How to Optimize Flow Rates and Particle Size for High-throughput Column Chromatography
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Flow Rate Optimization Background and Objectives
Column chromatography has evolved significantly since its inception in the early 20th century, transforming from a simple analytical technique to a sophisticated purification method essential in pharmaceutical, biotechnology, and chemical industries. The optimization of flow rates and particle size represents a critical frontier in advancing high-throughput column chromatography, directly impacting separation efficiency, resolution, and productivity.
Historically, chromatographic separations were time-consuming processes with limited throughput. The industry has progressively moved from gravity-fed systems to pressure-driven approaches, enabling faster separations while maintaining resolution. This evolution has been accompanied by innovations in stationary phase design, with particle sizes decreasing from traditional 50-100 μm particles to sub-2 μm particles in modern ultra-high-performance systems.
The relationship between flow rate and particle size follows complex hydrodynamic principles described by the van Deemter equation, which demonstrates how these parameters influence separation efficiency. As flow rates increase, the trade-off between speed and resolution becomes increasingly significant, particularly when working with smaller particle sizes that generate higher backpressure.
Current industry challenges include the need to process larger sample volumes while maintaining separation quality, reducing solvent consumption, and minimizing operational costs. These challenges are particularly acute in biopharmaceutical manufacturing, where chromatography often represents a significant bottleneck in downstream processing of therapeutic proteins and vaccines.
The primary objective of flow rate and particle size optimization is to establish operational parameters that maximize throughput without compromising separation quality. This involves determining the optimal combination of flow rate, particle size, column dimensions, and operating pressure for specific separation challenges.
Secondary objectives include reducing mobile phase consumption, minimizing analysis time, improving reproducibility, and enhancing overall process economics. For industrial applications, scalability of optimized conditions from analytical to preparative and production scales represents an additional critical goal.
Recent technological advances in instrumentation, including systems capable of withstanding ultra-high pressures (>1000 bar), have expanded the operational envelope for chromatographic separations. Simultaneously, innovations in stationary phase design, including monolithic columns, core-shell particles, and novel surface chemistries, have created new opportunities for optimization beyond traditional parameters.
The convergence of computational modeling, automation, and machine learning approaches with experimental chromatography has accelerated the optimization process, enabling more systematic exploration of the complex parameter space that defines chromatographic performance.
Historically, chromatographic separations were time-consuming processes with limited throughput. The industry has progressively moved from gravity-fed systems to pressure-driven approaches, enabling faster separations while maintaining resolution. This evolution has been accompanied by innovations in stationary phase design, with particle sizes decreasing from traditional 50-100 μm particles to sub-2 μm particles in modern ultra-high-performance systems.
The relationship between flow rate and particle size follows complex hydrodynamic principles described by the van Deemter equation, which demonstrates how these parameters influence separation efficiency. As flow rates increase, the trade-off between speed and resolution becomes increasingly significant, particularly when working with smaller particle sizes that generate higher backpressure.
Current industry challenges include the need to process larger sample volumes while maintaining separation quality, reducing solvent consumption, and minimizing operational costs. These challenges are particularly acute in biopharmaceutical manufacturing, where chromatography often represents a significant bottleneck in downstream processing of therapeutic proteins and vaccines.
The primary objective of flow rate and particle size optimization is to establish operational parameters that maximize throughput without compromising separation quality. This involves determining the optimal combination of flow rate, particle size, column dimensions, and operating pressure for specific separation challenges.
Secondary objectives include reducing mobile phase consumption, minimizing analysis time, improving reproducibility, and enhancing overall process economics. For industrial applications, scalability of optimized conditions from analytical to preparative and production scales represents an additional critical goal.
Recent technological advances in instrumentation, including systems capable of withstanding ultra-high pressures (>1000 bar), have expanded the operational envelope for chromatographic separations. Simultaneously, innovations in stationary phase design, including monolithic columns, core-shell particles, and novel surface chemistries, have created new opportunities for optimization beyond traditional parameters.
The convergence of computational modeling, automation, and machine learning approaches with experimental chromatography has accelerated the optimization process, enabling more systematic exploration of the complex parameter space that defines chromatographic performance.
