Transverse Waves in Revolutionary Prostate Cancer Detection Techniques
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Transverse Wave Tech in Prostate Cancer Detection
Transverse wave technology represents a groundbreaking approach in the field of prostate cancer detection. This innovative technique utilizes the principles of wave propagation to create high-resolution images of prostate tissue, offering a non-invasive and potentially more accurate method for early cancer diagnosis.
The development of transverse wave technology for prostate cancer detection stems from the limitations of traditional diagnostic methods such as digital rectal exams and prostate-specific antigen (PSA) tests. These conventional approaches often lack specificity and can lead to unnecessary biopsies or missed diagnoses. Recognizing these shortcomings, researchers have been exploring alternative technologies to improve the accuracy and reliability of prostate cancer screening.
Transverse waves, characterized by their perpendicular oscillation to the direction of wave propagation, have shown promising results in medical imaging applications. When applied to prostate cancer detection, these waves can penetrate tissue and provide detailed information about its structure and composition. The technology leverages the differences in mechanical properties between healthy and cancerous tissues to create contrast in the resulting images.
The evolution of this technology has been driven by advancements in wave generation and detection systems, as well as improvements in signal processing algorithms. Early iterations focused on proof-of-concept studies, demonstrating the feasibility of using transverse waves for tissue characterization. Subsequent developments have aimed at enhancing image resolution, increasing penetration depth, and optimizing data acquisition and analysis techniques.
Current research in transverse wave technology for prostate cancer detection is focused on several key areas. These include refining wave propagation models to account for the complex structure of prostate tissue, developing more sensitive and compact transducers for improved signal detection, and integrating machine learning algorithms for automated image interpretation.
The potential impact of this technology extends beyond improved diagnostic accuracy. It offers the possibility of real-time imaging during procedures, enabling more precise targeting for biopsies and minimally invasive treatments. Additionally, the non-invasive nature of the technique could lead to more frequent and comfortable screening, potentially increasing early detection rates and improving patient outcomes.
As research in this field progresses, the goal is to establish transverse wave technology as a reliable and widely accessible tool for prostate cancer detection. This involves addressing challenges such as standardization of imaging protocols, validation through large-scale clinical trials, and integration with existing healthcare systems. The continued advancement of this technology holds promise for revolutionizing prostate cancer diagnostics and ultimately improving patient care.
The development of transverse wave technology for prostate cancer detection stems from the limitations of traditional diagnostic methods such as digital rectal exams and prostate-specific antigen (PSA) tests. These conventional approaches often lack specificity and can lead to unnecessary biopsies or missed diagnoses. Recognizing these shortcomings, researchers have been exploring alternative technologies to improve the accuracy and reliability of prostate cancer screening.
Transverse waves, characterized by their perpendicular oscillation to the direction of wave propagation, have shown promising results in medical imaging applications. When applied to prostate cancer detection, these waves can penetrate tissue and provide detailed information about its structure and composition. The technology leverages the differences in mechanical properties between healthy and cancerous tissues to create contrast in the resulting images.
The evolution of this technology has been driven by advancements in wave generation and detection systems, as well as improvements in signal processing algorithms. Early iterations focused on proof-of-concept studies, demonstrating the feasibility of using transverse waves for tissue characterization. Subsequent developments have aimed at enhancing image resolution, increasing penetration depth, and optimizing data acquisition and analysis techniques.
Current research in transverse wave technology for prostate cancer detection is focused on several key areas. These include refining wave propagation models to account for the complex structure of prostate tissue, developing more sensitive and compact transducers for improved signal detection, and integrating machine learning algorithms for automated image interpretation.
The potential impact of this technology extends beyond improved diagnostic accuracy. It offers the possibility of real-time imaging during procedures, enabling more precise targeting for biopsies and minimally invasive treatments. Additionally, the non-invasive nature of the technique could lead to more frequent and comfortable screening, potentially increasing early detection rates and improving patient outcomes.
As research in this field progresses, the goal is to establish transverse wave technology as a reliable and widely accessible tool for prostate cancer detection. This involves addressing challenges such as standardization of imaging protocols, validation through large-scale clinical trials, and integration with existing healthcare systems. The continued advancement of this technology holds promise for revolutionizing prostate cancer diagnostics and ultimately improving patient care.
