How to Implement Inline Sampling for Column Chromatography Process Control — Architecture and Data Flow
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Inline Sampling Technology Background and Objectives
Column chromatography represents a cornerstone technology in biopharmaceutical manufacturing, analytical chemistry, and various industrial separation processes. The evolution of this technology has progressed from basic gravity-fed columns to sophisticated high-pressure systems with advanced detection capabilities. Despite these advancements, process monitoring and control remain challenging, particularly in real-time applications where immediate data is crucial for quality assurance and process optimization.
Inline sampling for column chromatography addresses this critical gap by enabling continuous, real-time monitoring of separation processes without disrupting the flow or compromising sample integrity. This technology has emerged as a response to increasing regulatory demands for Process Analytical Technology (PAT) implementation and the industry-wide shift toward continuous manufacturing paradigms.
The primary objective of inline sampling technology is to provide accurate, real-time data acquisition during chromatographic separations while maintaining process sterility and product quality. This enables immediate detection of process deviations, facilitates adaptive control strategies, and ultimately enhances process understanding and product consistency. Furthermore, it aims to reduce manual sampling requirements, minimizing human error and contamination risks.
Historical approaches to chromatography monitoring have relied heavily on offline analysis, introducing significant time delays between sample collection and actionable results. The technological progression toward inline sampling represents a paradigm shift from reactive to proactive process control, aligning with Quality by Design (QbD) principles that emphasize built-in quality rather than tested quality.
Current inline sampling systems face several technical challenges, including maintaining sample representativeness, preventing cross-contamination, ensuring compatibility with existing chromatography systems, and developing robust data integration architectures. The resolution of these challenges requires interdisciplinary approaches combining microfluidics, sensor technology, data analytics, and automation engineering.
The implementation of inline sampling technology aims to achieve multiple technical goals: minimizing dead volumes to prevent sample dilution, ensuring rapid response times for real-time decision making, maintaining sample integrity throughout the sampling process, and developing scalable solutions applicable across laboratory, pilot, and production environments.
As regulatory agencies increasingly emphasize continuous verification of critical quality attributes, inline sampling technology has become strategically important for pharmaceutical and biotechnology companies seeking to demonstrate robust process control. The technology's evolution is expected to continue toward more integrated, intelligent systems capable of not only monitoring but also predicting and autonomously adjusting process parameters to maintain optimal separation conditions.
Inline sampling for column chromatography addresses this critical gap by enabling continuous, real-time monitoring of separation processes without disrupting the flow or compromising sample integrity. This technology has emerged as a response to increasing regulatory demands for Process Analytical Technology (PAT) implementation and the industry-wide shift toward continuous manufacturing paradigms.
The primary objective of inline sampling technology is to provide accurate, real-time data acquisition during chromatographic separations while maintaining process sterility and product quality. This enables immediate detection of process deviations, facilitates adaptive control strategies, and ultimately enhances process understanding and product consistency. Furthermore, it aims to reduce manual sampling requirements, minimizing human error and contamination risks.
Historical approaches to chromatography monitoring have relied heavily on offline analysis, introducing significant time delays between sample collection and actionable results. The technological progression toward inline sampling represents a paradigm shift from reactive to proactive process control, aligning with Quality by Design (QbD) principles that emphasize built-in quality rather than tested quality.
Current inline sampling systems face several technical challenges, including maintaining sample representativeness, preventing cross-contamination, ensuring compatibility with existing chromatography systems, and developing robust data integration architectures. The resolution of these challenges requires interdisciplinary approaches combining microfluidics, sensor technology, data analytics, and automation engineering.
The implementation of inline sampling technology aims to achieve multiple technical goals: minimizing dead volumes to prevent sample dilution, ensuring rapid response times for real-time decision making, maintaining sample integrity throughout the sampling process, and developing scalable solutions applicable across laboratory, pilot, and production environments.
