Biosensor Improvements Utilizing Transverse Waves in Medical Diagnosis
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Transverse Wave Biosensors: Background and Objectives
Transverse wave biosensors represent a significant advancement in medical diagnostic technology, leveraging the unique properties of transverse waves to enhance sensitivity and accuracy in detecting various biomarkers. The development of these biosensors has its roots in the broader field of biosensing, which has seen rapid growth over the past few decades due to increasing demands for point-of-care diagnostics and personalized medicine.
The evolution of biosensor technology can be traced back to the 1960s with the development of the first glucose sensor. Since then, the field has expanded dramatically, incorporating various sensing mechanisms and targeting a wide range of analytes. The introduction of transverse wave technology to biosensors marks a new chapter in this progression, offering potential improvements in detection limits, response times, and overall performance.
Transverse waves, also known as shear waves, are characterized by oscillations perpendicular to the direction of wave propagation. In the context of biosensors, these waves can be generated and detected using various methods, including surface acoustic wave (SAW) devices, quartz crystal microbalances (QCM), and magnetoelastic sensors. The unique interaction of transverse waves with biological materials provides a novel approach to sensing that complements and potentially surpasses traditional biosensing methods.
The primary objective of incorporating transverse waves in biosensors is to enhance the sensitivity and specificity of medical diagnostics. By exploiting the properties of these waves, researchers aim to develop sensors capable of detecting minute quantities of biomarkers associated with various diseases, including cancer, cardiovascular disorders, and infectious diseases. This improved detection capability could lead to earlier diagnosis, more accurate monitoring of disease progression, and better-informed treatment decisions.
Another key goal is to create more robust and versatile biosensing platforms that can operate in complex biological environments. Transverse wave biosensors have shown promise in overcoming some of the limitations of conventional sensors, such as interference from non-specific binding and reduced performance in viscous media. This adaptability makes them particularly attractive for in vivo applications and continuous monitoring scenarios.
Furthermore, the development of transverse wave biosensors aligns with the broader trend towards miniaturization and integration in medical devices. The compact nature of many transverse wave sensing elements facilitates the creation of portable, lab-on-a-chip devices that can bring advanced diagnostic capabilities to resource-limited settings or enable real-time monitoring in clinical environments.
As research in this field progresses, the ultimate aim is to translate these technological advancements into practical, cost-effective diagnostic tools that can significantly impact healthcare delivery and patient outcomes. This involves not only refining the sensing mechanisms but also addressing challenges related to manufacturability, reliability, and integration with existing medical systems.
The evolution of biosensor technology can be traced back to the 1960s with the development of the first glucose sensor. Since then, the field has expanded dramatically, incorporating various sensing mechanisms and targeting a wide range of analytes. The introduction of transverse wave technology to biosensors marks a new chapter in this progression, offering potential improvements in detection limits, response times, and overall performance.
Transverse waves, also known as shear waves, are characterized by oscillations perpendicular to the direction of wave propagation. In the context of biosensors, these waves can be generated and detected using various methods, including surface acoustic wave (SAW) devices, quartz crystal microbalances (QCM), and magnetoelastic sensors. The unique interaction of transverse waves with biological materials provides a novel approach to sensing that complements and potentially surpasses traditional biosensing methods.
The primary objective of incorporating transverse waves in biosensors is to enhance the sensitivity and specificity of medical diagnostics. By exploiting the properties of these waves, researchers aim to develop sensors capable of detecting minute quantities of biomarkers associated with various diseases, including cancer, cardiovascular disorders, and infectious diseases. This improved detection capability could lead to earlier diagnosis, more accurate monitoring of disease progression, and better-informed treatment decisions.
Another key goal is to create more robust and versatile biosensing platforms that can operate in complex biological environments. Transverse wave biosensors have shown promise in overcoming some of the limitations of conventional sensors, such as interference from non-specific binding and reduced performance in viscous media. This adaptability makes them particularly attractive for in vivo applications and continuous monitoring scenarios.