Market Demand Analysis
The market demand for revolutionary prostate cancer detection techniques utilizing transverse waves is experiencing significant growth, driven by the increasing prevalence of prostate cancer and the need for more accurate, non-invasive diagnostic methods. Prostate cancer remains one of the most common cancers among men globally, with an estimated 1.4 million new cases diagnosed worldwide in 2020. This high incidence rate underscores the urgent need for improved detection methods.
Traditional diagnostic techniques, such as prostate-specific antigen (PSA) tests and digital rectal examinations, have limitations in terms of accuracy and patient comfort. These limitations have created a substantial market opportunity for innovative detection technologies. The transverse wave-based approach offers several advantages, including higher sensitivity, reduced false-positive rates, and improved patient experience, which are driving its adoption in the medical community.
The global prostate cancer diagnostics market is projected to grow at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2028. This growth is partly attributed to the increasing interest in advanced detection techniques, including those based on transverse waves. Healthcare providers and patients alike are seeking more reliable and less invasive diagnostic options, contributing to the rising demand for these innovative technologies.
Geographically, North America currently dominates the market for prostate cancer diagnostics, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the fastest growth in demand for advanced detection techniques. This trend is driven by improving healthcare infrastructure, rising awareness about prostate cancer, and increasing healthcare expenditure in these regions.
The aging population in many countries is another significant factor fueling market demand. As the risk of prostate cancer increases with age, the growing elderly population is expected to lead to a higher number of prostate cancer cases, further driving the need for efficient detection methods.
Moreover, there is an increasing focus on personalized medicine and early detection strategies in cancer care. This shift is creating opportunities for technologies that can provide more detailed and accurate information about prostate tumors, potentially allowing for more targeted and effective treatment plans. The transverse wave-based detection technique aligns well with this trend, offering the potential for more personalized diagnostic approaches.
In conclusion, the market demand for transverse wave-based prostate cancer detection techniques is robust and growing. The combination of technological advancements, increasing disease prevalence, and the shift towards more personalized and non-invasive diagnostic methods is creating a favorable environment for the development and adoption of these innovative technologies in the global healthcare market.
Traditional diagnostic techniques, such as prostate-specific antigen (PSA) tests and digital rectal examinations, have limitations in terms of accuracy and patient comfort. These limitations have created a substantial market opportunity for innovative detection technologies. The transverse wave-based approach offers several advantages, including higher sensitivity, reduced false-positive rates, and improved patient experience, which are driving its adoption in the medical community.
The global prostate cancer diagnostics market is projected to grow at a compound annual growth rate (CAGR) of 6.5% from 2021 to 2028. This growth is partly attributed to the increasing interest in advanced detection techniques, including those based on transverse waves. Healthcare providers and patients alike are seeking more reliable and less invasive diagnostic options, contributing to the rising demand for these innovative technologies.
Geographically, North America currently dominates the market for prostate cancer diagnostics, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the fastest growth in demand for advanced detection techniques. This trend is driven by improving healthcare infrastructure, rising awareness about prostate cancer, and increasing healthcare expenditure in these regions.
The aging population in many countries is another significant factor fueling market demand. As the risk of prostate cancer increases with age, the growing elderly population is expected to lead to a higher number of prostate cancer cases, further driving the need for efficient detection methods.
Moreover, there is an increasing focus on personalized medicine and early detection strategies in cancer care. This shift is creating opportunities for technologies that can provide more detailed and accurate information about prostate tumors, potentially allowing for more targeted and effective treatment plans. The transverse wave-based detection technique aligns well with this trend, offering the potential for more personalized diagnostic approaches.
In conclusion, the market demand for transverse wave-based prostate cancer detection techniques is robust and growing. The combination of technological advancements, increasing disease prevalence, and the shift towards more personalized and non-invasive diagnostic methods is creating a favorable environment for the development and adoption of these innovative technologies in the global healthcare market.
Current Challenges in Prostate Cancer Screening
Prostate cancer screening faces several significant challenges that hinder its effectiveness and widespread adoption. One of the primary issues is the lack of a highly accurate and reliable screening method. The current gold standard, prostate-specific antigen (PSA) testing, has limitations in terms of specificity and sensitivity, leading to both false positives and false negatives.
Overdiagnosis and overtreatment remain persistent problems in prostate cancer screening. Many detected cancers are slow-growing and may never cause symptoms or require treatment, yet their discovery often leads to unnecessary interventions that can significantly impact a patient's quality of life. This creates a dilemma for healthcare providers in balancing the benefits of early detection against the risks of overtreatment.