Market Analysis for High-throughput Chromatography Solutions
The high-throughput chromatography market has experienced substantial growth over the past decade, driven primarily by increasing demand in biopharmaceutical production, academic research, and clinical diagnostics. The global market for high-throughput chromatography solutions was valued at approximately $3.2 billion in 2022 and is projected to reach $5.7 billion by 2028, representing a compound annual growth rate of 10.1%.
Biopharmaceutical manufacturing represents the largest segment of this market, accounting for nearly 45% of total revenue. This dominance is attributed to the rising production of monoclonal antibodies, recombinant proteins, and vaccines, all of which require efficient purification processes. The COVID-19 pandemic further accelerated market growth as manufacturers scaled up vaccine production capabilities.
Academic and research institutions constitute the second-largest market segment at 25%, followed by clinical diagnostics at 15%. The remaining market share is distributed among food and beverage testing, environmental analysis, and other industrial applications. Geographically, North America leads with 38% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (7%).
Key market drivers include increasing R&D investments in biopharmaceuticals, growing adoption of single-use technologies, and rising demand for process analytical technologies (PAT) in chromatography workflows. The trend toward continuous manufacturing in biopharmaceutical production has created significant opportunities for high-throughput chromatography solutions that can integrate seamlessly with upstream and downstream processes.
Customer demands are evolving toward systems that offer greater flexibility, reduced footprint, lower buffer consumption, and improved automation capabilities. End-users increasingly seek chromatography solutions that optimize both flow rates and particle size to maximize throughput without compromising resolution or recovery.
Market challenges include high capital investment requirements, technical complexity in optimizing parameters for different biomolecules, and regulatory hurdles associated with implementing new purification technologies in GMP environments. Additionally, skilled operator shortages present obstacles to widespread adoption of advanced chromatography systems.
The competitive landscape features established players like Cytiva (formerly GE Healthcare Life Sciences), Thermo Fisher Scientific, Merck KGaA, Bio-Rad Laboratories, and Agilent Technologies. These companies collectively hold approximately 70% market share. Emerging players focusing on innovative technologies for flow rate and particle size optimization are gaining traction, particularly those offering solutions that reduce processing time while maintaining separation efficiency.
Biopharmaceutical manufacturing represents the largest segment of this market, accounting for nearly 45% of total revenue. This dominance is attributed to the rising production of monoclonal antibodies, recombinant proteins, and vaccines, all of which require efficient purification processes. The COVID-19 pandemic further accelerated market growth as manufacturers scaled up vaccine production capabilities.
Academic and research institutions constitute the second-largest market segment at 25%, followed by clinical diagnostics at 15%. The remaining market share is distributed among food and beverage testing, environmental analysis, and other industrial applications. Geographically, North America leads with 38% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (7%).
Key market drivers include increasing R&D investments in biopharmaceuticals, growing adoption of single-use technologies, and rising demand for process analytical technologies (PAT) in chromatography workflows. The trend toward continuous manufacturing in biopharmaceutical production has created significant opportunities for high-throughput chromatography solutions that can integrate seamlessly with upstream and downstream processes.
Customer demands are evolving toward systems that offer greater flexibility, reduced footprint, lower buffer consumption, and improved automation capabilities. End-users increasingly seek chromatography solutions that optimize both flow rates and particle size to maximize throughput without compromising resolution or recovery.
Market challenges include high capital investment requirements, technical complexity in optimizing parameters for different biomolecules, and regulatory hurdles associated with implementing new purification technologies in GMP environments. Additionally, skilled operator shortages present obstacles to widespread adoption of advanced chromatography systems.
The competitive landscape features established players like Cytiva (formerly GE Healthcare Life Sciences), Thermo Fisher Scientific, Merck KGaA, Bio-Rad Laboratories, and Agilent Technologies. These companies collectively hold approximately 70% market share. Emerging players focusing on innovative technologies for flow rate and particle size optimization are gaining traction, particularly those offering solutions that reduce processing time while maintaining separation efficiency.
Current Challenges in Column Chromatography Technology
Column chromatography technology, while well-established in analytical and preparative applications, faces several significant challenges when scaled for high-throughput operations. The primary limitation stems from the fundamental trade-off between resolution, speed, and capacity - optimizing one parameter typically compromises the others. This "separation trilemma" becomes particularly problematic in industrial settings where both throughput and purity are critical economic factors.