Furthermore, the development of transverse wave biosensors aligns with the broader trend towards miniaturization and integration in medical devices. The compact nature of many transverse wave sensing elements facilitates the creation of portable, lab-on-a-chip devices that can bring advanced diagnostic capabilities to resource-limited settings or enable real-time monitoring in clinical environments.
As research in this field progresses, the ultimate aim is to translate these technological advancements into practical, cost-effective diagnostic tools that can significantly impact healthcare delivery and patient outcomes. This involves not only refining the sensing mechanisms but also addressing challenges related to manufacturability, reliability, and integration with existing medical systems.
Market Analysis for Advanced Medical Diagnostics
The global market for advanced medical diagnostics is experiencing significant growth, driven by the increasing prevalence of chronic diseases, aging populations, and the demand for more accurate and efficient diagnostic tools. The biosensor market, particularly those utilizing transverse waves for medical diagnosis, is poised for substantial expansion within this broader landscape.
Current market trends indicate a shift towards personalized medicine and point-of-care testing, which aligns well with the capabilities of advanced biosensors. These devices offer rapid, sensitive, and specific detection of various biomarkers, making them invaluable in early disease detection and monitoring. The integration of transverse wave technology in biosensors has the potential to further enhance their performance, opening new avenues for market growth.
The market for biosensors in medical diagnostics is expected to grow at a robust rate over the next five years. This growth is fueled by advancements in nanotechnology, microfluidics, and signal processing, which have significantly improved biosensor sensitivity and reliability. The application of transverse waves in biosensors represents a cutting-edge development that could potentially disrupt the current market dynamics.
Key market segments for biosensors utilizing transverse waves include hospitals, diagnostic laboratories, research institutions, and the emerging home healthcare sector. The hospital segment currently holds the largest market share due to the high volume of diagnostic tests performed in clinical settings. However, the home healthcare segment is anticipated to show the fastest growth rate, driven by the increasing adoption of telemedicine and remote patient monitoring solutions.
Geographically, North America dominates the advanced medical diagnostics market, followed by Europe and Asia-Pacific. The United States, in particular, leads in terms of research and development activities related to biosensor technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid market growth due to improving healthcare infrastructure and increasing healthcare expenditure.
Challenges in the market include regulatory hurdles, particularly for novel biosensor technologies, and the need for standardization across different platforms. Additionally, the high cost of advanced biosensors and the requirement for skilled personnel to operate them may limit adoption in some healthcare settings.
Despite these challenges, the market outlook for biosensors utilizing transverse waves in medical diagnosis remains highly positive. The potential for these devices to provide faster, more accurate, and less invasive diagnostic solutions aligns well with the global trend towards precision medicine and patient-centric healthcare delivery.
Current market trends indicate a shift towards personalized medicine and point-of-care testing, which aligns well with the capabilities of advanced biosensors. These devices offer rapid, sensitive, and specific detection of various biomarkers, making them invaluable in early disease detection and monitoring. The integration of transverse wave technology in biosensors has the potential to further enhance their performance, opening new avenues for market growth.
The market for biosensors in medical diagnostics is expected to grow at a robust rate over the next five years. This growth is fueled by advancements in nanotechnology, microfluidics, and signal processing, which have significantly improved biosensor sensitivity and reliability. The application of transverse waves in biosensors represents a cutting-edge development that could potentially disrupt the current market dynamics.
Key market segments for biosensors utilizing transverse waves include hospitals, diagnostic laboratories, research institutions, and the emerging home healthcare sector. The hospital segment currently holds the largest market share due to the high volume of diagnostic tests performed in clinical settings. However, the home healthcare segment is anticipated to show the fastest growth rate, driven by the increasing adoption of telemedicine and remote patient monitoring solutions.
Geographically, North America dominates the advanced medical diagnostics market, followed by Europe and Asia-Pacific. The United States, in particular, leads in terms of research and development activities related to biosensor technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid market growth due to improving healthcare infrastructure and increasing healthcare expenditure.
