Column Chromatography for Small Molecule Purification: Troubleshooting Low Recovery and Peak Tailing
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Evolution and Purification Objectives
Column chromatography has evolved significantly since its inception in the early 20th century, transforming from a rudimentary separation technique to a sophisticated analytical and preparative tool essential in pharmaceutical, biotechnology, and chemical industries. The journey began with simple gravity-fed columns using silica or alumina as stationary phases, progressing through the development of high-performance liquid chromatography (HPLC) in the 1960s, which dramatically improved separation efficiency and analysis speed.
The 1980s witnessed the emergence of ultra-high-performance liquid chromatography (UHPLC), utilizing sub-2μm particles and higher pressures to achieve unprecedented resolution and throughput. Concurrently, advances in stationary phase chemistry expanded from traditional silica-based materials to include bonded phases, polymeric materials, and hybrid organic-inorganic substrates, each offering unique selectivity profiles for different molecular classes.
Recent technological innovations have focused on enhancing reproducibility, automation, and integration with other analytical techniques. Modern chromatography systems incorporate sophisticated detectors, including mass spectrometry interfaces, diode array detectors, and evaporative light scattering detectors, enabling multi-dimensional analysis and structural elucidation of complex mixtures.
The primary objectives in small molecule purification via column chromatography center on achieving high recovery rates, excellent separation efficiency, and minimal peak tailing. Recovery rates directly impact yield and economic viability, particularly critical in pharmaceutical manufacturing where target compounds may be expensive or difficult to synthesize. Separation efficiency determines the purity of isolated compounds, essential for regulatory compliance and downstream applications.
Peak tailing, characterized by asymmetrical chromatographic peaks with elongated trailing edges, represents a significant challenge that compromises both resolution and quantitative accuracy. This phenomenon often results from non-specific interactions between analytes and active sites on the stationary phase, improper column packing, or suboptimal mobile phase composition.
Current industry trends are moving toward green chromatography approaches, emphasizing reduced solvent consumption, environmentally friendly mobile phases, and sustainable stationary phase materials. Additionally, there is growing interest in miniaturization and microfluidic chromatography systems for point-of-care diagnostics and high-throughput screening applications.
The evolution trajectory suggests future developments will likely focus on intelligent chromatography systems incorporating machine learning algorithms for method development and optimization, predictive maintenance capabilities, and enhanced integration with complementary analytical techniques to form comprehensive analytical platforms.
The 1980s witnessed the emergence of ultra-high-performance liquid chromatography (UHPLC), utilizing sub-2μm particles and higher pressures to achieve unprecedented resolution and throughput. Concurrently, advances in stationary phase chemistry expanded from traditional silica-based materials to include bonded phases, polymeric materials, and hybrid organic-inorganic substrates, each offering unique selectivity profiles for different molecular classes.
Recent technological innovations have focused on enhancing reproducibility, automation, and integration with other analytical techniques. Modern chromatography systems incorporate sophisticated detectors, including mass spectrometry interfaces, diode array detectors, and evaporative light scattering detectors, enabling multi-dimensional analysis and structural elucidation of complex mixtures.
The primary objectives in small molecule purification via column chromatography center on achieving high recovery rates, excellent separation efficiency, and minimal peak tailing. Recovery rates directly impact yield and economic viability, particularly critical in pharmaceutical manufacturing where target compounds may be expensive or difficult to synthesize. Separation efficiency determines the purity of isolated compounds, essential for regulatory compliance and downstream applications.
Peak tailing, characterized by asymmetrical chromatographic peaks with elongated trailing edges, represents a significant challenge that compromises both resolution and quantitative accuracy. This phenomenon often results from non-specific interactions between analytes and active sites on the stationary phase, improper column packing, or suboptimal mobile phase composition.
Current industry trends are moving toward green chromatography approaches, emphasizing reduced solvent consumption, environmentally friendly mobile phases, and sustainable stationary phase materials. Additionally, there is growing interest in miniaturization and microfluidic chromatography systems for point-of-care diagnostics and high-throughput screening applications.