The invasive nature of current diagnostic procedures, such as prostate biopsies, presents another challenge. These procedures can cause discomfort, anxiety, and potential complications for patients, including infection and bleeding. The psychological impact of undergoing such procedures, especially in cases of false positives, cannot be underestimated.
There is also a lack of consensus on the optimal screening age and frequency, leading to inconsistent guidelines across different healthcare systems and organizations. This variability can result in confusion for both patients and healthcare providers, potentially leading to suboptimal screening practices.
The cost-effectiveness of prostate cancer screening programs is another significant challenge. The financial burden of widespread screening, including follow-up diagnostic tests and potential overtreatment, strains healthcare resources and may not always translate to improved overall health outcomes.
Additionally, there are disparities in access to screening and subsequent care, particularly among minority and socioeconomically disadvantaged populations. These disparities can lead to later-stage diagnoses and poorer outcomes for certain groups.
The interpretation of screening results and risk stratification pose challenges for clinicians. Current methods often struggle to differentiate between aggressive cancers that require immediate intervention and indolent tumors that may be safely monitored. This uncertainty can lead to difficult decision-making processes for both healthcare providers and patients.
Lastly, there is a need for improved patient education and shared decision-making regarding prostate cancer screening. Many men are unaware of the potential benefits and risks associated with screening, leading to uninformed choices or reluctance to participate in screening programs.
Overdiagnosis and overtreatment remain persistent problems in prostate cancer screening. Many detected cancers are slow-growing and may never cause symptoms or require treatment, yet their discovery often leads to unnecessary interventions that can significantly impact a patient's quality of life. This creates a dilemma for healthcare providers in balancing the benefits of early detection against the risks of overtreatment.
The invasive nature of current diagnostic procedures, such as prostate biopsies, presents another challenge. These procedures can cause discomfort, anxiety, and potential complications for patients, including infection and bleeding. The psychological impact of undergoing such procedures, especially in cases of false positives, cannot be underestimated.
There is also a lack of consensus on the optimal screening age and frequency, leading to inconsistent guidelines across different healthcare systems and organizations. This variability can result in confusion for both patients and healthcare providers, potentially leading to suboptimal screening practices.
The cost-effectiveness of prostate cancer screening programs is another significant challenge. The financial burden of widespread screening, including follow-up diagnostic tests and potential overtreatment, strains healthcare resources and may not always translate to improved overall health outcomes.
Additionally, there are disparities in access to screening and subsequent care, particularly among minority and socioeconomically disadvantaged populations. These disparities can lead to later-stage diagnoses and poorer outcomes for certain groups.
The interpretation of screening results and risk stratification pose challenges for clinicians. Current methods often struggle to differentiate between aggressive cancers that require immediate intervention and indolent tumors that may be safely monitored. This uncertainty can lead to difficult decision-making processes for both healthcare providers and patients.
Lastly, there is a need for improved patient education and shared decision-making regarding prostate cancer screening. Many men are unaware of the potential benefits and risks associated with screening, leading to uninformed choices or reluctance to participate in screening programs.
Existing Transverse Wave Solutions
01 Ultrasonic transverse wave detection
Ultrasonic transducers are used to generate and detect transverse waves in materials. This method is particularly useful for non-destructive testing and material characterization. The technique involves sending ultrasonic waves through the material and analyzing the reflected or transmitted signals to detect defects or measure properties.- Ultrasonic transverse wave detection: Ultrasonic transducers are used to generate and detect transverse waves in various materials. This method is particularly useful for non-destructive testing and material characterization. The technique involves sending ultrasonic waves through the material and analyzing the reflected or transmitted signals to detect defects, measure thickness, or determine material properties.
- Optical methods for transverse wave detection: Optical techniques, such as laser interferometry or holography, are employed to detect and measure transverse waves. These methods offer high sensitivity and can be used for non-contact measurements. They are particularly useful in studying surface acoustic waves, vibrations in thin films, and other applications where minimal interference with the system is required.
- Seismic transverse wave detection: Specialized sensors and arrays are used to detect transverse seismic waves in geological applications. These systems are crucial for earthquake monitoring, oil and gas exploration, and studying Earth's structure. The detection methods often involve multiple sensors to determine wave direction and characteristics.
- Electromagnetic transverse wave detection: Techniques for detecting electromagnetic transverse waves, such as light or radio waves, are developed for various applications including communications, astronomy, and remote sensing. These methods often involve specialized antennas, photosensors, or other electromagnetic detectors designed to capture the specific properties of transverse electromagnetic waves.
