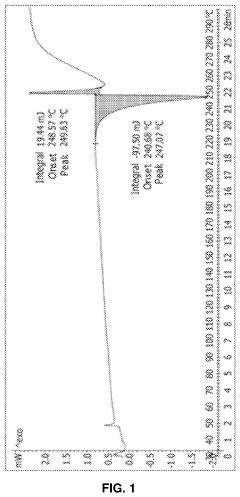
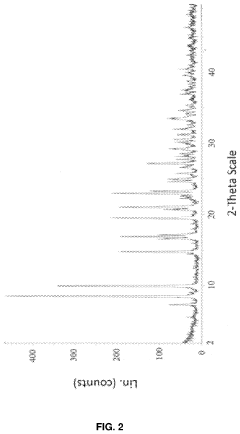
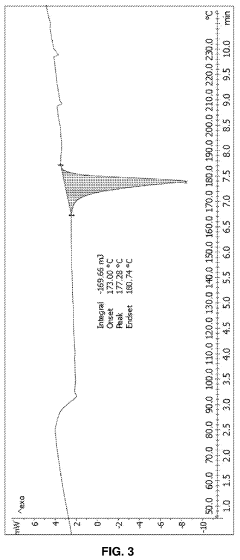
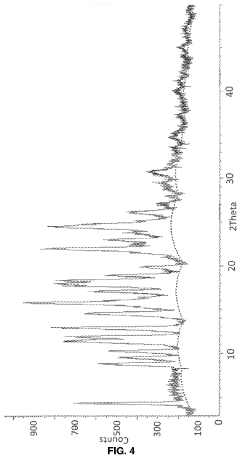
Case Study: Column Chromatography Process for API Intermediate Isolation — Yield, Purity and Scale Notes
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Evolution and Purification Objectives
Column chromatography has evolved significantly since its inception in the early 20th century, transforming from a rudimentary separation technique to a sophisticated purification method essential in pharmaceutical manufacturing. The journey began with simple gravity-fed columns using silica gel or alumina as stationary phases, progressing through increasingly refined methodologies that enhanced separation efficiency and reproducibility.
The 1960s marked a pivotal era with the development of high-performance liquid chromatography (HPLC), which dramatically improved resolution and reduced processing times. This advancement catalyzed the pharmaceutical industry's adoption of chromatographic techniques for API purification. The subsequent decades witnessed further innovations including the introduction of automated systems, specialized stationary phases, and continuous chromatography processes.
Recent technological evolution has focused on process intensification, with techniques such as simulated moving bed (SMB) chromatography enabling continuous operation and significantly improved productivity. Parallel developments in computational modeling and process analytical technology (PAT) have enhanced predictability and control of chromatographic separations, allowing for more efficient scale-up from laboratory to production environments.
In the context of API intermediate isolation, modern column chromatography aims to achieve multiple critical objectives simultaneously. Primary among these is maximizing product yield while maintaining stringent purity specifications—typically 99% or higher depending on the intermediate's position in the synthesis pathway. This balance between yield and purity represents a fundamental optimization challenge in process development.
Scale considerations introduce additional complexity, as processes must maintain performance characteristics when transitioning from laboratory (grams) to pilot (kilograms) and commercial manufacturing (tons). This necessitates careful attention to column dimensions, flow rates, and loading capacities to ensure consistent separation efficiency across scales.
Economic viability constitutes another key objective, requiring minimization of solvent consumption, reduction of processing time, and optimization of stationary phase utilization. These factors directly impact cost of goods and environmental footprint—increasingly important considerations in sustainable pharmaceutical manufacturing.
The technical trajectory points toward greater integration of chromatographic processes within continuous manufacturing paradigms, supported by advanced in-line monitoring and control systems. This evolution aligns with industry trends toward more flexible, efficient production methodologies capable of responding to market demands with agility while maintaining rigorous quality standards.
The 1960s marked a pivotal era with the development of high-performance liquid chromatography (HPLC), which dramatically improved resolution and reduced processing times. This advancement catalyzed the pharmaceutical industry's adoption of chromatographic techniques for API purification. The subsequent decades witnessed further innovations including the introduction of automated systems, specialized stationary phases, and continuous chromatography processes.