Flow rate optimization presents a complex challenge as increasing flow rates to improve throughput often leads to band broadening, reduced theoretical plate numbers, and diminished separation efficiency. This occurs due to increased longitudinal diffusion, mass transfer limitations, and eddy diffusion effects. Current systems struggle to maintain separation quality at elevated flow rates, especially for complex biological samples like monoclonal antibodies or recombinant proteins.
Particle size selection compounds these challenges. Smaller particles (sub-2μm) offer superior resolution and efficiency but generate extreme backpressure that exceeds the mechanical limitations of conventional equipment. Conversely, larger particles allow faster flow rates but sacrifice separation performance. This creates a technological bottleneck where the theoretical advantages of smaller particles cannot be fully realized in high-throughput applications.
Column packing heterogeneity remains a persistent issue, with inconsistencies in bed density creating preferential flow paths that reduce separation efficiency. This problem intensifies at higher flow rates where column compression and particle migration further disrupt the stationary phase uniformity. The industry lacks standardized methods to evaluate and ensure packing quality across different operational conditions.
Mobile phase viscosity and sample matrix effects introduce additional variables that complicate optimization. High-salt buffers or viscous samples require careful flow rate adjustments to prevent excessive system pressure while maintaining adequate mass transfer kinetics. Current predictive models often fail to account for these complex interactions, particularly with biological samples.
Scaling challenges persist when transferring methods from analytical to preparative scales. Parameters optimized at small scales frequently perform unpredictably when scaled up, necessitating extensive revalidation. This scale-up unpredictability increases development time and costs while reducing manufacturing flexibility.
Temperature control during high-flow operations presents another challenge, as frictional heating can create thermal gradients within columns that affect separation reproducibility. Current cooling technologies struggle to maintain uniform temperature profiles across larger diameter columns operating at elevated flow rates.
These technical limitations collectively constrain the advancement of high-throughput chromatography, creating significant opportunities for innovation in column design, particle engineering, and process control systems to overcome these fundamental challenges.
Flow rate optimization presents a complex challenge as increasing flow rates to improve throughput often leads to band broadening, reduced theoretical plate numbers, and diminished separation efficiency. This occurs due to increased longitudinal diffusion, mass transfer limitations, and eddy diffusion effects. Current systems struggle to maintain separation quality at elevated flow rates, especially for complex biological samples like monoclonal antibodies or recombinant proteins.
Particle size selection compounds these challenges. Smaller particles (sub-2μm) offer superior resolution and efficiency but generate extreme backpressure that exceeds the mechanical limitations of conventional equipment. Conversely, larger particles allow faster flow rates but sacrifice separation performance. This creates a technological bottleneck where the theoretical advantages of smaller particles cannot be fully realized in high-throughput applications.
Column packing heterogeneity remains a persistent issue, with inconsistencies in bed density creating preferential flow paths that reduce separation efficiency. This problem intensifies at higher flow rates where column compression and particle migration further disrupt the stationary phase uniformity. The industry lacks standardized methods to evaluate and ensure packing quality across different operational conditions.
Mobile phase viscosity and sample matrix effects introduce additional variables that complicate optimization. High-salt buffers or viscous samples require careful flow rate adjustments to prevent excessive system pressure while maintaining adequate mass transfer kinetics. Current predictive models often fail to account for these complex interactions, particularly with biological samples.
Scaling challenges persist when transferring methods from analytical to preparative scales. Parameters optimized at small scales frequently perform unpredictably when scaled up, necessitating extensive revalidation. This scale-up unpredictability increases development time and costs while reducing manufacturing flexibility.
Temperature control during high-flow operations presents another challenge, as frictional heating can create thermal gradients within columns that affect separation reproducibility. Current cooling technologies struggle to maintain uniform temperature profiles across larger diameter columns operating at elevated flow rates.
These technical limitations collectively constrain the advancement of high-throughput chromatography, creating significant opportunities for innovation in column design, particle engineering, and process control systems to overcome these fundamental challenges.