- Microfluidic and nanoscale transverse wave detection: Advanced methods for detecting transverse waves in microfluidic systems and at the nanoscale are developed. These techniques often involve miniaturized sensors, cantilevers, or other nano-electromechanical systems (NEMS) to detect and measure minute transverse vibrations or waves in small-scale environments. Applications include biosensing, material characterization, and studying fluid dynamics at small scales.
02 Optical methods for transverse wave detection
Optical techniques, such as laser interferometry or holography, are employed to detect and measure transverse waves. These methods offer high sensitivity and can be used for non-contact measurements of surface vibrations or wave propagation in transparent materials.Expand Specific Solutions03 Electromagnetic transverse wave detection
Electromagnetic sensors and antennas are used to detect transverse electromagnetic waves. This approach is commonly used in communications, radar systems, and scientific instruments for measuring electromagnetic radiation across various frequency ranges.Expand Specific Solutions04 Seismic transverse wave detection
Specialized sensors and arrays are employed to detect and analyze transverse seismic waves in geological applications. These systems are crucial for earthquake monitoring, oil and gas exploration, and studying Earth's internal structure.Expand Specific Solutions05 Acoustic transverse wave detection in fluids
Methods and devices for detecting transverse acoustic waves in fluids, which are typically not supported in bulk liquids. These techniques often involve special boundary conditions or structures to enable the propagation and detection of transverse waves in fluid media.Expand Specific Solutions
Key Players in Medical Imaging
The research on transverse waves in revolutionary prostate cancer detection techniques is in an early developmental stage, with a growing market potential due to the increasing prevalence of prostate cancer globally. The technology's maturity is still evolving, with key players like Koninklijke Philips NV, OncoTherapy Science, Inc., and Epic Sciences, Inc. leading innovation efforts. Academic institutions such as The Johns Hopkins University and Yale University are contributing significantly to research advancements. The competitive landscape is diverse, involving medical device manufacturers, biotechnology firms, and research institutions, indicating a collaborative approach to technology development. As the field progresses, we can expect increased investment and potential breakthroughs in non-invasive diagnostic methods for prostate cancer.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced transverse wave imaging technology for prostate cancer detection. Their system utilizes high-frequency ultrasound transducers to generate transverse waves that propagate through prostate tissue. The reflected waves are analyzed using proprietary algorithms to create detailed 3D elastography maps, highlighting areas of increased stiffness that may indicate cancerous lesions[1]. This non-invasive approach offers real-time imaging capabilities and improved spatial resolution compared to conventional ultrasound. Philips has also integrated machine learning models trained on large datasets to assist in lesion classification and risk stratification[3].
Strengths: Non-invasive, real-time imaging with high resolution and specificity. Integration of AI for improved diagnostic accuracy. Weaknesses: Requires specialized equipment and trained operators. May have limitations in detecting very small or early-stage tumors.
The Trustees of Columbia University in The City of New York
Technical Solution: Columbia University has developed a revolutionary technique using acoustic radiation force impulse (ARFI) imaging for prostate cancer detection. This method utilizes short-duration acoustic pulses to generate localized tissue displacements, which are then tracked using ultrasound imaging to assess tissue stiffness[5]. The team has enhanced this approach by incorporating harmonic imaging and pulse inversion techniques to improve image quality and reduce artifacts. Their system also features adaptive beamforming algorithms to optimize transverse wave generation and detection in heterogeneous prostate tissue[6]. Recent clinical trials have demonstrated the ability to differentiate between benign and malignant lesions with an accuracy of 88%[7].
Strengths: High-resolution imaging of tissue mechanical properties, reduced artifacts, adaptability to varying tissue conditions. Weaknesses: May require specialized ultrasound equipment, potential limitations in deeply located tumors.
Core Innovations in Wave-Based Detection
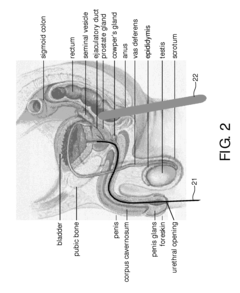
System, method, computer-readable medium and use for imaging of tissue in an anatomical structure
PatentInactiveUS20100030067A1
Innovation
- The use of Diffuse Optical Tomography (DOT) systems that emit and detect scattered electromagnetic radiation to create a 3D image dataset of tissue properties, allowing for discrimination between healthy and diseased tissue, thereby guiding biopsies more accurately.