As regulatory agencies increasingly emphasize continuous verification of critical quality attributes, inline sampling technology has become strategically important for pharmaceutical and biotechnology companies seeking to demonstrate robust process control. The technology's evolution is expected to continue toward more integrated, intelligent systems capable of not only monitoring but also predicting and autonomously adjusting process parameters to maintain optimal separation conditions.
Market Demand Analysis for Real-time Chromatography Monitoring
The global market for real-time chromatography monitoring systems is experiencing significant growth, driven by increasing demands for process analytical technology (PAT) in pharmaceutical and biopharmaceutical manufacturing. Current market research indicates that the biopharmaceutical process analytical technology sector is expanding at a compound annual growth rate of approximately 13% through 2025, with inline monitoring solutions representing one of the fastest-growing segments.
This growth is primarily fueled by regulatory pressures from agencies like the FDA and EMA, which are increasingly emphasizing Quality by Design (QbD) approaches and continuous manufacturing processes. These regulatory frameworks require manufacturers to implement robust monitoring systems that can provide real-time data on critical process parameters, making inline sampling for column chromatography a necessity rather than a luxury.
The biopharmaceutical industry represents the largest market segment for real-time chromatography monitoring, accounting for a substantial portion of the total market value. This is largely due to the critical role of chromatography in protein purification processes and the high value of biologic products, where batch failures can result in losses of millions of dollars. Consequently, companies are willing to invest significantly in technologies that reduce risk and increase process understanding.
From an end-user perspective, large pharmaceutical companies have been early adopters of inline monitoring technologies, but mid-sized biopharmaceutical companies are now rapidly implementing these systems as costs decrease and integration becomes more straightforward. Contract manufacturing organizations (CMOs) represent another growing segment, as they seek to differentiate their services through advanced process control capabilities.
Geographically, North America leads the market for real-time chromatography monitoring systems, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years due to increasing biopharmaceutical manufacturing capacity in countries like China, Singapore, and South Korea.
Key market drivers include the need for reduced production costs, improved product quality, and decreased time-to-market. Real-time monitoring enables faster decision-making during production, reducing hold times and allowing for immediate corrective actions when process deviations occur. This capability is particularly valuable in continuous manufacturing settings, where process understanding and control are paramount.
Customer surveys indicate that end-users prioritize reliability, ease of integration with existing systems, data integrity, and analytical accuracy when selecting real-time monitoring solutions. There is also growing demand for systems that can handle multiple analytical techniques simultaneously and provide predictive insights through advanced data analytics and machine learning algorithms.
This growth is primarily fueled by regulatory pressures from agencies like the FDA and EMA, which are increasingly emphasizing Quality by Design (QbD) approaches and continuous manufacturing processes. These regulatory frameworks require manufacturers to implement robust monitoring systems that can provide real-time data on critical process parameters, making inline sampling for column chromatography a necessity rather than a luxury.
The biopharmaceutical industry represents the largest market segment for real-time chromatography monitoring, accounting for a substantial portion of the total market value. This is largely due to the critical role of chromatography in protein purification processes and the high value of biologic products, where batch failures can result in losses of millions of dollars. Consequently, companies are willing to invest significantly in technologies that reduce risk and increase process understanding.
From an end-user perspective, large pharmaceutical companies have been early adopters of inline monitoring technologies, but mid-sized biopharmaceutical companies are now rapidly implementing these systems as costs decrease and integration becomes more straightforward. Contract manufacturing organizations (CMOs) represent another growing segment, as they seek to differentiate their services through advanced process control capabilities.
Geographically, North America leads the market for real-time chromatography monitoring systems, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years due to increasing biopharmaceutical manufacturing capacity in countries like China, Singapore, and South Korea.
Key market drivers include the need for reduced production costs, improved product quality, and decreased time-to-market. Real-time monitoring enables faster decision-making during production, reducing hold times and allowing for immediate corrective actions when process deviations occur. This capability is particularly valuable in continuous manufacturing settings, where process understanding and control are paramount.