Recent technological evolution has focused on process intensification, with techniques such as simulated moving bed (SMB) chromatography enabling continuous operation and significantly improved productivity. Parallel developments in computational modeling and process analytical technology (PAT) have enhanced predictability and control of chromatographic separations, allowing for more efficient scale-up from laboratory to production environments.
In the context of API intermediate isolation, modern column chromatography aims to achieve multiple critical objectives simultaneously. Primary among these is maximizing product yield while maintaining stringent purity specifications—typically 99% or higher depending on the intermediate's position in the synthesis pathway. This balance between yield and purity represents a fundamental optimization challenge in process development.
Scale considerations introduce additional complexity, as processes must maintain performance characteristics when transitioning from laboratory (grams) to pilot (kilograms) and commercial manufacturing (tons). This necessitates careful attention to column dimensions, flow rates, and loading capacities to ensure consistent separation efficiency across scales.
Economic viability constitutes another key objective, requiring minimization of solvent consumption, reduction of processing time, and optimization of stationary phase utilization. These factors directly impact cost of goods and environmental footprint—increasingly important considerations in sustainable pharmaceutical manufacturing.
The technical trajectory points toward greater integration of chromatographic processes within continuous manufacturing paradigms, supported by advanced in-line monitoring and control systems. This evolution aligns with industry trends toward more flexible, efficient production methodologies capable of responding to market demands with agility while maintaining rigorous quality standards.
Market Demand for High-Purity API Intermediates
The pharmaceutical industry's demand for high-purity Active Pharmaceutical Ingredient (API) intermediates has experienced substantial growth over the past decade, driven primarily by stringent regulatory requirements and the increasing complexity of drug molecules. Market research indicates that the global API intermediates market is currently valued at over 30 billion USD, with a compound annual growth rate exceeding 6% projected through 2028.
Column chromatography processes for API intermediate isolation have become increasingly critical as pharmaceutical companies face mounting pressure to achieve higher purity standards. Regulatory bodies worldwide, including the FDA and EMA, have progressively tightened specifications for impurity profiles, with some critical impurities now requiring control at levels below 10 parts per million. This regulatory landscape has transformed purification technologies from optional process improvements to essential manufacturing requirements.
The demand is particularly pronounced in the oncology and immunology therapeutic areas, where complex molecular structures necessitate sophisticated purification techniques. These high-value therapeutic areas represent approximately 45% of the current demand for advanced purification technologies, with column chromatography being a preferred method due to its scalability and reproducibility advantages.
Contract Development and Manufacturing Organizations (CDMOs) report that clients increasingly specify minimum purity requirements of 98-99% for late-stage intermediates, compared to 95% standards that were common just five years ago. This shift has created a premium market segment for purification technologies that can consistently deliver these enhanced purity profiles while maintaining acceptable yields at commercial scale.
Geographic analysis reveals that North America and Europe currently dominate demand for high-purity intermediates, collectively accounting for over 65% of the global market. However, the Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at approximately 9% annually, driven by expanding pharmaceutical manufacturing capabilities and increasing adoption of international quality standards.
Economic factors also significantly influence market dynamics, with purification processes typically representing 20-30% of the total manufacturing cost for complex APIs. Companies that can optimize chromatography processes to improve both yield and purity simultaneously gain substantial competitive advantages, as each percentage point improvement in yield can translate to millions in additional revenue for high-value pharmaceuticals.
The sustainability aspect of purification processes has emerged as an additional market driver, with pharmaceutical companies increasingly seeking chromatography solutions that minimize solvent usage and environmental impact while maintaining performance metrics. This trend aligns with broader industry commitments to reduce carbon footprints and implement green chemistry principles throughout manufacturing operations.
Column chromatography processes for API intermediate isolation have become increasingly critical as pharmaceutical companies face mounting pressure to achieve higher purity standards. Regulatory bodies worldwide, including the FDA and EMA, have progressively tightened specifications for impurity profiles, with some critical impurities now requiring control at levels below 10 parts per million. This regulatory landscape has transformed purification technologies from optional process improvements to essential manufacturing requirements.
The demand is particularly pronounced in the oncology and immunology therapeutic areas, where complex molecular structures necessitate sophisticated purification techniques. These high-value therapeutic areas represent approximately 45% of the current demand for advanced purification technologies, with column chromatography being a preferred method due to its scalability and reproducibility advantages.