The evolution trajectory suggests future developments will likely focus on intelligent chromatography systems incorporating machine learning algorithms for method development and optimization, predictive maintenance capabilities, and enhanced integration with complementary analytical techniques to form comprehensive analytical platforms.
Market Analysis for Small Molecule Purification Technologies
The global market for small molecule purification technologies has been experiencing steady growth, driven by increasing demand in pharmaceutical research, clinical diagnostics, and academic research sectors. The market size for chromatography equipment and consumables used in small molecule purification reached approximately $5.7 billion in 2022, with a compound annual growth rate (CAGR) of 6.8% projected through 2028.
Column chromatography remains the dominant technology in this space, accounting for nearly 65% of the total market share. High-performance liquid chromatography (HPLC) systems represent the largest segment within column chromatography technologies, followed by flash chromatography and preparative chromatography systems. The consumables market, including columns, resins, and solvents, generates recurring revenue streams and constitutes about 40% of the total market value.
Regionally, North America leads the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth due to expanding pharmaceutical manufacturing capabilities and increasing R&D investments.
Key market drivers include the growing pipeline of small molecule drugs, increasing adoption of precision medicine approaches, and technological advancements in chromatography systems that address traditional challenges like low recovery and peak tailing. The COVID-19 pandemic temporarily disrupted supply chains but ultimately accelerated demand for purification technologies in vaccine development and therapeutic research.
Customer segments show distinct needs: pharmaceutical companies prioritize scalability and reproducibility; academic institutions focus on versatility and cost-effectiveness; while contract research organizations value throughput and efficiency. The trend toward miniaturization and automation is gaining momentum, with integrated systems that reduce manual intervention and improve reproducibility becoming increasingly popular.
Challenges in small molecule purification, particularly low recovery rates and peak tailing issues, represent significant market opportunities. Companies offering solutions to these problems command premium pricing, with specialized columns designed to minimize sample loss and improve peak symmetry selling at 30-40% higher prices than standard columns.
The competitive landscape features established players like Waters Corporation, Agilent Technologies, and Thermo Fisher Scientific dominating with comprehensive product portfolios. However, specialized companies focusing on niche applications and innovative solutions for specific purification challenges are gaining market share through technological differentiation rather than price competition.
Column chromatography remains the dominant technology in this space, accounting for nearly 65% of the total market share. High-performance liquid chromatography (HPLC) systems represent the largest segment within column chromatography technologies, followed by flash chromatography and preparative chromatography systems. The consumables market, including columns, resins, and solvents, generates recurring revenue streams and constitutes about 40% of the total market value.
Regionally, North America leads the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth due to expanding pharmaceutical manufacturing capabilities and increasing R&D investments.
Key market drivers include the growing pipeline of small molecule drugs, increasing adoption of precision medicine approaches, and technological advancements in chromatography systems that address traditional challenges like low recovery and peak tailing. The COVID-19 pandemic temporarily disrupted supply chains but ultimately accelerated demand for purification technologies in vaccine development and therapeutic research.
Customer segments show distinct needs: pharmaceutical companies prioritize scalability and reproducibility; academic institutions focus on versatility and cost-effectiveness; while contract research organizations value throughput and efficiency. The trend toward miniaturization and automation is gaining momentum, with integrated systems that reduce manual intervention and improve reproducibility becoming increasingly popular.
Challenges in small molecule purification, particularly low recovery rates and peak tailing issues, represent significant market opportunities. Companies offering solutions to these problems command premium pricing, with specialized columns designed to minimize sample loss and improve peak symmetry selling at 30-40% higher prices than standard columns.
The competitive landscape features established players like Waters Corporation, Agilent Technologies, and Thermo Fisher Scientific dominating with comprehensive product portfolios. However, specialized companies focusing on niche applications and innovative solutions for specific purification challenges are gaining market share through technological differentiation rather than price competition.