Customer surveys indicate that end-users prioritize reliability, ease of integration with existing systems, data integrity, and analytical accuracy when selecting real-time monitoring solutions. There is also growing demand for systems that can handle multiple analytical techniques simultaneously and provide predictive insights through advanced data analytics and machine learning algorithms.
Current Challenges in Column Chromatography Process Control
Column chromatography remains a cornerstone technique in pharmaceutical manufacturing, biotechnology, and chemical processing industries. Despite its widespread adoption, several significant challenges persist in implementing effective process control systems, particularly when integrating inline sampling technologies.
Traditional column chromatography processes often rely on offline sampling methods that introduce considerable time delays between sample collection and analytical results. This temporal gap creates inefficiencies in real-time decision-making and process adjustments, leading to suboptimal yields and inconsistent product quality. The industry urgently needs solutions that bridge this gap through advanced inline sampling technologies.
Data integration presents another formidable challenge. Current chromatography systems frequently operate with disparate data management platforms that struggle to communicate effectively with each other. This fragmentation results in information silos where critical process data remains isolated, preventing holistic analysis and comprehensive process understanding. The lack of standardized data formats and communication protocols further exacerbates this issue.
Sensor technology limitations constitute a significant technical barrier. Existing inline sensors often lack the sensitivity, specificity, or robustness required for reliable real-time monitoring of complex biological mixtures. Many sensors suffer from fouling issues when exposed to high-protein environments or struggle to maintain calibration over extended production runs. Additionally, the diversity of analytes in chromatography processes demands multi-parameter sensing capabilities that current technologies cannot fully deliver.
Regulatory compliance adds another layer of complexity. Implementing inline sampling systems requires extensive validation to ensure data integrity and reliability. The pharmaceutical industry, in particular, faces stringent requirements for demonstrating that inline measurements correlate accurately with established offline reference methods. This validation burden often slows adoption of innovative monitoring approaches.
Scalability concerns also plague current implementations. Solutions that function effectively at laboratory scale frequently encounter unforeseen challenges when transferred to production environments. Differences in flow dynamics, pressure profiles, and material interactions can significantly alter sensor performance and data quality. Many existing architectures lack the flexibility to accommodate these scale-dependent variations without substantial reconfiguration.
Cost considerations remain a persistent obstacle. The initial investment for advanced inline sampling systems, including sensors, data management infrastructure, and integration components, represents a significant capital expenditure. Organizations must carefully evaluate the return on investment timeline, which can be difficult to quantify precisely due to the complex interplay of improved process control, reduced waste, and enhanced product quality.
Traditional column chromatography processes often rely on offline sampling methods that introduce considerable time delays between sample collection and analytical results. This temporal gap creates inefficiencies in real-time decision-making and process adjustments, leading to suboptimal yields and inconsistent product quality. The industry urgently needs solutions that bridge this gap through advanced inline sampling technologies.
Data integration presents another formidable challenge. Current chromatography systems frequently operate with disparate data management platforms that struggle to communicate effectively with each other. This fragmentation results in information silos where critical process data remains isolated, preventing holistic analysis and comprehensive process understanding. The lack of standardized data formats and communication protocols further exacerbates this issue.
Sensor technology limitations constitute a significant technical barrier. Existing inline sensors often lack the sensitivity, specificity, or robustness required for reliable real-time monitoring of complex biological mixtures. Many sensors suffer from fouling issues when exposed to high-protein environments or struggle to maintain calibration over extended production runs. Additionally, the diversity of analytes in chromatography processes demands multi-parameter sensing capabilities that current technologies cannot fully deliver.
Regulatory compliance adds another layer of complexity. Implementing inline sampling systems requires extensive validation to ensure data integrity and reliability. The pharmaceutical industry, in particular, faces stringent requirements for demonstrating that inline measurements correlate accurately with established offline reference methods. This validation burden often slows adoption of innovative monitoring approaches.
Scalability concerns also plague current implementations. Solutions that function effectively at laboratory scale frequently encounter unforeseen challenges when transferred to production environments. Differences in flow dynamics, pressure profiles, and material interactions can significantly alter sensor performance and data quality. Many existing architectures lack the flexibility to accommodate these scale-dependent variations without substantial reconfiguration.