Existing Methodologies for Flow Rate and Particle Size Control
01 Relationship between particle size and flow rate in column chromatography
The particle size of the stationary phase in column chromatography significantly affects the flow rate and separation efficiency. Smaller particles provide greater surface area and improved resolution but require higher pressure to maintain adequate flow rates. Conversely, larger particles allow for faster flow rates but may result in reduced separation efficiency. Optimizing this relationship is crucial for achieving effective chromatographic separations while maintaining practical operating conditions.- Relationship between particle size and flow rate in column chromatography: The particle size of the stationary phase in column chromatography significantly affects the flow rate and separation efficiency. Smaller particles provide better resolution but require higher pressure to maintain adequate flow rates. Conversely, larger particles allow for faster flow rates but may compromise separation quality. Optimizing this relationship is crucial for achieving efficient chromatographic separations while maintaining reasonable analysis times.
- Flow rate optimization techniques for different column types: Various techniques can be employed to optimize flow rates in different types of chromatography columns. These include adjusting mobile phase composition, column temperature, and pressure parameters. For high-performance liquid chromatography (HPLC), specific flow rate ranges are recommended based on column dimensions and particle characteristics. Optimization strategies differ between analytical and preparative chromatography applications, with the latter often requiring higher flow rates for increased throughput.
- Advanced particle technologies for enhanced chromatographic performance: Innovative particle technologies have been developed to improve chromatographic performance while maintaining reasonable flow rates. These include core-shell particles, monolithic columns, and superficially porous particles that offer reduced diffusion paths and lower back pressure compared to fully porous particles. Such technologies enable faster separations without sacrificing resolution, making them particularly valuable for high-throughput applications and complex sample analysis.
- Pressure considerations in relation to flow rate and particle size: System pressure is directly related to flow rate and inversely related to particle size in column chromatography. Smaller particles generate higher back pressure at equivalent flow rates, potentially exceeding instrument limitations. Modern ultra-high-performance liquid chromatography (UHPLC) systems are designed to handle these elevated pressures, allowing the use of sub-2μm particles at practical flow rates. Pressure-flow relationships must be carefully managed to prevent column damage and ensure consistent separation performance.
- Specialized flow rate applications for specific separation challenges: Certain separation challenges require specialized flow rate approaches. Gradient elution techniques may employ variable flow rates throughout the separation process. Size exclusion chromatography typically operates at lower flow rates to maximize resolution of differently sized molecules. Fast chromatography methods utilize optimized particle size and column dimensions to achieve rapid separations at higher flow rates. These specialized applications demonstrate the importance of tailoring flow rate parameters to specific analytical requirements.
02 Flow rate optimization techniques for chromatographic columns
Various techniques can be employed to optimize flow rates in column chromatography, including pressure adjustment, temperature control, and mobile phase composition modification. These techniques help maintain separation efficiency while achieving desired throughput. Advanced flow control systems can dynamically adjust parameters during separation processes to maintain optimal conditions as sample components move through the column, enhancing both speed and resolution of separations.Expand Specific Solutions03 Novel stationary phase materials and particle designs
Innovative stationary phase materials and particle designs have been developed to improve chromatographic performance. These include core-shell particles, monolithic columns, and functionalized surfaces that offer enhanced separation capabilities while allowing for higher flow rates. These advanced materials can maintain separation efficiency at increased flow rates by reducing diffusion path lengths and improving mass transfer kinetics, thereby addressing the traditional trade-off between speed and resolution.Expand Specific Solutions04 High-throughput chromatography systems
High-throughput chromatography systems incorporate specialized hardware and column designs to enable faster separations without compromising resolution. These systems often utilize parallel processing, automated sample handling, and optimized column dimensions to increase sample throughput. Advanced pumping systems capable of delivering consistent flow at higher pressures, combined with columns packed with smaller particles, allow for rapid separations in industrial and research applications.Expand Specific Solutions05 Analytical methods for characterizing flow dynamics and particle interactions
Sophisticated analytical methods have been developed to characterize flow dynamics and particle interactions within chromatographic columns. These methods include computational fluid dynamics modeling, real-time monitoring of separation processes, and advanced imaging techniques. Understanding these dynamics allows for better prediction of separation behavior and more effective optimization of flow rates based on particle size characteristics, leading to improved chromatographic methods development.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Chromatography
High-throughput column chromatography optimization is currently in a growth phase, with the market expanding due to increasing demand in pharmaceutical, biotechnology, and analytical sectors. The global market size is estimated to exceed $3 billion, growing at 7-8% annually. Technologically, the field is maturing with significant innovations from key players. Waters Technology, Agilent Technologies, and Shimadzu lead with advanced flow control systems, while Cytiva and Biotage focus on particle size optimization technologies. Dionex (now part of Thermo Fisher) has pioneered high-pressure systems, and academic institutions like Vrije Universiteit Brussel and University of Houston contribute fundamental research. The competitive landscape shows established players investing in automation and AI integration, while emerging companies develop specialized niche solutions for specific applications.