Contract Development and Manufacturing Organizations (CDMOs) report that clients increasingly specify minimum purity requirements of 98-99% for late-stage intermediates, compared to 95% standards that were common just five years ago. This shift has created a premium market segment for purification technologies that can consistently deliver these enhanced purity profiles while maintaining acceptable yields at commercial scale.
Geographic analysis reveals that North America and Europe currently dominate demand for high-purity intermediates, collectively accounting for over 65% of the global market. However, the Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at approximately 9% annually, driven by expanding pharmaceutical manufacturing capabilities and increasing adoption of international quality standards.
Economic factors also significantly influence market dynamics, with purification processes typically representing 20-30% of the total manufacturing cost for complex APIs. Companies that can optimize chromatography processes to improve both yield and purity simultaneously gain substantial competitive advantages, as each percentage point improvement in yield can translate to millions in additional revenue for high-value pharmaceuticals.
The sustainability aspect of purification processes has emerged as an additional market driver, with pharmaceutical companies increasingly seeking chromatography solutions that minimize solvent usage and environmental impact while maintaining performance metrics. This trend aligns with broader industry commitments to reduce carbon footprints and implement green chemistry principles throughout manufacturing operations.
Current Challenges in Column Chromatography Scaling
Column chromatography has emerged as a critical separation technique in pharmaceutical manufacturing, particularly for API intermediate isolation. However, scaling this process from laboratory to industrial production presents significant challenges that impact yield, purity, and overall process efficiency.
The primary challenge in scaling column chromatography lies in maintaining separation efficiency while increasing column dimensions. As column diameter increases, issues with flow distribution, wall effects, and bed homogeneity become more pronounced. Industrial-scale columns often experience channeling phenomena where the mobile phase follows preferential paths through the stationary phase, resulting in reduced separation efficiency and lower yields.
Pressure drop considerations become increasingly critical at larger scales. The relationship between flow rate, particle size, and pressure drop described by the Kozeny-Carman equation indicates that maintaining laboratory-scale flow rates in production columns requires exponentially higher pressures, often exceeding equipment limitations. This forces compromises in processing parameters that can negatively impact separation quality.
Loading capacity optimization presents another significant challenge. While laboratory-scale processes might achieve excellent separation with low sample-to-stationary phase ratios, such approaches become economically unfeasible at production scale. Increasing loading capacity without sacrificing purity requires extensive development work and often results in yield-purity tradeoffs that must be carefully balanced.
Solvent consumption scales dramatically with column size, creating economic and environmental sustainability concerns. Large-scale chromatography can require thousands of liters of solvents, necessitating recovery systems and raising questions about process greenness. The pharmaceutical industry faces increasing pressure to reduce solvent usage while maintaining product quality.
Reproducibility between batches becomes more difficult at scale. Variations in packing density, stationary phase lot-to-lot consistency, and environmental conditions can significantly impact separation profiles. Establishing robust control strategies that ensure consistent API intermediate quality requires sophisticated monitoring approaches and potentially adaptive process control systems.
Time considerations also become critical at scale. While laboratory separations might be completed in hours, industrial-scale columns may require days for a single cycle, creating production bottlenecks. This extended processing time increases the risk of sample degradation for sensitive API intermediates and reduces manufacturing flexibility.
Equipment limitations further constrain scaling efforts. Specialized large-diameter columns, distribution systems, and fraction collection equipment require significant capital investment. The mechanical challenges of handling large volumes of stationary phase and ensuring uniform packing density throughout industrial columns remain significant engineering hurdles.
The primary challenge in scaling column chromatography lies in maintaining separation efficiency while increasing column dimensions. As column diameter increases, issues with flow distribution, wall effects, and bed homogeneity become more pronounced. Industrial-scale columns often experience channeling phenomena where the mobile phase follows preferential paths through the stationary phase, resulting in reduced separation efficiency and lower yields.
Pressure drop considerations become increasingly critical at larger scales. The relationship between flow rate, particle size, and pressure drop described by the Kozeny-Carman equation indicates that maintaining laboratory-scale flow rates in production columns requires exponentially higher pressures, often exceeding equipment limitations. This forces compromises in processing parameters that can negatively impact separation quality.
Loading capacity optimization presents another significant challenge. While laboratory-scale processes might achieve excellent separation with low sample-to-stationary phase ratios, such approaches become economically unfeasible at production scale. Increasing loading capacity without sacrificing purity requires extensive development work and often results in yield-purity tradeoffs that must be carefully balanced.