Challenges in the market include regulatory hurdles, particularly for novel biosensor technologies, and the need for standardization across different platforms. Additionally, the high cost of advanced biosensors and the requirement for skilled personnel to operate them may limit adoption in some healthcare settings.
Despite these challenges, the market outlook for biosensors utilizing transverse waves in medical diagnosis remains highly positive. The potential for these devices to provide faster, more accurate, and less invasive diagnostic solutions aligns well with the global trend towards precision medicine and patient-centric healthcare delivery.
Current Challenges in Transverse Wave Biosensor Technology
Transverse wave biosensors represent a promising frontier in medical diagnostics, yet they face several significant challenges that hinder their widespread adoption and optimal performance. One of the primary obstacles is the complexity of signal generation and detection in biological environments. Unlike longitudinal waves, transverse waves require a more sophisticated apparatus to produce and measure, especially in viscous media like bodily fluids.
The sensitivity and specificity of transverse wave biosensors are also areas of concern. While these sensors have shown potential for detecting minute changes in biological samples, they often struggle with distinguishing between target analytes and similar molecules, leading to false positives or negatives. This issue is particularly pronounced in complex biological matrices where multiple interfering substances are present.
Another challenge lies in the miniaturization and integration of transverse wave biosensor systems. Current designs are often bulky and require external equipment for signal processing, limiting their portability and point-of-care applications. Developing compact, self-contained devices that maintain high performance is a significant engineering hurdle.
The stability and reproducibility of transverse wave biosensors in varied environmental conditions pose additional difficulties. Factors such as temperature fluctuations, pH changes, and ionic strength variations can significantly affect sensor performance, leading to inconsistent results across different testing scenarios.
Furthermore, the biocompatibility of materials used in transverse wave biosensors is a critical concern, especially for in vivo applications. Ensuring that sensor components do not trigger adverse biological responses while maintaining their functional properties is a delicate balance that researchers are still working to achieve.
The power consumption of these biosensors is another area requiring improvement. Current systems often demand substantial energy input, which limits their use in portable or implantable devices where long-term operation without frequent recharging is crucial.
Lastly, the cost-effectiveness of transverse wave biosensor technology remains a significant barrier to widespread adoption. The sophisticated components and manufacturing processes required for these sensors result in high production costs, making them less competitive compared to established diagnostic methods.
Addressing these challenges will require interdisciplinary collaboration, combining expertise from fields such as materials science, bioengineering, and signal processing. As research progresses, overcoming these hurdles will be crucial for realizing the full potential of transverse wave biosensors in revolutionizing medical diagnostics.
The sensitivity and specificity of transverse wave biosensors are also areas of concern. While these sensors have shown potential for detecting minute changes in biological samples, they often struggle with distinguishing between target analytes and similar molecules, leading to false positives or negatives. This issue is particularly pronounced in complex biological matrices where multiple interfering substances are present.
Another challenge lies in the miniaturization and integration of transverse wave biosensor systems. Current designs are often bulky and require external equipment for signal processing, limiting their portability and point-of-care applications. Developing compact, self-contained devices that maintain high performance is a significant engineering hurdle.
The stability and reproducibility of transverse wave biosensors in varied environmental conditions pose additional difficulties. Factors such as temperature fluctuations, pH changes, and ionic strength variations can significantly affect sensor performance, leading to inconsistent results across different testing scenarios.
Furthermore, the biocompatibility of materials used in transverse wave biosensors is a critical concern, especially for in vivo applications. Ensuring that sensor components do not trigger adverse biological responses while maintaining their functional properties is a delicate balance that researchers are still working to achieve.
The power consumption of these biosensors is another area requiring improvement. Current systems often demand substantial energy input, which limits their use in portable or implantable devices where long-term operation without frequent recharging is crucial.
