Column Packing Procedures for Reproducible Separations — SOP and Troubleshooting Table
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Column Packing Background and Objectives
Chromatography column packing has evolved significantly since its inception in the early 20th century. Initially developed as a simple separation technique, chromatography has transformed into a sophisticated analytical and preparative methodology essential across pharmaceutical, biotechnology, and chemical industries. The evolution of column packing techniques has paralleled advancements in particle technology, from large irregular particles to today's sub-2-micron spherical particles, enabling unprecedented separation efficiencies.
The reproducibility of chromatographic separations remains a critical challenge in analytical laboratories worldwide. Despite technological advancements in instrumentation and column manufacturing, the manual process of column packing continues to introduce variability that affects separation performance. Industry data suggests that approximately 30-40% of chromatographic method failures can be attributed to column-related issues, with improper packing being a significant contributor.
Standard Operating Procedures (SOPs) for column packing have become increasingly important as regulatory requirements for analytical method validation have become more stringent. The FDA, EMA, and other regulatory bodies now require comprehensive documentation of column preparation procedures as part of method validation packages, particularly for methods used in quality control of pharmaceutical products.
The primary objective of developing robust column packing procedures is to ensure consistent chromatographic performance across different analysts, laboratories, and time periods. Key performance indicators include theoretical plate count, asymmetry factor, resolution between critical pairs, and pressure drop characteristics. A well-packed column typically demonstrates less than 5% relative standard deviation in these parameters across multiple packing cycles.
Current technological trends in column packing include automation of the packing process, development of self-packing columns, and implementation of real-time monitoring systems to assess packing quality during the procedure. These innovations aim to reduce the human factor in column preparation and enhance reproducibility.
The economic impact of improved column packing procedures is substantial. Industry estimates suggest that optimized column packing can extend column lifetime by 30-50%, reduce solvent consumption by up to 25%, and significantly decrease method development time. For high-throughput analytical laboratories, these efficiencies translate to millions in annual savings.
This technical research report aims to comprehensively examine the state-of-the-art in chromatography column packing procedures, identify critical parameters affecting reproducibility, establish a standardized SOP framework applicable across various chromatographic modes, and develop a systematic troubleshooting approach for common packing-related issues. The ultimate goal is to provide a practical resource that bridges theoretical understanding with practical implementation, enabling laboratories to achieve consistent, high-quality chromatographic separations.
The reproducibility of chromatographic separations remains a critical challenge in analytical laboratories worldwide. Despite technological advancements in instrumentation and column manufacturing, the manual process of column packing continues to introduce variability that affects separation performance. Industry data suggests that approximately 30-40% of chromatographic method failures can be attributed to column-related issues, with improper packing being a significant contributor.
Standard Operating Procedures (SOPs) for column packing have become increasingly important as regulatory requirements for analytical method validation have become more stringent. The FDA, EMA, and other regulatory bodies now require comprehensive documentation of column preparation procedures as part of method validation packages, particularly for methods used in quality control of pharmaceutical products.
The primary objective of developing robust column packing procedures is to ensure consistent chromatographic performance across different analysts, laboratories, and time periods. Key performance indicators include theoretical plate count, asymmetry factor, resolution between critical pairs, and pressure drop characteristics. A well-packed column typically demonstrates less than 5% relative standard deviation in these parameters across multiple packing cycles.
Current technological trends in column packing include automation of the packing process, development of self-packing columns, and implementation of real-time monitoring systems to assess packing quality during the procedure. These innovations aim to reduce the human factor in column preparation and enhance reproducibility.
The economic impact of improved column packing procedures is substantial. Industry estimates suggest that optimized column packing can extend column lifetime by 30-50%, reduce solvent consumption by up to 25%, and significantly decrease method development time. For high-throughput analytical laboratories, these efficiencies translate to millions in annual savings.