Waters Technology Corp.
Technical Solution: Waters Technology has developed the ACQUITY Premier Solution for high-throughput column chromatography, featuring hybrid particle technology with MaxPeak surfaces that minimize non-specific adsorption. Their approach combines sub-2μm particle sizes (typically 1.7μm) with UPLC (Ultra Performance Liquid Chromatography) technology to optimize flow rates while maintaining separation efficiency. The system utilizes pressure-tolerant columns capable of withstanding up to 15,000 psi, allowing for faster flow rates without compromising resolution. Waters' Charged Surface Hybrid (CSH) particles incorporate both silica and polymer characteristics, enabling operation across wider pH ranges (2-11) while maintaining mechanical stability at high pressures and flow rates. Their Advanced Polymer Technology (APT) columns feature precisely controlled particle size distributions (PSDs) with relative standard deviations <10%, significantly improving column packing uniformity and chromatographic performance[1][3].
Strengths: Superior peak shape and resolution due to hybrid particle technology; reduced sample loss through MaxPeak surfaces; exceptional reproducibility with tight particle size distribution control. Weaknesses: Higher cost compared to conventional systems; requires specialized training for optimal operation; higher backpressure necessitates more robust instrumentation.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered the InfinityLab approach to high-throughput column chromatography optimization, focusing on the interplay between particle size, column dimensions, and flow rates. Their technology employs superficially porous particles (SPP) with solid cores and porous shells (2.7μm Poroshell 120 particles) that reduce diffusion paths while maintaining surface area for separation. This design allows for faster flow rates with minimal efficiency loss compared to fully porous particles. Agilent's Advanced Automated Calibration (AAC) system dynamically adjusts flow rates based on real-time monitoring of column backpressure and temperature, maintaining optimal linear velocity regardless of solvent composition changes. Their columns incorporate proprietary end-fitting technology that ensures uniform flow distribution across the column diameter, minimizing band broadening even at elevated flow rates. The InfinityLab system also features predictive algorithms that recommend optimal particle size and flow rate combinations based on specific separation requirements, balancing throughput against resolution needs[2][5].
Strengths: Superficially porous particles provide excellent efficiency at higher flow rates; intelligent software optimization reduces method development time; modular design allows customization for specific applications. Weaknesses: Performance advantages diminish for smaller molecules; higher initial investment compared to conventional HPLC; requires high-quality samples to prevent clogging of narrow-bore columns.
Key Patents and Innovations in High-throughput Chromatography
An extract of persea
PatentInactiveUS20220361541A1
Innovation
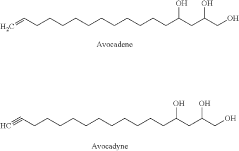
- A process involving extraction with organic solvents, saponification, addition of calcium to precipitate calcium soaps, and subsequent separation and purification using ethyl acetate or water, allowing for the selective extraction of avocadene and avocadyne without the need for chromatographic steps, achieving high purity without toxic solvents like hexane.
Fluid pump having low pressure metering and high pressure delivering
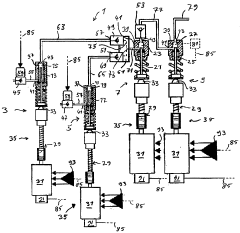
PatentWO2006087036A1
Innovation
- A pumping apparatus comprising multiple metering devices connected in parallel, a booster device, and a damping device in series, allowing for precise blending and pressure increase of fluids at low pressure before delivering a ripple-free, high-pressure stream, with a control unit to stabilize output pressure and synchronize device operations.