Lastly, the cost-effectiveness of transverse wave biosensor technology remains a significant barrier to widespread adoption. The sophisticated components and manufacturing processes required for these sensors result in high production costs, making them less competitive compared to established diagnostic methods.
Addressing these challenges will require interdisciplinary collaboration, combining expertise from fields such as materials science, bioengineering, and signal processing. As research progresses, overcoming these hurdles will be crucial for realizing the full potential of transverse wave biosensors in revolutionizing medical diagnostics.
Existing Transverse Wave Biosensor Solutions
01 Improved sensing materials and techniques
Advancements in sensing materials and techniques have led to more sensitive and accurate biosensors. These improvements include the development of novel nanomaterials, enhanced surface functionalization methods, and innovative signal amplification strategies. Such advancements result in biosensors with lower detection limits, higher specificity, and broader dynamic ranges.- Improved sensitivity and detection methods: Advancements in biosensor technology focus on enhancing sensitivity and detection methods. This includes developing novel sensing materials, optimizing signal transduction mechanisms, and implementing advanced data processing algorithms. These improvements allow for more accurate and reliable detection of target analytes, even at lower concentrations.
- Miniaturization and portability: Efforts are being made to reduce the size of biosensors while maintaining or improving their performance. This trend towards miniaturization enables the development of portable, handheld devices suitable for point-of-care diagnostics and field testing. Microfluidic technologies and nanotechnology play crucial roles in achieving compact biosensor designs.
- Integration of multiple sensing modalities: Biosensors are being designed to incorporate multiple sensing modalities within a single device. This integration allows for simultaneous detection of various analytes or parameters, providing a more comprehensive analysis. Such multi-modal biosensors offer improved versatility and efficiency in diverse applications, from medical diagnostics to environmental monitoring.
- Enhanced biocompatibility and stability: Improving the biocompatibility and stability of biosensors is crucial for their long-term performance and reliability, especially in in vivo applications. Research focuses on developing new materials and surface modifications that reduce biofouling, enhance sensor longevity, and maintain sensitivity over extended periods. This includes exploring novel immobilization techniques for biological recognition elements.
- Integration with data analytics and IoT: Biosensors are increasingly being integrated with advanced data analytics and Internet of Things (IoT) technologies. This integration enables real-time data collection, processing, and analysis, facilitating remote monitoring and decision-making. Machine learning algorithms are being employed to improve data interpretation and predictive capabilities, enhancing the overall functionality and applicability of biosensor systems.
02 Integration of microfluidics and lab-on-a-chip technologies
The incorporation of microfluidic systems and lab-on-a-chip technologies has significantly enhanced biosensor performance. These integrated platforms allow for precise sample handling, reduced reagent consumption, and improved reaction kinetics. This integration results in more compact, portable, and user-friendly biosensor devices suitable for point-of-care diagnostics and field applications.Expand Specific Solutions03 Advanced signal processing and data analysis
Improvements in signal processing algorithms and data analysis techniques have enhanced the performance of biosensors. Machine learning and artificial intelligence approaches are being applied to interpret complex biosensor data, leading to more accurate and reliable results. These advancements enable real-time monitoring, noise reduction, and improved selectivity in biosensor systems.Expand Specific Solutions04 Multiplexing and high-throughput capabilities
Biosensors have been improved to allow for simultaneous detection of multiple analytes or high-throughput screening. This has been achieved through the development of sensor arrays, multimodal detection systems, and advanced surface patterning techniques. These improvements enable more comprehensive and efficient analysis in various applications, including drug discovery and environmental monitoring.Expand Specific Solutions05 Enhanced biocompatibility and long-term stability