This technical research report aims to comprehensively examine the state-of-the-art in chromatography column packing procedures, identify critical parameters affecting reproducibility, establish a standardized SOP framework applicable across various chromatographic modes, and develop a systematic troubleshooting approach for common packing-related issues. The ultimate goal is to provide a practical resource that bridges theoretical understanding with practical implementation, enabling laboratories to achieve consistent, high-quality chromatographic separations.
Market Demand Analysis for Reproducible Separation Technologies
The global market for reproducible separation technologies has been experiencing significant growth, driven by increasing demands in pharmaceutical research, biotechnology, and analytical chemistry sectors. The compound annual growth rate (CAGR) for chromatography instruments and consumables market is projected to maintain steady growth through 2028, with particular emphasis on technologies that ensure reproducibility and precision.
Pharmaceutical and biopharmaceutical industries represent the largest market segment for reproducible separation technologies, accounting for approximately 40% of the total market share. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where reproducible separations are critical for ensuring batch-to-batch consistency and product safety.
Academic and research institutions constitute another significant market segment, driven by the need for reliable analytical methods in scientific research. The growing emphasis on reproducibility in scientific publications has further accelerated demand for standardized separation procedures and protocols.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) have emerged as rapidly growing consumers of reproducible separation technologies. As outsourcing continues to trend upward in pharmaceutical development, these organizations require robust and transferable analytical methods to maintain consistency across different sites and projects.
Regional analysis indicates North America leads the market for reproducible separation technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, primarily due to expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing investments in research infrastructure.
Key market drivers include increasing regulatory scrutiny on manufacturing processes, growing complexity of biological samples requiring sophisticated separation techniques, and rising demand for high-throughput analytical methods. The push toward process analytical technology (PAT) in pharmaceutical manufacturing has further emphasized the need for reproducible separation methods that can be integrated into continuous monitoring systems.
Customer pain points consistently highlight challenges with method transfer between laboratories, column-to-column variability, and difficulties in troubleshooting separation problems. This creates specific market opportunities for standardized column packing procedures and comprehensive troubleshooting guides that can address these challenges directly.
The market shows particular interest in digital solutions that can document and standardize separation procedures, with growing demand for software tools that can track column performance, predict maintenance needs, and suggest optimization strategies based on historical data.
Pharmaceutical and biopharmaceutical industries represent the largest market segment for reproducible separation technologies, accounting for approximately 40% of the total market share. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where reproducible separations are critical for ensuring batch-to-batch consistency and product safety.
Academic and research institutions constitute another significant market segment, driven by the need for reliable analytical methods in scientific research. The growing emphasis on reproducibility in scientific publications has further accelerated demand for standardized separation procedures and protocols.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) have emerged as rapidly growing consumers of reproducible separation technologies. As outsourcing continues to trend upward in pharmaceutical development, these organizations require robust and transferable analytical methods to maintain consistency across different sites and projects.
Regional analysis indicates North America leads the market for reproducible separation technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, primarily due to expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing investments in research infrastructure.
Key market drivers include increasing regulatory scrutiny on manufacturing processes, growing complexity of biological samples requiring sophisticated separation techniques, and rising demand for high-throughput analytical methods. The push toward process analytical technology (PAT) in pharmaceutical manufacturing has further emphasized the need for reproducible separation methods that can be integrated into continuous monitoring systems.
Customer pain points consistently highlight challenges with method transfer between laboratories, column-to-column variability, and difficulties in troubleshooting separation problems. This creates specific market opportunities for standardized column packing procedures and comprehensive troubleshooting guides that can address these challenges directly.
The market shows particular interest in digital solutions that can document and standardize separation procedures, with growing demand for software tools that can track column performance, predict maintenance needs, and suggest optimization strategies based on historical data.
Current Challenges in Column Packing Methodologies
Despite significant advancements in chromatographic technologies, column packing remains one of the most challenging aspects in achieving reproducible separations. Current methodologies face several persistent challenges that impact separation efficiency, column longevity, and result reproducibility. The variability in packing density represents a primary concern, as inconsistent compression of stationary phase particles leads to channeling, void formation, and irregular flow patterns that compromise separation performance.