Current Challenges in Column Chromatography Recovery
Column chromatography for small molecule purification faces several significant challenges that impact recovery rates and separation efficiency. Low recovery rates, often below 70-80%, represent a major concern for researchers and industrial applications alike. This issue is particularly problematic when working with high-value compounds where material loss translates directly to economic inefficiency. Multiple factors contribute to this challenge, including irreversible adsorption to stationary phases, sample degradation during separation, and inefficient elution protocols.
Peak tailing presents another persistent challenge, characterized by asymmetrical peak shapes with elongated trailing edges. This phenomenon compromises resolution between closely eluting compounds and complicates accurate quantification. The primary causes include overloading of sample components, mixed retention mechanisms, and the presence of secondary interactions between analytes and active sites on the stationary phase. These secondary interactions often involve silanol groups on silica-based columns or metal impurities that create unwanted retention.
Equipment-related issues further exacerbate recovery problems. Suboptimal column packing leads to channeling and uneven flow distribution, while system dead volumes contribute to band broadening and sample dilution. Modern HPLC and UHPLC systems have reduced but not eliminated these concerns, particularly when working with older equipment or when systems are improperly maintained.
Method development challenges also impact recovery rates. The selection of inappropriate mobile phase compositions can result in poor solubility of target compounds or inadequate elution strength. pH control issues may alter the ionization state of analytes, changing their retention behavior unpredictably. Temperature fluctuations during separation can cause peak broadening and reduced resolution, especially for thermally sensitive compounds.
Sample preparation represents another critical factor affecting recovery. Incomplete dissolution, matrix effects from complex samples, and sample degradation prior to injection all contribute to diminished recovery. Filtration steps, while necessary to remove particulates, can also cause sample loss through adsorption to filter materials.
Recent technological advances have attempted to address these challenges through the development of new stationary phases with reduced secondary interactions, improved end-capping procedures to minimize silanol activity, and hybrid organic-inorganic materials that offer enhanced pH stability. However, these solutions often come with trade-offs in terms of cost, column lifetime, or compatibility with existing methods.
The economic impact of these challenges is substantial, particularly in pharmaceutical and fine chemical industries where purification costs can represent 50-80% of total production expenses. Improving recovery rates by even a few percentage points can translate to significant cost savings at industrial scales.
Peak tailing presents another persistent challenge, characterized by asymmetrical peak shapes with elongated trailing edges. This phenomenon compromises resolution between closely eluting compounds and complicates accurate quantification. The primary causes include overloading of sample components, mixed retention mechanisms, and the presence of secondary interactions between analytes and active sites on the stationary phase. These secondary interactions often involve silanol groups on silica-based columns or metal impurities that create unwanted retention.
Equipment-related issues further exacerbate recovery problems. Suboptimal column packing leads to channeling and uneven flow distribution, while system dead volumes contribute to band broadening and sample dilution. Modern HPLC and UHPLC systems have reduced but not eliminated these concerns, particularly when working with older equipment or when systems are improperly maintained.
Method development challenges also impact recovery rates. The selection of inappropriate mobile phase compositions can result in poor solubility of target compounds or inadequate elution strength. pH control issues may alter the ionization state of analytes, changing their retention behavior unpredictably. Temperature fluctuations during separation can cause peak broadening and reduced resolution, especially for thermally sensitive compounds.
Sample preparation represents another critical factor affecting recovery. Incomplete dissolution, matrix effects from complex samples, and sample degradation prior to injection all contribute to diminished recovery. Filtration steps, while necessary to remove particulates, can also cause sample loss through adsorption to filter materials.
Recent technological advances have attempted to address these challenges through the development of new stationary phases with reduced secondary interactions, improved end-capping procedures to minimize silanol activity, and hybrid organic-inorganic materials that offer enhanced pH stability. However, these solutions often come with trade-offs in terms of cost, column lifetime, or compatibility with existing methods.