Cost considerations remain a persistent obstacle. The initial investment for advanced inline sampling systems, including sensors, data management infrastructure, and integration components, represents a significant capital expenditure. Organizations must carefully evaluate the return on investment timeline, which can be difficult to quantify precisely due to the complex interplay of improved process control, reduced waste, and enhanced product quality.
Current Inline Sampling Architectures and Implementations
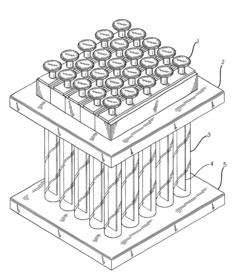
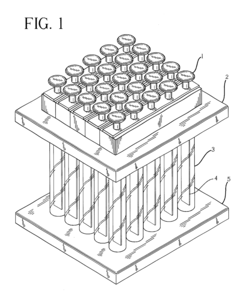
01 Inline sampling systems for chromatography monitoring
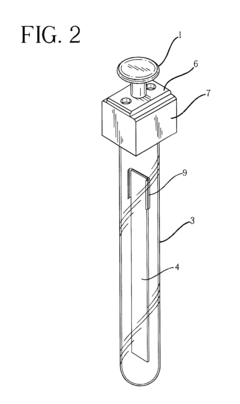
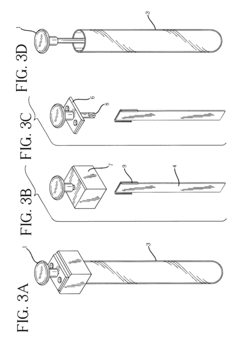
Inline sampling systems are designed to extract samples directly from chromatography columns during operation without disrupting the process flow. These systems typically include specialized valves, connectors, and sampling ports that allow for the withdrawal of representative samples at critical points in the chromatography process. The samples can then be analyzed to determine the composition, purity, and concentration of the target compounds, enabling real-time monitoring and control of the separation process.- Automated inline sampling systems for chromatography: Automated systems for inline sampling in column chromatography enable real-time monitoring and control of the separation process. These systems incorporate automated sampling valves, flow diverters, and programmable controllers that can extract samples at predetermined intervals without disrupting the main process flow. The automation reduces manual intervention, minimizes contamination risks, and provides consistent sampling for quality control purposes.
- Real-time analysis and detection methods: Various detection and analysis methods are employed for inline monitoring of column chromatography processes. These include spectroscopic techniques (UV-Vis, IR, Raman), mass spectrometry, and conductivity measurements that can be integrated directly into the chromatography flow path. These detection systems provide immediate feedback on separation efficiency, component purity, and process parameters, allowing for rapid adjustments to optimize chromatographic performance.
- Microfluidic and miniaturized sampling devices: Miniaturized sampling devices utilize microfluidic technology to extract and analyze minute sample volumes from chromatography columns. These systems feature micro-channels, nano-sensors, and integrated analytical components that enable high-precision sampling with minimal impact on the main separation process. The reduced scale allows for faster analysis, lower reagent consumption, and integration with automated process control systems.
- Sample preparation and conditioning techniques: Specialized techniques for conditioning and preparing inline samples ensure accurate analysis of chromatography processes. These include filtration systems, dilution mechanisms, buffer exchange modules, and temperature control devices that process samples before analysis. Proper sample conditioning removes particulates, adjusts concentration, and stabilizes analytes to prevent interference with detection systems and ensure representative sampling of the chromatographic process.
- Integration with process control systems: Advanced integration of inline sampling with process control systems enables closed-loop control of chromatography processes. These systems combine sampling data with process parameters to make automated adjustments to flow rates, buffer composition, and column conditions. The integration includes data management software, predictive algorithms, and feedback control mechanisms that optimize separation efficiency and product quality while maintaining process consistency.
