex transformations. Weaknesses: Safety concerns due to perchloric acid's reactivity, potential environmental impact, and the need for specialized handling equipment.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda Pharmaceutical has integrated perchloric acid into their biomedical research and drug development processes, focusing on its applications in analytical chemistry and drug purification. The company utilizes perchloric acid in high-performance liquid chromatography (HPLC) methods for the quantification and characterization of drug molecules and their metabolites[4]. Takeda has also developed a proprietary extraction technique using perchloric acid to isolate and purify bioactive compounds from natural sources, enhancing their drug discovery efforts[5]. Additionally, the company employs perchloric acid in the preparation of biological samples for mass spectrometry analysis, improving the detection and quantification of target molecules in complex matrices[6]. Takeda's approach includes rigorous safety protocols and specialized waste management systems to address the challenges associated with perchloric acid use.
Strengths: Enhanced analytical capabilities, improved drug purification processes, and versatile applications in biomedical research. Weaknesses: Requires strict safety measures, potential for equipment corrosion, and the need for specialized disposal methods.
Innovative Applications of Perchloric Acid in Biomedicine
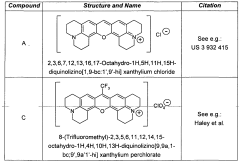
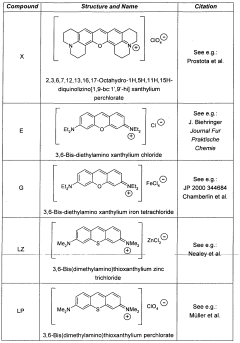
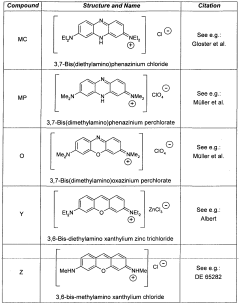
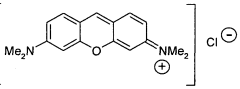
3,6-disubstituted xanthylium salts
PatentWO2010067078A2
Innovation
- Development of specific xanthylium compounds that act as tau protein aggregation inhibitors, disrupting the structure of paired helical filaments and reversing proteolytic stability, thereby preventing the progression of tauopathies.
Hydrochloride salt of ((1S,2S,4R)-4-{4-[(1S)-2,3-dihydro-1H-inden-1-ylamino] -7H-pyrrolo [2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl)methyl sulfamate
PatentInactiveAU2013204831A1
Innovation
- Novel hydrochloride salt form of ((1S,2S,4R)-4-{4-[(1S)-2,3-dihydro-1H-inden-1-ylamino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl)methyl sulfamate with improved physical properties for manufacturing and formulation.
- Development of crystalline forms of the hydrochloride salt with specific properties suitable for large-scale manufacturing, pharmaceutical formulation, and storage.
- Creation of pharmaceutical compositions comprising the hydrochloride salt or its crystalline forms for treating various diseases related to E1 activity inhibition.
Safety Regulations for Perchloric Acid in Laboratories
The use of perchloric acid in laboratories, particularly for biomedical applications and drug development, necessitates stringent safety regulations due to its highly reactive and potentially explosive nature. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the National Fire Protection Association (NFPA) have established comprehensive guidelines for the handling, storage, and disposal of perchloric acid.
Laboratory personnel must undergo specialized training before working with perchloric acid. This training covers proper handling techniques, personal protective equipment (PPE) requirements, and emergency response procedures. PPE for perchloric acid handling typically includes chemical-resistant gloves, splash-proof goggles, and a lab coat or chemical-resistant apron.
Storage regulations for perchloric acid are particularly strict. It must be kept in dedicated, properly labeled containers made of glass or other inert materials. These containers should be stored in a cool, well-ventilated area, away from organic materials, dehydrating agents, and other incompatible substances. Secondary containment is often required to prevent spills from spreading.
Ventilation is a critical aspect of laboratory safety when working with perchloric acid. Fume hoods used for perchloric acid work must be specially designed with wash-down systems to prevent the accumulation of explosive perchlorates. Regular cleaning and maintenance of these hoods are mandatory to ensure their effectiveness and prevent potential hazards.
Waste disposal of perchloric acid and its byproducts requires careful consideration. Neutralization and dilution procedures must be followed before disposal, and in many cases, specialized waste management services are required. Laboratories must maintain detailed records of perchloric acid usage, storage, and disposal to comply with regulatory requirements.
Emergency response plans for perchloric acid incidents must be in place and regularly reviewed. These plans should include procedures for spill containment, evacuation protocols, and first aid measures. Specialized spill kits designed for perchloric acid must be readily available in areas where it is used or stored.
Regular safety audits and inspections are essential to ensure compliance with perchloric acid regulations. These audits should assess storage conditions, ventilation systems, PPE availability, and adherence to handling protocols. Any deficiencies identified must be promptly addressed to maintain a safe working environment.
In the context of biomedical applications and drug development, additional regulations may apply. For instance, Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) guidelines may impose further requirements on the use of perchloric acid in research and production settings. These regulations aim to ensure the quality and reproducibility of scientific data while maintaining the highest safety standards.
Laboratory personnel must undergo specialized training before working with perchloric acid. This training covers proper handling techniques, personal protective equipment (PPE) requirements, and emergency response procedures. PPE for perchloric acid handling typically includes chemical-resistant gloves, splash-proof goggles, and a lab coat or chemical-resistant apron.