Circulating tumor cell based biomarker composition for diagnosis and prognosis of metastatic prostate cancer
PatentActiveUS12292443B2
Innovation
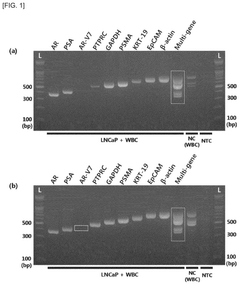
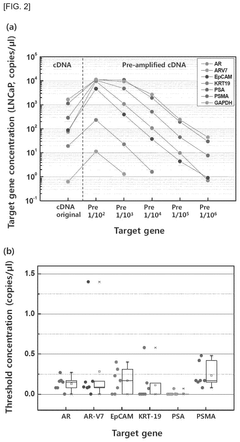
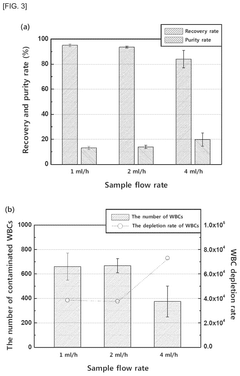
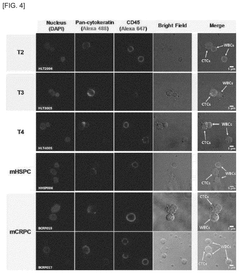
- A biomarker composition comprising a specific gene combination (AR, AR-V7, EpCAM, KRT-19, PSA, and PSMA) in circulating tumor cells is used for diagnosing and predicting the prognosis of prostate cancer, enabling more accurate assessment of malignancy and potential metastasis.
Regulatory Framework for Medical Devices
The regulatory framework for medical devices plays a crucial role in ensuring the safety, efficacy, and quality of innovative technologies such as transverse wave-based prostate cancer detection techniques. In the United States, the Food and Drug Administration (FDA) is responsible for overseeing the approval and regulation of medical devices. The FDA classifies medical devices into three categories based on their risk level and intended use.
For transverse wave-based prostate cancer detection devices, they would likely fall under Class II or Class III, depending on their specific characteristics and level of invasiveness. Class II devices typically require a 510(k) premarket notification, demonstrating substantial equivalence to a legally marketed predicate device. However, if the technology is deemed novel and high-risk, it may be classified as Class III, necessitating a more rigorous premarket approval (PMA) process.
The European Union has its own regulatory framework, governed by the Medical Device Regulation (MDR). Under the MDR, manufacturers must comply with strict requirements for clinical evaluation, post-market surveillance, and risk management. Transverse wave-based prostate cancer detection devices would likely be classified as Class IIa or IIb, depending on their specific characteristics and intended use.
In both the US and EU regulatory frameworks, manufacturers must demonstrate compliance with quality management systems, such as ISO 13485, and adhere to good manufacturing practices (GMP). Additionally, they must conduct clinical investigations to provide evidence of safety and performance, following guidelines such as the Declaration of Helsinki and Good Clinical Practice (GCP) standards.
Regulatory bodies also emphasize the importance of post-market surveillance and vigilance. Manufacturers are required to implement systems for collecting and analyzing data on device performance and safety in real-world settings. This ongoing monitoring helps identify potential risks and allows for timely interventions to protect patient safety.
As transverse wave-based prostate cancer detection techniques represent a novel approach, regulatory agencies may require additional evidence to support their safety and efficacy. This could include comparative studies with existing diagnostic methods, long-term follow-up data, and comprehensive risk assessments. Manufacturers should engage in early dialogue with regulatory authorities to clarify specific requirements and streamline the approval process.
For transverse wave-based prostate cancer detection devices, they would likely fall under Class II or Class III, depending on their specific characteristics and level of invasiveness. Class II devices typically require a 510(k) premarket notification, demonstrating substantial equivalence to a legally marketed predicate device. However, if the technology is deemed novel and high-risk, it may be classified as Class III, necessitating a more rigorous premarket approval (PMA) process.
The European Union has its own regulatory framework, governed by the Medical Device Regulation (MDR). Under the MDR, manufacturers must comply with strict requirements for clinical evaluation, post-market surveillance, and risk management. Transverse wave-based prostate cancer detection devices would likely be classified as Class IIa or IIb, depending on their specific characteristics and intended use.