Improvements in biosensor biocompatibility and long-term stability have been achieved through the development of novel surface modification techniques and encapsulation methods. These advancements reduce biofouling, improve sensor longevity, and enable continuous monitoring in complex biological environments. Such improvements are particularly crucial for implantable biosensors and long-term environmental monitoring applications.Expand Specific Solutions
Key Players in Biosensor and Medical Diagnostics Industry
The biosensor improvements utilizing transverse waves in medical diagnosis field is in a growth phase, with increasing market size and technological advancements. The global biosensors market is expected to expand significantly due to rising demand for rapid and accurate diagnostic tools. Technological maturity varies across different applications, with some areas more developed than others. Key players like Koninklijke Philips, Hitachi, and Toshiba Medical Systems are driving innovation through R&D investments. Academic institutions such as MIT and University of Manitoba are contributing to fundamental research, while companies like Samsung Electronics and Misonix are focusing on commercialization. The competitive landscape is diverse, with both established medical device manufacturers and emerging startups vying for market share in this promising field.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced biosensors utilizing transverse waves for medical diagnosis. Their technology incorporates piezoelectric materials to generate and detect shear waves, enabling high-resolution imaging of soft tissues[1]. The company's biosensors employ microelectromechanical systems (MEMS) to create miniaturized, highly sensitive devices capable of detecting subtle biomolecular interactions[3]. Philips has also integrated machine learning algorithms to enhance signal processing and improve diagnostic accuracy, particularly in applications such as cancer detection and cardiovascular health monitoring[5].
Strengths: High sensitivity and specificity in biomolecular detection, miniaturization capabilities, and integration with AI for improved diagnostics. Weaknesses: Potentially higher cost compared to traditional biosensors, and may require specialized training for healthcare professionals.
Hitachi Ltd.
Technical Solution: Hitachi has pioneered the development of surface acoustic wave (SAW) biosensors that utilize transverse waves for medical diagnostics. Their technology employs interdigital transducers (IDTs) on piezoelectric substrates to generate and detect SAWs, allowing for label-free and real-time detection of biomolecules[2]. Hitachi's biosensors incorporate nanomaterials such as graphene to enhance sensitivity and expand the range of detectable analytes[4]. The company has also developed multi-channel SAW devices for simultaneous detection of multiple biomarkers, improving diagnostic efficiency in clinical settings[6].
Strengths: Label-free and real-time detection capabilities, high sensitivity due to nanomaterial integration, and multi-analyte detection. Weaknesses: Potential for interference from non-specific binding and challenges in mass production of consistent devices.
Core Innovations in Transverse Wave Biosensor Technology
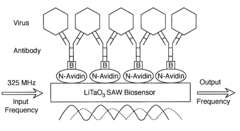
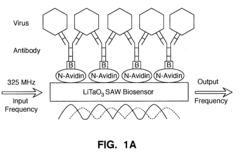
Detection of bioagents using a shear horizontal surface acoustic wave biosensor
PatentActiveUS20110053139A1
Innovation
- A shear horizontal surface acoustic wave (SH-SAW) biosensor using a lithium tantalate (LiTaO3) wafer with surface-bound ligands that specifically bind to target agents, generating measurable acoustic waves for detection, allowing for real-time identification and quantification of bioagents without pre-processing of samples.
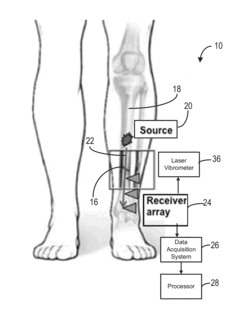
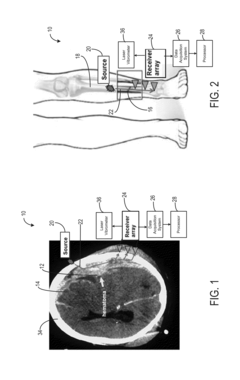
System and method for analyzing tissue using shear waves
PatentActiveUS20150148675A1
Innovation
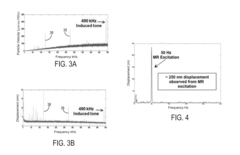
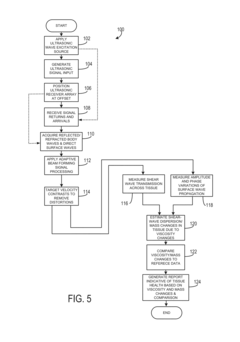
- The system employs transverse ultrasonic wave excitation sources and receivers that minimize surface wave interference, combined with adaptive beam-forming signal processing to compensate for tissue heterogeneity, allowing for the detection and imaging of internal damage by estimating shear-wave dispersion due to viscosity and mass changes in tissues.