Storage regulations for perchloric acid are particularly strict. It must be kept in dedicated, properly labeled containers made of glass or other inert materials. These containers should be stored in a cool, well-ventilated area, away from organic materials, dehydrating agents, and other incompatible substances. Secondary containment is often required to prevent spills from spreading.
Ventilation is a critical aspect of laboratory safety when working with perchloric acid. Fume hoods used for perchloric acid work must be specially designed with wash-down systems to prevent the accumulation of explosive perchlorates. Regular cleaning and maintenance of these hoods are mandatory to ensure their effectiveness and prevent potential hazards.
Waste disposal of perchloric acid and its byproducts requires careful consideration. Neutralization and dilution procedures must be followed before disposal, and in many cases, specialized waste management services are required. Laboratories must maintain detailed records of perchloric acid usage, storage, and disposal to comply with regulatory requirements.
Emergency response plans for perchloric acid incidents must be in place and regularly reviewed. These plans should include procedures for spill containment, evacuation protocols, and first aid measures. Specialized spill kits designed for perchloric acid must be readily available in areas where it is used or stored.
Regular safety audits and inspections are essential to ensure compliance with perchloric acid regulations. These audits should assess storage conditions, ventilation systems, PPE availability, and adherence to handling protocols. Any deficiencies identified must be promptly addressed to maintain a safe working environment.
In the context of biomedical applications and drug development, additional regulations may apply. For instance, Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) guidelines may impose further requirements on the use of perchloric acid in research and production settings. These regulations aim to ensure the quality and reproducibility of scientific data while maintaining the highest safety standards.
Environmental Impact of Perchloric Acid in Drug Manufacturing
The use of perchloric acid in drug manufacturing processes has raised significant environmental concerns due to its potential impact on ecosystems and human health. As a strong oxidizing agent, perchloric acid can persist in the environment and contaminate soil and water sources if not properly managed. The release of perchlorate ions, a byproduct of perchloric acid, into the environment has been linked to various ecological and health issues.
One of the primary environmental concerns is the contamination of groundwater and surface water. Perchlorate ions are highly soluble and mobile in water, making them difficult to remove once they enter aquatic systems. This contamination can affect drinking water sources and pose risks to aquatic life. Studies have shown that perchlorate can accumulate in plants and animals, potentially disrupting thyroid function and affecting growth and development in various species.
Soil contamination is another significant issue associated with perchloric acid use in drug manufacturing. Improper disposal or accidental spills can lead to the accumulation of perchlorate in soil, which may persist for extended periods. This can impact soil fertility and affect the growth of vegetation in contaminated areas. Furthermore, perchlorate in soil can be taken up by plants, potentially entering the food chain and posing risks to both wildlife and human consumers.
Air pollution is also a concern, particularly in facilities where perchloric acid is used in large quantities. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during manufacturing processes involving perchloric acid. These emissions can contribute to local air quality issues and potentially affect the health of workers and nearby communities.
To mitigate these environmental impacts, strict regulations and best practices have been implemented in the pharmaceutical industry. These include advanced wastewater treatment systems to remove perchlorate ions, proper handling and storage protocols to prevent spills, and air pollution control technologies to minimize emissions. Additionally, some manufacturers are exploring alternative oxidizing agents or developing new synthesis routes that reduce or eliminate the need for perchloric acid in drug production.
Research into more environmentally friendly alternatives is ongoing, with a focus on green chemistry principles. This includes the development of less hazardous oxidizing agents, catalytic processes that reduce the amount of perchloric acid required, and closed-loop systems that minimize waste generation and environmental release. These efforts aim to balance the need for efficient drug manufacturing processes with environmental protection and sustainability goals.
One of the primary environmental concerns is the contamination of groundwater and surface water. Perchlorate ions are highly soluble and mobile in water, making them difficult to remove once they enter aquatic systems. This contamination can affect drinking water sources and pose risks to aquatic life. Studies have shown that perchlorate can accumulate in plants and animals, potentially disrupting thyroid function and affecting growth and development in various species.
Soil contamination is another significant issue associated with perchloric acid use in drug manufacturing. Improper disposal or accidental spills can lead to the accumulation of perchlorate in soil, which may persist for extended periods. This can impact soil fertility and affect the growth of vegetation in contaminated areas. Furthermore, perchlorate in soil can be taken up by plants, potentially entering the food chain and posing risks to both wildlife and human consumers.
Air pollution is also a concern, particularly in facilities where perchloric acid is used in large quantities. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during manufacturing processes involving perchloric acid. These emissions can contribute to local air quality issues and potentially affect the health of workers and nearby communities.
To mitigate these environmental impacts, strict regulations and best practices have been implemented in the pharmaceutical industry. These include advanced wastewater treatment systems to remove perchlorate ions, proper handling and storage protocols to prevent spills, and air pollution control technologies to minimize emissions. Additionally, some manufacturers are exploring alternative oxidizing agents or developing new synthesis routes that reduce or eliminate the need for perchloric acid in drug production.
Research into more environmentally friendly alternatives is ongoing, with a focus on green chemistry principles. This includes the development of less hazardous oxidizing agents, catalytic processes that reduce the amount of perchloric acid required, and closed-loop systems that minimize waste generation and environmental release. These efforts aim to balance the need for efficient drug manufacturing processes with environmental protection and sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!