Solvent consumption scales dramatically with column size, creating economic and environmental sustainability concerns. Large-scale chromatography can require thousands of liters of solvents, necessitating recovery systems and raising questions about process greenness. The pharmaceutical industry faces increasing pressure to reduce solvent usage while maintaining product quality.
Reproducibility between batches becomes more difficult at scale. Variations in packing density, stationary phase lot-to-lot consistency, and environmental conditions can significantly impact separation profiles. Establishing robust control strategies that ensure consistent API intermediate quality requires sophisticated monitoring approaches and potentially adaptive process control systems.
Time considerations also become critical at scale. While laboratory separations might be completed in hours, industrial-scale columns may require days for a single cycle, creating production bottlenecks. This extended processing time increases the risk of sample degradation for sensitive API intermediates and reduces manufacturing flexibility.
Equipment limitations further constrain scaling efforts. Specialized large-diameter columns, distribution systems, and fraction collection equipment require significant capital investment. The mechanical challenges of handling large volumes of stationary phase and ensuring uniform packing density throughout industrial columns remain significant engineering hurdles.
Current Industrial-Scale Chromatography Solutions
01 Optimization of column chromatography parameters for improved yield and purity
Various parameters in column chromatography can be optimized to enhance both yield and purity of the target compounds. These parameters include flow rate, column dimensions, stationary phase selection, and mobile phase composition. By carefully adjusting these parameters, the separation efficiency can be significantly improved, leading to higher product yield and purity. Optimization techniques may involve gradient elution, temperature control, and pressure adjustments to achieve optimal separation conditions.- Optimization of column chromatography parameters for improved yield and purity: Various parameters in column chromatography can be optimized to enhance both yield and purity of the target compounds. These parameters include flow rate, column dimensions, stationary phase selection, and mobile phase composition. By carefully adjusting these parameters, the separation efficiency can be significantly improved, leading to higher product yield and purity. Optimization techniques may involve gradient elution, temperature control, and pressure adjustments to achieve optimal separation conditions.
- Novel stationary phases for enhanced separation efficiency: The development and use of novel stationary phases can significantly impact the yield and purity in column chromatography processes. These innovative materials may include modified silica gels, polymeric resins, monolithic columns, and functionalized supports with specific binding properties. Such advanced stationary phases can provide better selectivity, reduced peak broadening, and improved resolution, ultimately leading to higher purity products and increased process yields.
- Continuous and automated chromatography systems: Continuous and automated chromatography systems represent significant advancements in improving yield and purity. These systems utilize techniques such as simulated moving bed chromatography, sequential injection, and multi-column configurations to enable continuous processing. Automation of sample loading, fraction collection, and system monitoring reduces human error and increases reproducibility. These approaches can significantly enhance throughput while maintaining or improving product purity compared to traditional batch processes.
- Purification strategies for complex mixtures: Specialized purification strategies have been developed for handling complex mixtures in column chromatography. These include multi-step chromatography approaches, orthogonal separation techniques, and selective pre-treatment methods. For particularly challenging separations, combining different chromatographic modes (such as size exclusion, ion exchange, and affinity chromatography) in sequence can dramatically improve both yield and purity. These strategies are particularly valuable for biological compounds, pharmaceuticals, and natural product extracts.
- Scale-up and industrial applications of column chromatography: Scaling up column chromatography from laboratory to industrial scale while maintaining yield and purity presents unique challenges. Innovations in this area include the development of larger diameter columns, improved packing methods, and specialized equipment for handling increased volumes. Process analytical technologies for real-time monitoring and control help maintain separation quality during scale-up. Economic considerations such as solvent recycling, reduced processing time, and minimized material losses are also addressed to make large-scale chromatographic separations more efficient and cost-effective.