02 Real-time analytical techniques for process control
Various analytical techniques can be integrated with column chromatography systems to provide real-time data for process control. These techniques include spectroscopic methods (UV-Vis, IR, Raman), mass spectrometry, and conductivity measurements that can be coupled directly to the chromatography column via inline sampling interfaces. The continuous stream of analytical data allows operators to make informed decisions about process parameters, such as flow rates, buffer compositions, and collection points, optimizing the separation efficiency and product quality.Expand Specific Solutions03 Automated sampling and feedback control systems
Automated sampling systems incorporate programmable logic controllers and software algorithms to collect samples at predetermined intervals or based on specific process conditions. These systems can analyze the samples and automatically adjust process parameters based on the results, creating a closed-loop feedback control mechanism. The automation reduces operator intervention, minimizes human error, and enables consistent process performance, particularly important for large-scale industrial chromatography operations in pharmaceutical and biotechnology manufacturing.Expand Specific Solutions04 Specialized sampling devices and interfaces
Specialized sampling devices have been developed to address the unique challenges of inline sampling in column chromatography. These include micro-sampling probes that can extract small volumes without disturbing the column bed, membrane-based sampling interfaces that allow selective permeation of analytes, and flow-through cells that enable continuous monitoring without sample extraction. These devices are designed to maintain the integrity of the chromatographic process while providing representative samples for analysis.Expand Specific Solutions05 Integration of inline sampling with PAT frameworks
Process Analytical Technology (PAT) frameworks incorporate inline sampling as a key component for quality assurance in chromatography processes. These integrated systems combine sampling, analysis, data management, and process control into a comprehensive approach for ensuring product quality. The PAT approach emphasizes the importance of understanding process variables and their impact on product attributes, using inline sampling to gather critical data throughout the manufacturing process rather than relying solely on end-product testing.Expand Specific Solutions
Key Industry Players in Chromatography Process Control
The inline sampling for column chromatography process control market is currently in a growth phase, characterized by increasing adoption across pharmaceutical and biotechnology industries. The global market size is estimated to be expanding at a CAGR of 5-7%, driven by demand for real-time process analytics and quality control. From a technological maturity perspective, established players like Waters Technology Corp. and Shimadzu Corp. lead with comprehensive solutions, while Cytiva Sweden AB and Agilent Technologies offer specialized platforms with advanced data integration capabilities. ChromaCon AG and Merck Patent GmbH are innovating with novel chromatographic principles and continuous processing technologies. Emerging companies like Suzhou Aidimai Medical Technology are entering with cost-effective alternatives, while academic institutions such as Karlsruher Institut für Technologie and Zhejiang University contribute significant research advancements in data flow architecture optimization.
Waters Technology Corp.
Technical Solution: Waters has developed an advanced inline sampling architecture for column chromatography that integrates their ACQUITY UPLC technology with real-time process monitoring capabilities. Their system employs a multi-tier architecture where sampling nodes are strategically positioned along the chromatography column to extract micro-volume samples without disrupting the separation process. The data flow follows a streamlined path: samples are automatically withdrawn via microfluidic channels, immediately diluted if necessary, and analyzed through integrated UV/Vis spectroscopy, conductivity measurements, and mass spectrometry detection. Waters' implementation incorporates their Empower CDS (Chromatography Data System) for centralized data processing, featuring adaptive sampling rates that automatically increase frequency during critical separation events. Their architecture includes edge computing capabilities that perform preliminary data analysis at the sampling point before transmitting results to a central control system, reducing network load and enabling near real-time process adjustments[1][3]. The system utilizes predictive algorithms to anticipate separation trends and automatically adjusts sampling parameters, while maintaining 21 CFR Part 11 compliance for pharmaceutical applications.
Strengths: Waters' system offers exceptional sensitivity with detection limits in the nanogram range and superior integration with existing chromatography workflows. Their architecture provides industry-leading sampling frequency (up to 20 Hz) without compromising separation efficiency. Weaknesses: The implementation requires significant initial investment and specialized training for operators. The system's complexity can present challenges for troubleshooting and maintenance in production environments.