Scale-up Considerations for Industrial Applications
Scaling up column chromatography from laboratory to industrial scale presents significant engineering challenges that must be addressed systematically. When transitioning from small-scale operations to industrial production, flow rate and particle size optimization become critical factors affecting separation efficiency, throughput, and economic viability. Industrial applications typically require processing volumes thousands of times larger than laboratory settings, necessitating careful consideration of hydrodynamic effects and column geometry.
The relationship between particle size and flow rate becomes more complex at industrial scale due to increased column diameters and heights. While smaller particles generally provide better resolution, they also create higher backpressure, limiting flow rates and potentially causing operational issues in large columns. Industrial systems typically employ particles in the 50-100 μm range as a compromise between separation efficiency and operational feasibility, compared to the 5-10 μm particles often used in analytical applications.
Wall effects, which are negligible in laboratory columns, become significant in industrial-scale operations. As column diameter increases, the ratio of wall surface area to column volume decreases, altering flow distribution patterns. This phenomenon requires adjustment of flow rates to maintain separation efficiency and prevent channeling. Mathematical models such as the van Deemter equation must be modified to account for these scale-dependent effects when predicting optimal flow conditions.
Pressure drop considerations become paramount in industrial applications. The pressure drop across a chromatography column increases with the square of flow rate and inversely with the square of particle diameter. Industrial systems must operate within equipment pressure limitations while maintaining adequate flow rates for economic production. This often necessitates the use of specialized column designs with improved flow distributors and collectors to ensure uniform flow across the entire column cross-section.
Heat dissipation represents another scale-up challenge rarely encountered in laboratory settings. The friction generated by high flow rates through packed beds creates heat that can affect separation performance, particularly for temperature-sensitive biomolecules. Industrial systems require effective temperature control strategies, including jacketed columns or segmented cooling zones, to maintain consistent separation conditions throughout the column volume.
Economic considerations ultimately drive industrial scale-up decisions. The capital investment in larger columns and supporting infrastructure must be balanced against operational costs and productivity. Higher flow rates reduce processing time but may compromise separation quality or increase buffer consumption. Optimizing this balance requires pilot-scale testing with progressively larger columns to validate scale-up parameters before full industrial implementation.
The relationship between particle size and flow rate becomes more complex at industrial scale due to increased column diameters and heights. While smaller particles generally provide better resolution, they also create higher backpressure, limiting flow rates and potentially causing operational issues in large columns. Industrial systems typically employ particles in the 50-100 μm range as a compromise between separation efficiency and operational feasibility, compared to the 5-10 μm particles often used in analytical applications.
Wall effects, which are negligible in laboratory columns, become significant in industrial-scale operations. As column diameter increases, the ratio of wall surface area to column volume decreases, altering flow distribution patterns. This phenomenon requires adjustment of flow rates to maintain separation efficiency and prevent channeling. Mathematical models such as the van Deemter equation must be modified to account for these scale-dependent effects when predicting optimal flow conditions.
Pressure drop considerations become paramount in industrial applications. The pressure drop across a chromatography column increases with the square of flow rate and inversely with the square of particle diameter. Industrial systems must operate within equipment pressure limitations while maintaining adequate flow rates for economic production. This often necessitates the use of specialized column designs with improved flow distributors and collectors to ensure uniform flow across the entire column cross-section.
Heat dissipation represents another scale-up challenge rarely encountered in laboratory settings. The friction generated by high flow rates through packed beds creates heat that can affect separation performance, particularly for temperature-sensitive biomolecules. Industrial systems require effective temperature control strategies, including jacketed columns or segmented cooling zones, to maintain consistent separation conditions throughout the column volume.
Economic considerations ultimately drive industrial scale-up decisions. The capital investment in larger columns and supporting infrastructure must be balanced against operational costs and productivity. Higher flow rates reduce processing time but may compromise separation quality or increase buffer consumption. Optimizing this balance requires pilot-scale testing with progressively larger columns to validate scale-up parameters before full industrial implementation.