02 Novel stationary phases for enhanced separation efficiency
The development and use of novel stationary phases can significantly impact the yield and purity in column chromatography processes. These innovative materials may include modified silica gels, polymeric resins, monolithic columns, and functionalized supports with specific binding properties. Such advanced stationary phases can provide better selectivity, reduced peak broadening, and improved resolution, ultimately leading to higher purity products and increased process yields.Expand Specific Solutions03 Continuous and automated chromatography systems
Continuous and automated chromatography systems represent a significant advancement in improving yield and purity. These systems utilize techniques such as simulated moving bed (SMB) chromatography, sequential injection, and multi-column configurations to enable continuous processing. Automation of sample loading, fraction collection, and system monitoring reduces human error and increases reproducibility. These approaches can significantly enhance throughput while maintaining or improving product purity compared to traditional batch processes.Expand Specific Solutions04 Post-chromatography purification techniques
Various post-chromatography techniques can be employed to further enhance the purity of isolated compounds. These methods include crystallization, precipitation, liquid-liquid extraction, and membrane filtration. When combined with column chromatography, these secondary purification steps can significantly increase the final purity of the target compounds while preserving overall process yield. The selection of appropriate post-processing methods depends on the specific physicochemical properties of the target compounds and impurities.Expand Specific Solutions05 Scale-up strategies for industrial chromatography processes
Scaling up chromatography processes from laboratory to industrial scale while maintaining yield and purity presents significant challenges. Effective strategies include linear scale-up approaches, maintaining bed height while increasing column diameter, adjusting flow rates proportionally, and implementing process analytical technology (PAT) for real-time monitoring. These methods help ensure that separation efficiency is preserved during scale-up, allowing for consistent product quality and economically viable large-scale purification processes.Expand Specific Solutions
Leading Pharmaceutical Process Development Organizations
Column chromatography for API intermediate isolation is currently in a mature growth phase, with the global market estimated at $3.5 billion and growing at 7-8% annually. The technology landscape features established leaders like Amgen and Novartis who have developed proprietary high-efficiency chromatography platforms, alongside specialized equipment manufacturers such as Waters Technology, ChromaCon AG, and Cytiva Sweden AB who provide innovative solutions for yield optimization and scale-up challenges. Emerging players like Shimadzu and Bio-Rad are advancing automation and continuous processing technologies, while academic-industry partnerships with institutions like University of Michigan are driving next-generation approaches focusing on sustainability and process intensification. The field is characterized by increasing competition in developing cost-effective solutions that maintain purity while improving yield at commercial scale.
Waters Technology Corp.
Technical Solution: Waters has developed the ACQUITY Advanced Polymer Chromatography (APC) system specifically adapted for API intermediate isolation. This platform utilizes sub-2μm particle technology combined with ultra-high performance liquid chromatography (UHPLC) principles to achieve superior resolution while significantly reducing run times. Their approach incorporates specialized column chemistries with enhanced stability under aggressive solvent conditions often required for API processing. Waters' system features proprietary detector technologies including photodiode array (PDA) and mass detection capabilities that enable precise fraction collection based on multiple quality parameters simultaneously. The platform includes advanced software algorithms for method development and optimization, reducing development time by up to 60%. Their technology has been validated for scale-up from analytical to preparative scales with predictable performance, maintaining separation efficiency while increasing throughput by factors of 3-5x compared to conventional approaches.
Strengths: Industry-leading resolution capabilities for structurally similar compounds; significant reduction in processing time and solvent consumption; comprehensive software suite simplifies method transfer between development and production. Weaknesses: Higher pressure requirements necessitate specialized equipment; more sensitive to sample preparation quality; higher initial investment compared to traditional HPLC systems.
Repligen Corp.
Technical Solution: Repligen has developed the OPUS® (Open Platform User-Specified) chromatography technology specifically adapted for API intermediate isolation. Their approach centers on pre-packed columns with consistent bed density and performance characteristics, eliminating variability associated with manual packing procedures. The technology incorporates a range of specialized media options optimized for different separation challenges, including reversed-phase, ion exchange, and mixed-mode chromatography. Repligen's system features a unique flow distribution design that ensures uniform sample application and elution across the entire column diameter, significantly improving separation efficiency and yield recovery. Their platform includes comprehensive validation protocols with extensive testing for extractables and leachables to ensure product quality and safety. The technology has demonstrated consistent performance across multiple manufacturing sites, with reproducible yield (>88%) and purity profiles that meet or exceed regulatory requirements for pharmaceutical intermediates.
Strengths: Pre-packed format eliminates variability associated with column packing; reduced implementation time compared to traditional approaches; comprehensive validation documentation simplifies regulatory compliance. Weaknesses: Less flexibility for custom media configurations; higher cost per separation compared to self-packed columns; limited to predetermined column dimensions.