Cytiva Sweden AB
Technical Solution: Cytiva has developed an innovative inline sampling architecture specifically optimized for biopharmaceutical chromatography processes. Their system is built around the UNICORN process control platform and features a multi-point sampling approach designed to minimize product loss while maximizing process information. The architecture employs specialized low-dead-volume sampling interfaces that can be positioned at critical points throughout the chromatography skid, including pre-column, post-column, and at intermediate points for multi-column continuous processes. The data flow begins with microfluidic sampling that diverts minimal product volumes (typically 50-200 μL) to integrated analytical modules including UV spectroscopy, conductivity measurement, and optional specialized detectors for protein characterization. Cytiva's implementation features a parallel processing approach where multiple analytical streams operate simultaneously, enabling comprehensive real-time monitoring of product attributes and process parameters[6]. Their architecture incorporates sophisticated data fusion algorithms that combine signals from multiple detectors to provide enhanced analytical resolution and specificity. The system employs a model-predictive control framework that utilizes real-time analytical data to make dynamic adjustments to chromatography parameters such as flow rates, buffer compositions, and collection triggers. Cytiva's implementation includes specialized software modules for specific chromatography applications, including protein A affinity, ion exchange, and multimodal chromatography, with optimized sampling strategies for each mode.
Strengths: Cytiva's system offers exceptional integration with existing bioprocess equipment and workflows, creating a seamless implementation path for biopharmaceutical manufacturers. Their architecture provides specialized features for continuous chromatography processes, including multi-column periodic counter-current systems. Weaknesses: The system has more limited application outside of biopharmaceutical processing compared to more general-purpose chromatography platforms. The specialized nature of the system can create challenges when adapting to novel separation modes or unconventional process configurations.
Core Technologies for Real-time Chromatographic Data Acquisition
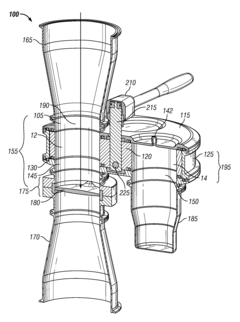
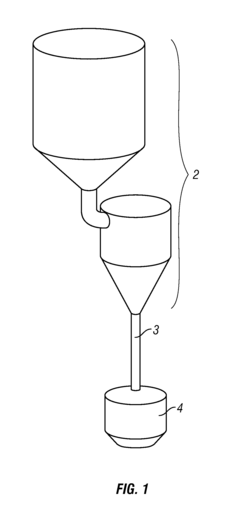
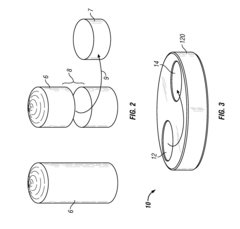
Systems and methods for inline sampling
PatentActiveUS9038488B2
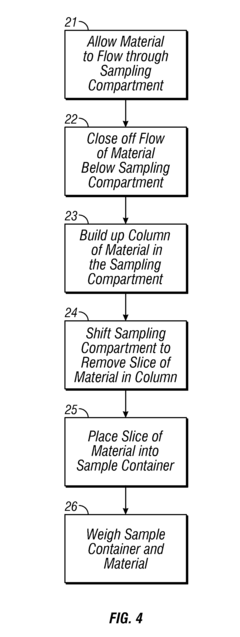
Innovation
- The implementation of an inline sampling method and device that allows material to flow through a sampling compartment, where a column is built up and a fixed volume is removed for precise weighing, minimizing process interruption and reducing sampling errors by avoiding the introduction of disturbances in the material flow.
Method and apparatus for developing thin layer chromatography plates for maximizing mobile phase conditions in column chromatography
PatentInactiveUS6264893B1
Innovation
- An apparatus and method utilizing a grid of multiple TLC developing chambers to conduct simultaneous tests with varying solvents and concentrations, optimizing mobile phase conditions for the smallest spot sizes and greatest Rf differences, allowing for the translation of these conditions to achieve the narrowest bands and greatest distance between bands in column chromatography.