Regulatory Framework for Medical Diagnostic Devices
The regulatory framework for medical diagnostic devices plays a crucial role in ensuring the safety, efficacy, and quality of biosensors utilizing transverse waves in medical diagnosis. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing these devices. The FDA classifies medical devices into three categories based on their risk level and intended use, with most biosensors falling under Class II or Class III.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device. This process involves providing detailed information on the device's design, performance, and safety features. Class III devices, which are considered high-risk, require a more rigorous premarket approval (PMA) process, including extensive clinical trials to demonstrate safety and effectiveness.
In the European Union, medical diagnostic devices are regulated under the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. The IVDR introduces a risk-based classification system similar to the FDA's, with four classes (A, B, C, and D) of increasing risk. Biosensors utilizing transverse waves would likely fall under Class C or D, requiring conformity assessment by a notified body and more stringent clinical evidence requirements.
Both the FDA and EU regulations emphasize the importance of post-market surveillance and vigilance. Manufacturers are required to implement systems for collecting and analyzing data on device performance and safety in real-world settings. This ongoing monitoring helps identify potential issues and allows for timely interventions to protect patient safety.
The regulatory landscape also addresses the unique challenges posed by innovative technologies like transverse wave biosensors. Regulatory bodies are increasingly adopting adaptive approaches to keep pace with technological advancements. For instance, the FDA's Digital Health Software Precertification (Pre-Cert) Program aims to streamline the review process for software-based medical devices, which may include certain types of biosensors.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), are working to align regulatory requirements across different countries. This harmonization can potentially reduce the regulatory burden on manufacturers and accelerate the global availability of innovative diagnostic technologies.
As biosensor technology continues to evolve, regulatory frameworks must adapt to address emerging challenges, such as data privacy concerns, cybersecurity risks, and the integration of artificial intelligence in diagnostic devices. Ongoing collaboration between regulatory bodies, industry stakeholders, and healthcare professionals is essential to ensure that the regulatory framework remains robust and responsive to technological advancements in the field of medical diagnostics.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device. This process involves providing detailed information on the device's design, performance, and safety features. Class III devices, which are considered high-risk, require a more rigorous premarket approval (PMA) process, including extensive clinical trials to demonstrate safety and effectiveness.
In the European Union, medical diagnostic devices are regulated under the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. The IVDR introduces a risk-based classification system similar to the FDA's, with four classes (A, B, C, and D) of increasing risk. Biosensors utilizing transverse waves would likely fall under Class C or D, requiring conformity assessment by a notified body and more stringent clinical evidence requirements.
Both the FDA and EU regulations emphasize the importance of post-market surveillance and vigilance. Manufacturers are required to implement systems for collecting and analyzing data on device performance and safety in real-world settings. This ongoing monitoring helps identify potential issues and allows for timely interventions to protect patient safety.
The regulatory landscape also addresses the unique challenges posed by innovative technologies like transverse wave biosensors. Regulatory bodies are increasingly adopting adaptive approaches to keep pace with technological advancements. For instance, the FDA's Digital Health Software Precertification (Pre-Cert) Program aims to streamline the review process for software-based medical devices, which may include certain types of biosensors.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), are working to align regulatory requirements across different countries. This harmonization can potentially reduce the regulatory burden on manufacturers and accelerate the global availability of innovative diagnostic technologies.