Critical Patents in API Intermediate Isolation Technology
3-((r)-2-(amino-2-phenylethyl)-1-(2-fluoro-6 trifluoromethyl benzyl)-5-iodo-6-methyl-1h-pyrimidine-2,4-dione or a salt thereof, process for its preparation, and its use in the synthesis of elagolix
PatentActiveUS20220411382A1
Innovation
- Identification of a new intermediate in elagolix synthesis that can be isolated in solid form, enabling purification without column chromatography.
- Development of a process that avoids column chromatography purification steps while maintaining high purity of the final API.
- Direct conversion of intermediate (II) to compound (Ia) using iodine monochloride, potentially improving overall yield compared to previous multi-step processes.
Highly pure linezolid, substantally free of impurities
PatentInactiveIN2850DEL2013A
Innovation
- Development of a method to produce highly pure Linezolid with HPLC purity greater than 99.9% by identifying and removing specific impurities such as Linezolid N-oxide, dihydroxy, ditosyl, O-acetyl amine, N-acetyl amine, oxazoline, hydroxy amide, and amino alcohol impurities, using techniques like HPLC, NMR, and mass spectroscopy to characterize and isolate these impurities.
Regulatory Compliance for Pharmaceutical Processing
Regulatory compliance in pharmaceutical processing represents a critical framework governing the column chromatography process for API intermediate isolation. The pharmaceutical industry operates under stringent regulatory oversight from agencies such as the FDA, EMA, and ICH, which establish comprehensive guidelines for manufacturing processes, including chromatographic separation techniques.
For column chromatography processes specifically, compliance requirements focus on several key areas. Process validation documentation must demonstrate consistent yield and purity across multiple batches, with particular attention to scale-up parameters when transitioning from laboratory to production environments. The case study highlights the importance of establishing robust validation protocols that account for variations in column performance at different scales.
Quality control measures must be implemented throughout the chromatographic process, including in-process testing and final product analysis. Regulatory bodies require detailed documentation of analytical methods used to determine purity profiles, with validation data supporting method sensitivity, specificity, and reproducibility. The column chromatography process must demonstrate capability to consistently remove process-related impurities to levels below established safety thresholds.
Equipment qualification represents another significant compliance consideration. Chromatography systems must undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) procedures. Documentation must verify that equipment operates within specified parameters and produces consistent results across production runs. This includes validation of column packing procedures, flow rates, and pressure tolerances.
Raw material control systems must be established for all chromatography components, including resins, buffers, and solvents. Suppliers must be qualified, and materials must meet predetermined specifications. For API intermediate isolation, particular attention must be paid to leachables and extractables from chromatography media that could potentially contaminate the final product.
Change management protocols are essential for maintaining regulatory compliance when modifications to the chromatography process become necessary. Any changes to column dimensions, packing materials, or operating parameters must undergo formal review and risk assessment before implementation. The case study demonstrates how scale-up modifications require careful documentation and justification to maintain regulatory acceptance.
Environmental monitoring programs must be implemented to ensure chromatography operations occur under appropriate conditions. This includes controls for microbial contamination, particulate matter, and temperature fluctuations that could impact column performance or product quality. Waste handling procedures must also comply with environmental regulations, particularly for organic solvents and other hazardous materials used in the chromatographic process.
For column chromatography processes specifically, compliance requirements focus on several key areas. Process validation documentation must demonstrate consistent yield and purity across multiple batches, with particular attention to scale-up parameters when transitioning from laboratory to production environments. The case study highlights the importance of establishing robust validation protocols that account for variations in column performance at different scales.
Quality control measures must be implemented throughout the chromatographic process, including in-process testing and final product analysis. Regulatory bodies require detailed documentation of analytical methods used to determine purity profiles, with validation data supporting method sensitivity, specificity, and reproducibility. The column chromatography process must demonstrate capability to consistently remove process-related impurities to levels below established safety thresholds.
Equipment qualification represents another significant compliance consideration. Chromatography systems must undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) procedures. Documentation must verify that equipment operates within specified parameters and produces consistent results across production runs. This includes validation of column packing procedures, flow rates, and pressure tolerances.
Raw material control systems must be established for all chromatography components, including resins, buffers, and solvents. Suppliers must be qualified, and materials must meet predetermined specifications. For API intermediate isolation, particular attention must be paid to leachables and extractables from chromatography media that could potentially contaminate the final product.