Data Flow Optimization and Integration Strategies
Optimizing data flow in inline sampling systems for column chromatography requires a comprehensive integration strategy that addresses both hardware and software components. The architecture must facilitate seamless data transmission from sampling points to analytical instruments and control systems. Real-time data acquisition represents the foundation of this process, where strategically positioned sensors capture critical process parameters including UV absorbance, conductivity, pH, and pressure at predetermined intervals.
Data preprocessing mechanisms must be implemented directly at the sampling interface to filter noise, normalize readings, and perform preliminary validation. This edge processing significantly reduces the volume of transmitted data while preserving analytical integrity. The implementation of data compression algorithms specifically designed for chromatography signals can further optimize bandwidth utilization without compromising resolution in critical peak detection regions.
Integration protocols between sampling systems and process control platforms require standardized communication frameworks. Industry standards such as OPC UA, MQTT, or REST APIs provide robust options for establishing bidirectional data exchange. The selection should consider factors including latency requirements, security protocols, and compatibility with existing manufacturing execution systems (MES).
Buffer management strategies become essential when dealing with high-frequency sampling data. Implementing circular buffers with intelligent overflow handling ensures continuous operation even during network disruptions or processing bottlenecks. Critical data points can be flagged for priority transmission and storage, ensuring decision-critical information remains available regardless of system load.
Data synchronization mechanisms must account for potential timing discrepancies between sampling events and analytical results. Timestamp standardization across all system components, preferably using network time protocol (NTP), ensures accurate correlation between process conditions and analytical outcomes. This temporal alignment is particularly crucial when implementing feedback control loops based on inline measurements.
Scalability considerations should inform the data flow architecture design from inception. Microservices-based approaches allow for modular expansion of analytical capabilities without disrupting existing workflows. Container orchestration technologies facilitate deployment across distributed processing environments, enabling flexible allocation of computational resources based on current analytical demands.
Security protocols must be embedded throughout the data flow pipeline, implementing encryption for data in transit and at rest. Role-based access controls should govern data visibility and system configuration capabilities, with comprehensive audit logging to maintain regulatory compliance and facilitate troubleshooting of process deviations.
Data preprocessing mechanisms must be implemented directly at the sampling interface to filter noise, normalize readings, and perform preliminary validation. This edge processing significantly reduces the volume of transmitted data while preserving analytical integrity. The implementation of data compression algorithms specifically designed for chromatography signals can further optimize bandwidth utilization without compromising resolution in critical peak detection regions.
Integration protocols between sampling systems and process control platforms require standardized communication frameworks. Industry standards such as OPC UA, MQTT, or REST APIs provide robust options for establishing bidirectional data exchange. The selection should consider factors including latency requirements, security protocols, and compatibility with existing manufacturing execution systems (MES).
Buffer management strategies become essential when dealing with high-frequency sampling data. Implementing circular buffers with intelligent overflow handling ensures continuous operation even during network disruptions or processing bottlenecks. Critical data points can be flagged for priority transmission and storage, ensuring decision-critical information remains available regardless of system load.
Data synchronization mechanisms must account for potential timing discrepancies between sampling events and analytical results. Timestamp standardization across all system components, preferably using network time protocol (NTP), ensures accurate correlation between process conditions and analytical outcomes. This temporal alignment is particularly crucial when implementing feedback control loops based on inline measurements.
Scalability considerations should inform the data flow architecture design from inception. Microservices-based approaches allow for modular expansion of analytical capabilities without disrupting existing workflows. Container orchestration technologies facilitate deployment across distributed processing environments, enabling flexible allocation of computational resources based on current analytical demands.
Security protocols must be embedded throughout the data flow pipeline, implementing encryption for data in transit and at rest. Role-based access controls should govern data visibility and system configuration capabilities, with comprehensive audit logging to maintain regulatory compliance and facilitate troubleshooting of process deviations.