Manual packing procedures introduce significant operator-dependent variability, with differences in technique, applied pressure, and slurry preparation methods resulting in column-to-column inconsistencies even when performed by the same technician. This human factor remains difficult to standardize despite detailed SOPs and training programs.
The industry also struggles with scalability issues, as packing procedures optimized at analytical scale often fail to translate effectively to preparative or industrial scales. The physics of particle settling and compression changes dramatically with column diameter, requiring substantial modifications to packing parameters that are not always well understood or documented.
Material inconsistencies present another significant challenge, with batch-to-batch variations in particle size distribution, surface characteristics, and pore structure of stationary phases affecting packing homogeneity. Even minor differences in these properties can necessitate adjustments to established packing protocols.
Equipment limitations further complicate reproducible column packing. Many laboratories utilize improvised setups rather than specialized column packing stations, leading to inconsistent pressure application, flow rates, and slurry delivery. The lack of standardized equipment specifications across the industry makes it difficult to establish universal best practices.
Modern high-efficiency columns with sub-2μm particles present unique challenges, requiring extremely high pressures for proper packing while being more susceptible to bed collapse and over-compression. The narrow particle size distribution tolerances leave minimal room for error in packing procedures.
Quality assessment of packed columns remains problematic, with traditional metrics like theoretical plate count and asymmetry factor providing only partial insights into column bed homogeneity. Advanced techniques such as magnetic resonance imaging of packed beds remain confined to research settings rather than routine quality control.
Documentation and knowledge transfer represent persistent challenges, with many organizations relying on tribal knowledge rather than comprehensive, scientifically-validated protocols. The troubleshooting of packing failures often follows trial-and-error approaches rather than systematic problem-solving methodologies based on fundamental understanding of the underlying physical processes.
Manual packing procedures introduce significant operator-dependent variability, with differences in technique, applied pressure, and slurry preparation methods resulting in column-to-column inconsistencies even when performed by the same technician. This human factor remains difficult to standardize despite detailed SOPs and training programs.
The industry also struggles with scalability issues, as packing procedures optimized at analytical scale often fail to translate effectively to preparative or industrial scales. The physics of particle settling and compression changes dramatically with column diameter, requiring substantial modifications to packing parameters that are not always well understood or documented.
Material inconsistencies present another significant challenge, with batch-to-batch variations in particle size distribution, surface characteristics, and pore structure of stationary phases affecting packing homogeneity. Even minor differences in these properties can necessitate adjustments to established packing protocols.
Equipment limitations further complicate reproducible column packing. Many laboratories utilize improvised setups rather than specialized column packing stations, leading to inconsistent pressure application, flow rates, and slurry delivery. The lack of standardized equipment specifications across the industry makes it difficult to establish universal best practices.
Modern high-efficiency columns with sub-2μm particles present unique challenges, requiring extremely high pressures for proper packing while being more susceptible to bed collapse and over-compression. The narrow particle size distribution tolerances leave minimal room for error in packing procedures.
Quality assessment of packed columns remains problematic, with traditional metrics like theoretical plate count and asymmetry factor providing only partial insights into column bed homogeneity. Advanced techniques such as magnetic resonance imaging of packed beds remain confined to research settings rather than routine quality control.
Documentation and knowledge transfer represent persistent challenges, with many organizations relying on tribal knowledge rather than comprehensive, scientifically-validated protocols. The troubleshooting of packing failures often follows trial-and-error approaches rather than systematic problem-solving methodologies based on fundamental understanding of the underlying physical processes.
Standard Operating Procedures for Column Packing
01 Standardized column packing protocols
Implementing standardized protocols for column packing procedures significantly improves reproducibility in chromatographic separations. These protocols include specific guidelines for slurry preparation, flow rates during packing, compression techniques, and post-packing conditioning. By following consistent methodologies, variations between different batches of packed columns can be minimized, leading to more reliable analytical results across multiple experiments.- Standardized column packing protocols: Implementing standardized protocols for column packing procedures significantly improves reproducibility in chromatographic separations. These protocols include detailed step-by-step instructions for slurry preparation, packing pressure control, and consolidation techniques. By following consistent methodologies, variations between different operators and packing instances can be minimized, leading to more reliable and reproducible column performance across multiple preparations.