As biosensor technology continues to evolve, regulatory frameworks must adapt to address emerging challenges, such as data privacy concerns, cybersecurity risks, and the integration of artificial intelligence in diagnostic devices. Ongoing collaboration between regulatory bodies, industry stakeholders, and healthcare professionals is essential to ensure that the regulatory framework remains robust and responsive to technological advancements in the field of medical diagnostics.
Biosensor Data Privacy and Security Considerations
The integration of transverse wave technology in biosensors for medical diagnosis brings forth critical considerations regarding data privacy and security. As these advanced biosensors collect and process sensitive health information, robust measures must be implemented to safeguard patient data and maintain confidentiality.
One primary concern is the secure transmission of biosensor data. Transverse wave-based biosensors may collect real-time physiological information, which needs to be transmitted to medical professionals or centralized databases. Encryption protocols, such as end-to-end encryption and secure socket layer (SSL) technology, should be employed to protect data during transmission, preventing unauthorized access or interception.
Data storage and management present another significant challenge. Healthcare providers and biosensor manufacturers must ensure that patient information is stored securely, with access restricted to authorized personnel only. Implementing multi-factor authentication, role-based access controls, and regular security audits can help maintain data integrity and prevent breaches.
The potential for data breaches and cyber attacks targeting biosensor systems cannot be overlooked. As these devices become more interconnected and integrated into broader healthcare networks, they may become attractive targets for malicious actors. Robust firewalls, intrusion detection systems, and regular security updates are essential to mitigate these risks and protect sensitive medical information.
Privacy concerns also extend to the use and sharing of biosensor data. Clear policies and consent mechanisms must be established to inform patients about how their data will be used, stored, and potentially shared for research or other purposes. Compliance with data protection regulations, such as GDPR in Europe or HIPAA in the United States, is crucial to ensure legal and ethical handling of patient information.
The potential for re-identification of anonymized data is another consideration. As biosensors collect increasingly detailed and unique physiological data, there is a risk that even de-identified information could be linked back to specific individuals. Advanced anonymization techniques and strict data access controls are necessary to minimize this risk and protect patient privacy.
Lastly, the long-term storage and management of biosensor data raise questions about data retention policies and patient rights. Healthcare providers and biosensor manufacturers must establish clear guidelines for data retention periods, data deletion processes, and mechanisms for patients to access or request the removal of their information.
One primary concern is the secure transmission of biosensor data. Transverse wave-based biosensors may collect real-time physiological information, which needs to be transmitted to medical professionals or centralized databases. Encryption protocols, such as end-to-end encryption and secure socket layer (SSL) technology, should be employed to protect data during transmission, preventing unauthorized access or interception.
Data storage and management present another significant challenge. Healthcare providers and biosensor manufacturers must ensure that patient information is stored securely, with access restricted to authorized personnel only. Implementing multi-factor authentication, role-based access controls, and regular security audits can help maintain data integrity and prevent breaches.
The potential for data breaches and cyber attacks targeting biosensor systems cannot be overlooked. As these devices become more interconnected and integrated into broader healthcare networks, they may become attractive targets for malicious actors. Robust firewalls, intrusion detection systems, and regular security updates are essential to mitigate these risks and protect sensitive medical information.
Privacy concerns also extend to the use and sharing of biosensor data. Clear policies and consent mechanisms must be established to inform patients about how their data will be used, stored, and potentially shared for research or other purposes. Compliance with data protection regulations, such as GDPR in Europe or HIPAA in the United States, is crucial to ensure legal and ethical handling of patient information.
The potential for re-identification of anonymized data is another consideration. As biosensors collect increasingly detailed and unique physiological data, there is a risk that even de-identified information could be linked back to specific individuals. Advanced anonymization techniques and strict data access controls are necessary to minimize this risk and protect patient privacy.
Lastly, the long-term storage and management of biosensor data raise questions about data retention policies and patient rights. Healthcare providers and biosensor manufacturers must establish clear guidelines for data retention periods, data deletion processes, and mechanisms for patients to access or request the removal of their information.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!