Regulatory Compliance for Analytical Process Control Systems
Implementing inline sampling for column chromatography process control requires strict adherence to regulatory frameworks governing analytical processes in pharmaceutical and biotechnology industries. The regulatory landscape for such systems is complex and multifaceted, with requirements varying across different regions and regulatory bodies.
The FDA's guidance on Process Analytical Technology (PAT) framework, part of the broader Quality by Design (QbD) initiative, provides the foundation for implementing inline sampling technologies. This framework emphasizes real-time quality assurance rather than end-product testing, aligning perfectly with the objectives of inline chromatography monitoring. Compliance with 21 CFR Part 11 for electronic records and signatures is mandatory when implementing digital data flows from inline sampling systems.
In the European context, the European Medicines Agency (EMA) has established guidelines that emphasize the validation of analytical procedures used in process control. The EU GMP Annex 11 specifically addresses computerized systems in GxP environments, requiring thorough documentation of system architecture and data flow pathways for inline sampling implementations.
For global operations, ICH guidelines, particularly Q8 (Pharmaceutical Development), Q9 (Quality Risk Management), and Q10 (Pharmaceutical Quality System), provide harmonized approaches to quality assurance that must be considered when designing inline sampling architectures. These guidelines emphasize risk-based approaches to validation and verification of analytical control systems.
Data integrity requirements represent another critical regulatory consideration. ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be embedded in the system architecture and data flow design. This ensures that data generated through inline sampling maintains its integrity throughout the process control lifecycle.
Validation protocols for inline sampling systems must follow GAMP 5 guidelines, with particular attention to the categorization of software components and the appropriate validation approach for each category. The data flow architecture must include audit trail capabilities that allow for reconstruction of events related to the creation, modification, or deletion of GxP-relevant records.
Change management procedures compliant with regulatory expectations must be established for any modifications to the inline sampling system architecture or data flow pathways. This includes impact assessments on data integrity, product quality, and regulatory compliance before implementing changes.
Regular system reviews and periodic revalidation are necessary to maintain the validated state of inline sampling systems, especially after significant updates or changes to connected systems that might affect data flow integrity or analytical performance.
The FDA's guidance on Process Analytical Technology (PAT) framework, part of the broader Quality by Design (QbD) initiative, provides the foundation for implementing inline sampling technologies. This framework emphasizes real-time quality assurance rather than end-product testing, aligning perfectly with the objectives of inline chromatography monitoring. Compliance with 21 CFR Part 11 for electronic records and signatures is mandatory when implementing digital data flows from inline sampling systems.
In the European context, the European Medicines Agency (EMA) has established guidelines that emphasize the validation of analytical procedures used in process control. The EU GMP Annex 11 specifically addresses computerized systems in GxP environments, requiring thorough documentation of system architecture and data flow pathways for inline sampling implementations.
For global operations, ICH guidelines, particularly Q8 (Pharmaceutical Development), Q9 (Quality Risk Management), and Q10 (Pharmaceutical Quality System), provide harmonized approaches to quality assurance that must be considered when designing inline sampling architectures. These guidelines emphasize risk-based approaches to validation and verification of analytical control systems.
Data integrity requirements represent another critical regulatory consideration. ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be embedded in the system architecture and data flow design. This ensures that data generated through inline sampling maintains its integrity throughout the process control lifecycle.
Validation protocols for inline sampling systems must follow GAMP 5 guidelines, with particular attention to the categorization of software components and the appropriate validation approach for each category. The data flow architecture must include audit trail capabilities that allow for reconstruction of events related to the creation, modification, or deletion of GxP-relevant records.
Change management procedures compliant with regulatory expectations must be established for any modifications to the inline sampling system architecture or data flow pathways. This includes impact assessments on data integrity, product quality, and regulatory compliance before implementing changes.
Regular system reviews and periodic revalidation are necessary to maintain the validated state of inline sampling systems, especially after significant updates or changes to connected systems that might affect data flow integrity or analytical performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!