In both the US and EU regulatory frameworks, manufacturers must demonstrate compliance with quality management systems, such as ISO 13485, and adhere to good manufacturing practices (GMP). Additionally, they must conduct clinical investigations to provide evidence of safety and performance, following guidelines such as the Declaration of Helsinki and Good Clinical Practice (GCP) standards.
Regulatory bodies also emphasize the importance of post-market surveillance and vigilance. Manufacturers are required to implement systems for collecting and analyzing data on device performance and safety in real-world settings. This ongoing monitoring helps identify potential risks and allows for timely interventions to protect patient safety.
As transverse wave-based prostate cancer detection techniques represent a novel approach, regulatory agencies may require additional evidence to support their safety and efficacy. This could include comparative studies with existing diagnostic methods, long-term follow-up data, and comprehensive risk assessments. Manufacturers should engage in early dialogue with regulatory authorities to clarify specific requirements and streamline the approval process.
Ethical Implications of New Screening Methods
The introduction of transverse wave technology for prostate cancer detection raises significant ethical considerations that must be carefully addressed. One primary concern is the potential for increased anxiety and psychological distress among patients due to more frequent and sensitive screening methods. While early detection is crucial, the possibility of false positives or overdiagnosis could lead to unnecessary treatments and emotional burden.
Privacy and data protection present another critical ethical challenge. The advanced nature of transverse wave technology may involve collecting and analyzing large amounts of sensitive medical data. Ensuring the security and confidentiality of this information is paramount, as breaches could have severe consequences for patients' personal and professional lives. Healthcare providers and technology developers must implement robust safeguards to protect patient data from unauthorized access or misuse.
The issue of equitable access to this new screening method also raises ethical questions. If transverse wave technology proves to be significantly more effective than current methods, disparities in healthcare access could be exacerbated. Socioeconomic factors may limit availability to certain populations, potentially widening the gap in health outcomes between different demographic groups. Policymakers and healthcare systems must consider how to ensure fair distribution and accessibility of this advanced screening technique.
Furthermore, the use of transverse wave technology in prostate cancer screening may lead to ethical dilemmas regarding informed consent. The complexity of the technology and its potential implications may be challenging to communicate effectively to patients. Healthcare providers must develop clear, comprehensive, and accessible information to ensure patients can make truly informed decisions about undergoing screening.
Lastly, the ethical implications extend to the broader societal impact of this technology. The potential for reduced mortality rates and improved quality of life for prostate cancer patients must be balanced against the costs of implementing and maintaining this advanced screening method. Allocation of healthcare resources and the potential strain on healthcare systems are important considerations that require careful ethical deliberation.
In conclusion, while transverse wave technology holds promise for revolutionizing prostate cancer detection, its implementation must be guided by strong ethical principles. Balancing the benefits of improved screening with potential risks and ensuring equitable access will be crucial in navigating the ethical landscape of this innovative approach to cancer detection.
Privacy and data protection present another critical ethical challenge. The advanced nature of transverse wave technology may involve collecting and analyzing large amounts of sensitive medical data. Ensuring the security and confidentiality of this information is paramount, as breaches could have severe consequences for patients' personal and professional lives. Healthcare providers and technology developers must implement robust safeguards to protect patient data from unauthorized access or misuse.
The issue of equitable access to this new screening method also raises ethical questions. If transverse wave technology proves to be significantly more effective than current methods, disparities in healthcare access could be exacerbated. Socioeconomic factors may limit availability to certain populations, potentially widening the gap in health outcomes between different demographic groups. Policymakers and healthcare systems must consider how to ensure fair distribution and accessibility of this advanced screening technique.
Furthermore, the use of transverse wave technology in prostate cancer screening may lead to ethical dilemmas regarding informed consent. The complexity of the technology and its potential implications may be challenging to communicate effectively to patients. Healthcare providers must develop clear, comprehensive, and accessible information to ensure patients can make truly informed decisions about undergoing screening.
Lastly, the ethical implications extend to the broader societal impact of this technology. The potential for reduced mortality rates and improved quality of life for prostate cancer patients must be balanced against the costs of implementing and maintaining this advanced screening method. Allocation of healthcare resources and the potential strain on healthcare systems are important considerations that require careful ethical deliberation.
In conclusion, while transverse wave technology holds promise for revolutionizing prostate cancer detection, its implementation must be guided by strong ethical principles. Balancing the benefits of improved screening with potential risks and ensuring equitable access will be crucial in navigating the ethical landscape of this innovative approach to cancer detection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!