- Automated packing systems: Automated column packing systems enhance reproducibility by reducing human intervention and associated variability. These systems control critical parameters such as flow rate, pressure, and packing duration with high precision. Computerized monitoring and adjustment of packing conditions ensure consistent bed density and column efficiency. Automation also enables detailed documentation of packing parameters for future reference and troubleshooting, contributing to improved batch-to-batch consistency.
- Quality control methods for packed columns: Implementing robust quality control methods is essential for verifying column packing reproducibility. These methods include testing column efficiency, asymmetry, pressure drop, and retention time reproducibility. Advanced techniques such as pulse testing and residence time distribution analysis can detect subtle variations in packing quality. Regular performance monitoring using standard test mixtures helps identify deviations from expected behavior, allowing for timely intervention and maintenance to maintain consistent separation performance.
- Slurry preparation techniques: The preparation of consistent slurries is fundamental to achieving reproducible column packing. Key factors include particle size distribution control, slurry concentration optimization, and appropriate solvent selection. Techniques such as sonication, degassing, and temperature control during slurry preparation help prevent particle aggregation and ensure uniform suspension. Standardized mixing protocols and timing between slurry preparation and column packing contribute significantly to consistent bed formation and homogeneity.
- Environmental and operational parameter control: Controlling environmental and operational parameters during column packing significantly impacts reproducibility. Factors such as temperature, humidity, and vibration must be carefully regulated. Consistent packing pressure profiles, flow rates, and compression factors are critical for achieving uniform bed density. Systematic approaches to equilibration and conditioning of newly packed columns ensure stable baseline performance. Detailed documentation of environmental conditions during packing enables correlation analysis with column performance and facilitates troubleshooting of reproducibility issues.
02 Automated packing systems
Automated column packing systems enhance reproducibility by reducing human error and ensuring consistent application of pressure, flow rates, and packing times. These systems can precisely control critical parameters such as slurry concentration, compression force, and solvent flow during the packing process. The automation technology provides detailed documentation of packing conditions, allowing for better troubleshooting and method transfer between different laboratories.Expand Specific Solutions03 Quality control testing methods
Specific quality control tests are essential for verifying column packing reproducibility. These include measurements of column efficiency, asymmetry factor, pressure drop, and retention time reproducibility. Advanced testing methods may incorporate pulse testing, van Deemter curve analysis, and porosity determination. Implementing standardized quality control procedures ensures that packed columns meet predefined performance criteria before use in analytical or preparative applications.Expand Specific Solutions04 Slurry preparation techniques
The preparation of stationary phase slurries significantly impacts column packing reproducibility. Key factors include particle size distribution, slurry concentration, solvent selection, and sonication or stirring methods. Optimized slurry preparation techniques ensure uniform particle suspension and prevent aggregation, leading to homogeneous bed formation during the packing process. Consistent slurry preparation methodologies are fundamental to achieving reproducible chromatographic performance across multiple columns.Expand Specific Solutions05 Environmental and equipment factors
Environmental conditions and equipment specifications play crucial roles in column packing reproducibility. Temperature fluctuations, vibrations, and pressure system stability can significantly affect packing quality. Maintaining consistent laboratory conditions and using qualified equipment with proper calibration ensures reproducible column performance. Additionally, factors such as tubing dimensions, connector designs, and frit specifications must be standardized to minimize system-related variables that could impact packing reproducibility.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Separation Science
The column packing procedures for reproducible separations market is currently in a growth phase, characterized by increasing demand for standardized protocols in chromatography applications. The global market size is expanding steadily, driven by biopharmaceutical manufacturing needs and analytical testing requirements. Technologically, the field shows moderate maturity with established players like Cytiva, Repligen, and Bio-Rad Laboratories leading innovation in column packing technologies. Emerging companies such as Biotechflow and PhyNexus are introducing specialized solutions, while established chemical manufacturers including BASF, Air Liquide, and Wanhua Chemical provide supporting materials. The competitive landscape features a mix of specialized bioprocessing companies and diversified scientific instrument providers like Hitachi High-Tech and EMD Millipore, with increasing focus on automation and reproducibility to address industry standardization challenges.