The economic impact of these challenges is substantial, particularly in pharmaceutical and fine chemical industries where purification costs can represent 50-80% of total production expenses. Improving recovery rates by even a few percentage points can translate to significant cost savings at industrial scales.
Established Solutions for Peak Tailing and Recovery Issues
01 Mobile phase optimization for peak tailing reduction
Optimizing the mobile phase composition can significantly reduce peak tailing in column chromatography. This includes adjusting pH levels, ionic strength, and adding modifiers such as ion-pairing reagents. The proper selection of organic solvents and their ratios can improve peak symmetry and enhance separation efficiency. Buffer selection and concentration are also critical factors that influence the interaction between analytes and stationary phase, thereby minimizing tailing effects.- Mobile phase optimization for peak tailing reduction: Optimizing the mobile phase composition is crucial for reducing peak tailing in column chromatography. This includes adjusting pH levels, ionic strength, and organic modifier concentrations to minimize secondary interactions between analytes and the stationary phase. Proper selection of buffer systems and additives like ion-pairing reagents can significantly improve peak symmetry and enhance chromatographic resolution, leading to better recovery and more accurate quantification of target compounds.
- Stationary phase modifications to improve recovery: Modifications to the stationary phase can significantly improve chromatographic recovery and reduce peak tailing. These modifications include end-capping of residual silanol groups, bonding specific functional groups to the silica surface, and using hybrid particle technologies. Advanced stationary phases with reduced metal content and improved surface homogeneity minimize unwanted interactions with analytes, resulting in more symmetrical peaks and higher recovery rates, particularly for basic compounds and biomolecules.
- Column conditioning and equilibration techniques: Proper column conditioning and equilibration are essential for consistent chromatographic performance and reduced peak tailing. This involves systematic washing procedures, temperature control during equilibration, and adequate column saturation with the mobile phase before sample injection. Implementing standardized pre-conditioning protocols ensures column stability, improves run-to-run reproducibility, and maximizes recovery of analytes, particularly for complex biological samples and pharmaceutical compounds.
- Advanced detection and quantification methods: Implementing advanced detection and quantification methods can compensate for peak tailing effects and improve overall recovery in column chromatography. These include mathematical peak modeling, deconvolution algorithms, and multi-detector approaches that provide complementary information about eluting compounds. Modern data processing techniques can correct for peak asymmetry during quantification, while specialized detectors with enhanced sensitivity can detect and accurately quantify compounds even when chromatographic separation is not ideal.
- Sample preparation strategies for improved chromatography: Effective sample preparation strategies are critical for minimizing peak tailing and maximizing recovery in column chromatography. These include selective extraction techniques, sample clean-up procedures to remove interfering compounds, and proper dissolution methods to ensure sample compatibility with the mobile phase. Adjusting sample pH before injection, using appropriate diluents, and implementing techniques like solid-phase extraction or liquid-liquid extraction can significantly reduce matrix effects that contribute to peak tailing and poor recovery.