Validation and Quality Control Protocols
Validation and quality control protocols are essential components of high-throughput column chromatography optimization. Establishing robust validation procedures ensures consistent performance and reliable results across multiple runs and different batches of materials. These protocols must address both the initial validation of optimized parameters and ongoing quality control during routine operations.
System suitability tests represent the foundation of validation protocols for column chromatography. These tests typically include measurements of resolution, peak symmetry, theoretical plate count, and retention time reproducibility. For high-throughput applications specifically, additional parameters such as back-pressure stability and column lifetime under accelerated flow conditions must be evaluated. Validation protocols should establish acceptance criteria for each parameter based on the specific separation requirements.
Method validation for optimized flow rates and particle size configurations requires demonstration of linearity, accuracy, precision, specificity, detection limit, and quantitation limit. This validation process should follow established guidelines such as ICH Q2(R1) or USP <1225>, adapted specifically for high-throughput conditions. The validation must verify that the optimized parameters maintain separation efficiency while increasing throughput.
Real-time monitoring systems play a crucial role in quality control during high-throughput chromatography. Implementation of process analytical technology (PAT) enables continuous assessment of critical parameters including pressure fluctuations, flow rate consistency, and detector response. Modern systems incorporate automated feedback mechanisms that can make minor adjustments to maintain optimal conditions throughout extended runs.
Statistical process control (SPC) methodologies should be integrated into quality control protocols to identify trends and deviations before they impact separation quality. Control charts for key performance indicators help visualize system performance over time and establish warning and action limits. For high-throughput applications, multivariate statistical approaches may be necessary to monitor the complex interrelationships between flow rates, particle size, and separation efficiency.
Robustness testing constitutes another vital element of validation protocols. This testing evaluates how small, deliberate variations in method parameters affect the reliability of results. For flow rate and particle size optimization, robustness testing should examine the impact of minor fluctuations in temperature, buffer composition, sample load, and instrument variability. A well-optimized high-throughput method should demonstrate acceptable performance across these variations.
Documentation and training requirements complete the validation framework. Comprehensive standard operating procedures (SOPs) must detail all aspects of the optimized method, including troubleshooting guidelines for common issues encountered at accelerated flow rates. Personnel training programs should emphasize critical control points specific to high-throughput operations and the importance of adherence to established protocols.
System suitability tests represent the foundation of validation protocols for column chromatography. These tests typically include measurements of resolution, peak symmetry, theoretical plate count, and retention time reproducibility. For high-throughput applications specifically, additional parameters such as back-pressure stability and column lifetime under accelerated flow conditions must be evaluated. Validation protocols should establish acceptance criteria for each parameter based on the specific separation requirements.
Method validation for optimized flow rates and particle size configurations requires demonstration of linearity, accuracy, precision, specificity, detection limit, and quantitation limit. This validation process should follow established guidelines such as ICH Q2(R1) or USP <1225>, adapted specifically for high-throughput conditions. The validation must verify that the optimized parameters maintain separation efficiency while increasing throughput.
Real-time monitoring systems play a crucial role in quality control during high-throughput chromatography. Implementation of process analytical technology (PAT) enables continuous assessment of critical parameters including pressure fluctuations, flow rate consistency, and detector response. Modern systems incorporate automated feedback mechanisms that can make minor adjustments to maintain optimal conditions throughout extended runs.
Statistical process control (SPC) methodologies should be integrated into quality control protocols to identify trends and deviations before they impact separation quality. Control charts for key performance indicators help visualize system performance over time and establish warning and action limits. For high-throughput applications, multivariate statistical approaches may be necessary to monitor the complex interrelationships between flow rates, particle size, and separation efficiency.
Robustness testing constitutes another vital element of validation protocols. This testing evaluates how small, deliberate variations in method parameters affect the reliability of results. For flow rate and particle size optimization, robustness testing should examine the impact of minor fluctuations in temperature, buffer composition, sample load, and instrument variability. A well-optimized high-throughput method should demonstrate acceptable performance across these variations.
Documentation and training requirements complete the validation framework. Comprehensive standard operating procedures (SOPs) must detail all aspects of the optimized method, including troubleshooting guidelines for common issues encountered at accelerated flow rates. Personnel training programs should emphasize critical control points specific to high-throughput operations and the importance of adherence to established protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!