Cytiva BioProcess R&D AB
Technical Solution: Cytiva has developed a comprehensive column packing procedure for chromatography separations that focuses on reproducibility and scalability. Their approach includes standardized protocols for different types of resins (protein A, ion exchange, hydrophobic interaction) with specific flow rates and compression factors for each. The company employs a multi-stage packing process that includes pre-column preparation, slurry preparation with controlled concentration (50-70% slurry), and adaptive compression techniques. Their system incorporates real-time monitoring of bed height, pressure, and flow distribution using specialized sensors to ensure column homogeneity. Cytiva's procedures include validation steps with HETP (Height Equivalent to a Theoretical Plate) and asymmetry measurements to verify packing quality, with acceptance criteria of asymmetry between 0.8-1.5 and HETP values below specified thresholds for different column diameters.
Strengths: Highly reproducible results across different scales (laboratory to production); comprehensive troubleshooting guides specific to different resin types; validated protocols that meet regulatory requirements. Weaknesses: Requires specialized equipment that increases initial investment; protocols may be overly complex for simple applications; system is less flexible for novel resin types not covered by existing procedures.
Repligen Corp.
Technical Solution: Repligen has pioneered an automated column packing technology called OPUS (Open Platform User Specified) that addresses reproducibility challenges in chromatography separations. Their system utilizes pre-packed columns with standardized protocols that eliminate variability in manual packing procedures. The technology incorporates precision flow controllers that maintain consistent linear velocity during the packing process, critical for achieving uniform bed density. Repligen's approach includes a proprietary slurry preparation method that prevents air entrapment and ensures homogeneous distribution of resin particles. Their SOP includes specific parameters for different resin types, with automated pressure ramping profiles that optimize compression without damaging sensitive chromatography media. The system also features integrated quality verification using conductivity and UV sensors to perform automated HETP and asymmetry testing, with results automatically documented for compliance purposes.
Strengths: Eliminates operator-dependent variability through automation; reduces labor costs and time requirements; provides comprehensive electronic documentation for regulatory compliance. Weaknesses: Higher capital investment compared to manual packing systems; limited flexibility for customization of packing parameters; requires specialized training for operation and maintenance.
Critical Parameters Affecting Column Packing Reproducibility
Column packing apparatus and packing method
PatentInactiveUS20170248559A1
Innovation
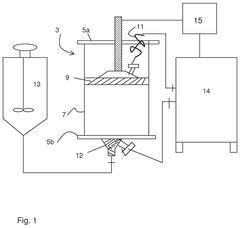
- A column packing apparatus and method featuring a tubular structure with a packing material supplier and a filling controller, including a resistive element to maintain a predetermined flow rate and pressure level, ensuring uniform pressure and consistent filler material deposition.
Column Packing Method
PatentPendingUS20250018313A1
Innovation
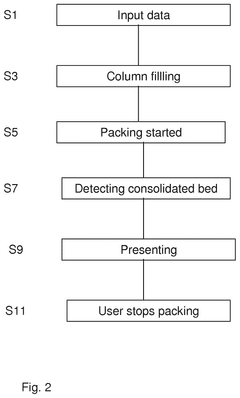
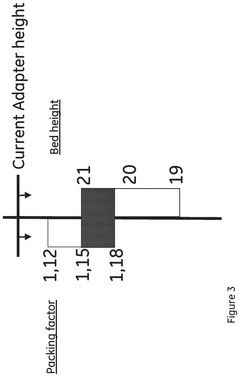
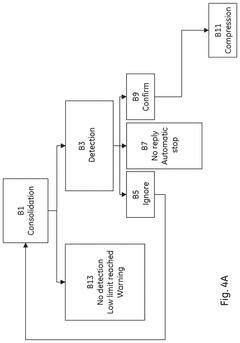
- A column packing system and method that uses a control unit with software to guide the user through the packing process, providing input data for media type, slurry concentration, and target bed height, automatically controlling the filling and compression of the bed to achieve an optimal packing factor within acceptable ranges, and includes warnings and automatic stopping to prevent damage.