Change management protocols are essential for maintaining regulatory compliance when modifications to the chromatography process become necessary. Any changes to column dimensions, packing materials, or operating parameters must undergo formal review and risk assessment before implementation. The case study demonstrates how scale-up modifications require careful documentation and justification to maintain regulatory acceptance.
Environmental monitoring programs must be implemented to ensure chromatography operations occur under appropriate conditions. This includes controls for microbial contamination, particulate matter, and temperature fluctuations that could impact column performance or product quality. Waste handling procedures must also comply with environmental regulations, particularly for organic solvents and other hazardous materials used in the chromatographic process.
Economic Analysis of Chromatography Process Scale-Up
The economic analysis of chromatography process scale-up represents a critical component in determining the viability of column chromatography for industrial applications. When scaling from laboratory to production levels, the cost dynamics change significantly, often in non-linear ways that must be carefully evaluated.
Initial capital expenditure for large-scale chromatography equipment can be substantial, with industrial-scale columns, pumps, detectors, and fraction collectors requiring investments ranging from $500,000 to several million dollars depending on the required throughput and level of automation. These costs must be amortized over the expected production volume and lifetime of the equipment.
Operational expenses follow a different scaling pattern than traditional chemical processes. While some economies of scale apply, the relationship between column size and processing capacity is not directly proportional. Doubling column diameter increases cross-sectional area by a factor of four, potentially increasing throughput, but flow rates cannot always be scaled proportionally due to pressure limitations and mass transfer considerations.
Consumables represent a significant recurring cost in chromatography processes. Stationary phase materials for large columns can cost $10,000-$100,000 per filling, with lifespans varying based on process conditions. Solvent consumption scales with column volume, with recovery and recycling systems becoming economically essential at production scale to mitigate environmental impact and reduce costs.
Labor requirements do not scale linearly with production volume. While laboratory-scale operations might require constant attention, automated production-scale systems can operate with minimal supervision, though specialized personnel for maintenance and troubleshooting remain necessary.
Yield and purity considerations dramatically impact economic viability. The case study demonstrates that achieving 98% purity with 85% yield requires optimization of loading conditions, flow rates, and fraction collection parameters. Each percentage point of yield improvement can translate to hundreds of thousands of dollars in annual savings for high-value API intermediates.
Process validation and regulatory compliance add significant costs that are often underestimated in preliminary analyses. These include method validation, cleaning validation, and documentation requirements that increase with scale and regulatory scrutiny.
Time-to-market considerations must also factor into economic analyses, as chromatography development and scale-up typically require 6-18 months. This timeline impacts return on investment calculations, especially for products with limited patent life or competitive market pressures.
Initial capital expenditure for large-scale chromatography equipment can be substantial, with industrial-scale columns, pumps, detectors, and fraction collectors requiring investments ranging from $500,000 to several million dollars depending on the required throughput and level of automation. These costs must be amortized over the expected production volume and lifetime of the equipment.
Operational expenses follow a different scaling pattern than traditional chemical processes. While some economies of scale apply, the relationship between column size and processing capacity is not directly proportional. Doubling column diameter increases cross-sectional area by a factor of four, potentially increasing throughput, but flow rates cannot always be scaled proportionally due to pressure limitations and mass transfer considerations.
Consumables represent a significant recurring cost in chromatography processes. Stationary phase materials for large columns can cost $10,000-$100,000 per filling, with lifespans varying based on process conditions. Solvent consumption scales with column volume, with recovery and recycling systems becoming economically essential at production scale to mitigate environmental impact and reduce costs.
Labor requirements do not scale linearly with production volume. While laboratory-scale operations might require constant attention, automated production-scale systems can operate with minimal supervision, though specialized personnel for maintenance and troubleshooting remain necessary.
Yield and purity considerations dramatically impact economic viability. The case study demonstrates that achieving 98% purity with 85% yield requires optimization of loading conditions, flow rates, and fraction collection parameters. Each percentage point of yield improvement can translate to hundreds of thousands of dollars in annual savings for high-value API intermediates.
Process validation and regulatory compliance add significant costs that are often underestimated in preliminary analyses. These include method validation, cleaning validation, and documentation requirements that increase with scale and regulatory scrutiny.
Time-to-market considerations must also factor into economic analyses, as chromatography development and scale-up typically require 6-18 months. This timeline impacts return on investment calculations, especially for products with limited patent life or competitive market pressures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!