Quality Control Metrics for Packed Columns
Quality control metrics for packed columns are essential for ensuring reproducible separations in chromatography processes. The establishment of standardized metrics allows for objective evaluation of column performance and facilitates troubleshooting when separation issues arise. Key metrics include theoretical plate count (N), which measures separation efficiency by quantifying the number of theoretical equilibration stages within the column. Higher plate counts indicate better separation capabilities, with modern analytical columns typically achieving 5,000-15,000 plates per column.
Asymmetry factor (As) represents another critical metric, measuring peak shape quality by comparing the front and back portions of chromatographic peaks. Ideal values range between 0.9-1.2, with deviations indicating potential issues such as overloading, void formation, or chemical interactions between analytes and stationary phase. Regular monitoring of asymmetry factors provides early warning of column deterioration.
Resolution (Rs) quantifies the degree of separation between adjacent peaks and serves as a direct measure of separation performance. Values above 1.5 indicate baseline separation, while lower values suggest inadequate separation. Resolution depends on retention factor, selectivity, and column efficiency, making it a comprehensive metric for overall column performance assessment.
Pressure drop across the column represents a physical parameter that indicates packing uniformity and stability. Unexpected increases in pressure may signal particulate contamination or bed collapse, while decreases might indicate channeling or void formation. Establishing baseline pressure values after column packing allows for meaningful trend analysis during the column's lifecycle.
Retention time reproducibility serves as a practical metric for day-to-day operations, with variations below 2% generally considered acceptable for well-packed columns. Larger deviations warrant investigation into potential issues with mobile phase composition, temperature fluctuations, or changes in flow rate.
Implementation of these metrics requires establishing acceptance criteria specific to each application. Documentation of initial column performance creates a reference point for future comparisons. Regular testing intervals should be defined based on usage frequency and sample complexity, with more frequent testing for columns handling complex or dirty samples. Automated data collection and analysis systems can streamline this process, generating trend charts that visualize column performance over time.
When metrics fall outside established parameters, a systematic troubleshooting approach using the provided troubleshooting table can identify root causes and appropriate corrective actions, extending column lifetime and ensuring consistent separation quality.
Asymmetry factor (As) represents another critical metric, measuring peak shape quality by comparing the front and back portions of chromatographic peaks. Ideal values range between 0.9-1.2, with deviations indicating potential issues such as overloading, void formation, or chemical interactions between analytes and stationary phase. Regular monitoring of asymmetry factors provides early warning of column deterioration.
Resolution (Rs) quantifies the degree of separation between adjacent peaks and serves as a direct measure of separation performance. Values above 1.5 indicate baseline separation, while lower values suggest inadequate separation. Resolution depends on retention factor, selectivity, and column efficiency, making it a comprehensive metric for overall column performance assessment.
Pressure drop across the column represents a physical parameter that indicates packing uniformity and stability. Unexpected increases in pressure may signal particulate contamination or bed collapse, while decreases might indicate channeling or void formation. Establishing baseline pressure values after column packing allows for meaningful trend analysis during the column's lifecycle.
Retention time reproducibility serves as a practical metric for day-to-day operations, with variations below 2% generally considered acceptable for well-packed columns. Larger deviations warrant investigation into potential issues with mobile phase composition, temperature fluctuations, or changes in flow rate.
Implementation of these metrics requires establishing acceptance criteria specific to each application. Documentation of initial column performance creates a reference point for future comparisons. Regular testing intervals should be defined based on usage frequency and sample complexity, with more frequent testing for columns handling complex or dirty samples. Automated data collection and analysis systems can streamline this process, generating trend charts that visualize column performance over time.
When metrics fall outside established parameters, a systematic troubleshooting approach using the provided troubleshooting table can identify root causes and appropriate corrective actions, extending column lifetime and ensuring consistent separation quality.
Automation Potential in Column Packing Processes
The column packing process in chromatography represents a critical yet labor-intensive procedure that significantly impacts separation reproducibility. Current manual packing methods involve numerous variables including slurry concentration, packing pressure, and consolidation time—all requiring precise human intervention. Automation technologies offer substantial potential to standardize these processes, reducing human error and improving consistency across operations.
Robotic systems equipped with precision liquid handling capabilities could revolutionize slurry preparation by ensuring exact solvent ratios and particle suspension homogeneity. These systems can maintain consistent stirring speeds and durations, eliminating variations that commonly occur with manual preparation. Pressure control automation represents another significant opportunity, where computerized systems can implement precise pressure ramps and holds according to optimized protocols.
Sensor integration presents perhaps the most transformative automation potential. Real-time monitoring of bed formation using optical density sensors, pressure differential measurements, and even acoustic monitoring could provide immediate feedback during the packing process. These data streams enable adaptive control systems to make micro-adjustments to packing parameters, potentially creating self-optimizing packing procedures.
Machine learning algorithms could analyze historical packing data to identify optimal parameters for specific column types and stationary phases. By correlating packing variables with separation performance metrics, predictive models could recommend ideal conditions before packing begins, significantly reducing trial-and-error approaches currently prevalent in the industry.
Automated quality assessment represents another frontier, where technologies such as computed tomography or magnetic resonance imaging could non-destructively evaluate packed column homogeneity. These systems could automatically detect channeling, voids, or wall effects—common issues that compromise separation performance—before the column enters service.
The economic case for automation appears compelling when considering the high costs associated with inconsistent separations, particularly in pharmaceutical manufacturing where batch failures can result in significant financial losses. Initial investment in automation technology could be offset by improved column longevity, reduced packing material waste, and decreased operator time requirements.
Implementation challenges include developing flexible systems capable of handling various column dimensions and stationary phase types, as well as creating user-friendly interfaces that allow operators to transition from manual to automated workflows. Regulatory considerations will also need addressing, particularly for GMP environments where automated systems require thorough validation.
Robotic systems equipped with precision liquid handling capabilities could revolutionize slurry preparation by ensuring exact solvent ratios and particle suspension homogeneity. These systems can maintain consistent stirring speeds and durations, eliminating variations that commonly occur with manual preparation. Pressure control automation represents another significant opportunity, where computerized systems can implement precise pressure ramps and holds according to optimized protocols.
Sensor integration presents perhaps the most transformative automation potential. Real-time monitoring of bed formation using optical density sensors, pressure differential measurements, and even acoustic monitoring could provide immediate feedback during the packing process. These data streams enable adaptive control systems to make micro-adjustments to packing parameters, potentially creating self-optimizing packing procedures.
Machine learning algorithms could analyze historical packing data to identify optimal parameters for specific column types and stationary phases. By correlating packing variables with separation performance metrics, predictive models could recommend ideal conditions before packing begins, significantly reducing trial-and-error approaches currently prevalent in the industry.
Automated quality assessment represents another frontier, where technologies such as computed tomography or magnetic resonance imaging could non-destructively evaluate packed column homogeneity. These systems could automatically detect channeling, voids, or wall effects—common issues that compromise separation performance—before the column enters service.
The economic case for automation appears compelling when considering the high costs associated with inconsistent separations, particularly in pharmaceutical manufacturing where batch failures can result in significant financial losses. Initial investment in automation technology could be offset by improved column longevity, reduced packing material waste, and decreased operator time requirements.
Implementation challenges include developing flexible systems capable of handling various column dimensions and stationary phase types, as well as creating user-friendly interfaces that allow operators to transition from manual to automated workflows. Regulatory considerations will also need addressing, particularly for GMP environments where automated systems require thorough validation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!