02 Stationary phase modifications to improve recovery
Modifications to the stationary phase can significantly improve chromatographic recovery and reduce peak tailing. These modifications include end-capping of residual silanol groups, bonding specific functional groups to the silica surface, and using hybrid organic-inorganic materials. Advanced stationary phases with reduced metal content and controlled pore size distribution can minimize unwanted interactions with analytes, resulting in improved peak shapes and higher recovery rates.Expand Specific Solutions03 Column conditioning and equilibration techniques
Proper column conditioning and equilibration are essential for reducing peak tailing and improving recovery in chromatography. This involves systematic washing procedures, temperature control during equilibration, and ensuring adequate column saturation with the mobile phase before sample injection. Pre-conditioning with specific additives can mask active sites on the stationary phase that might cause tailing. Maintaining consistent equilibration protocols between runs enhances reproducibility and peak symmetry.Expand Specific Solutions04 Sample preparation methods for enhanced chromatographic performance
Advanced sample preparation techniques can significantly improve chromatographic performance by reducing matrix effects that contribute to peak tailing. These methods include selective extraction procedures, sample clean-up using solid-phase extraction, and pre-concentration techniques. Adjusting sample solvent composition to match the mobile phase, controlling sample pH, and removing particulates can prevent injection-related peak distortions and improve overall recovery of target analytes.Expand Specific Solutions05 Instrumental parameters and system optimization
Optimizing instrumental parameters is crucial for minimizing peak tailing and maximizing recovery in column chromatography. This includes adjusting flow rates, controlling column temperature, optimizing injection volume, and reducing dead volumes in the chromatographic system. Advanced detection techniques with higher sensitivity and selectivity can improve signal-to-noise ratios and peak resolution. Regular system maintenance, including replacement of frits and connectors, helps prevent system-related causes of peak tailing.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Chromatography
Column chromatography for small molecule purification faces challenges in a competitive landscape currently in the growth phase. The market is expanding rapidly, with an estimated size of $3-4 billion and projected annual growth of 8-10%. Technologically, the field shows varying maturity levels across different applications. Industry leaders like Waters Technology Corp. and Hitachi High-Tech America demonstrate advanced capabilities in instrumentation, while pharmaceutical companies such as Amgen, Janssen Biotech, and UCB Biopharma focus on application-specific optimizations. EMD Millipore and Micromass UK have made significant progress in addressing recovery and peak tailing issues through innovative stationary phase designs and detection technologies. The convergence of analytical expertise from established players and novel approaches from emerging companies is driving continuous improvement in purification efficiency.
Amgen, Inc.
Technical Solution: Amgen has developed sophisticated column chromatography approaches for small molecule purification as part of their drug development pipeline. Their technology addresses recovery and peak tailing issues through a combination of specialized stationary phases and optimized separation conditions. Amgen employs a systematic approach to method development using automated column screening systems that evaluate multiple stationary phases simultaneously to identify optimal selectivity for challenging separations. Their methodology incorporates the use of hybrid organic-inorganic stationary phases with reduced silanol activity and controlled metal content to minimize secondary interactions causing peak tailing. Amgen has pioneered the application of superficially porous particles (core-shell technology) for small molecule purification, which provides enhanced mass transfer characteristics while maintaining reasonable backpressure, particularly beneficial for larger small molecules. The company utilizes specialized mobile phase additives like amine modifiers and competing bases that effectively block active sites on the column surface. Their purification platform includes sophisticated gradient optimization algorithms that model and predict chromatographic behavior to develop methods with maximum recovery and minimal tailing, particularly important for high-value pharmaceutical intermediates and APIs.
Strengths: Extensive experience with diverse chemical structures from drug discovery pipeline; integrated analytical to preparative scale capabilities; sophisticated modeling and prediction tools for method development. Weaknesses: Solutions often developed for internal compounds may not be universally applicable; highly optimized methods may be sensitive to minor variations in conditions; some approaches may be proprietary and not fully disclosed in scientific literature.
Micromass UK Ltd.
Technical Solution: Micromass UK (now part of Waters Corporation) has developed specialized column chromatography solutions integrating with mass spectrometry for enhanced small molecule purification. Their technology addresses peak tailing and recovery issues through innovative column chemistry and advanced detection methods. The company's ACQUITY QDa Mass Detector integration with chromatography systems provides real-time monitoring of analytes, allowing for precise fraction collection even with tailing peaks. Their columns feature specialized bonding chemistry with reduced silanol activity and controlled metal content to minimize unwanted secondary interactions. Micromass has pioneered the use of 2D chromatography approaches where compounds exhibiting poor behavior in one separation mode are automatically transferred to a complementary mode, significantly improving overall recovery. Their systems incorporate intelligent peak detection algorithms that can identify and collect tailing peaks based on mass spectral information rather than UV profile alone. The company has also developed specialized mobile phase additives compatible with MS detection that improve peak shape without introducing ion suppression effects, a common challenge in LC-MS applications for small molecule purification.
Strengths: Seamless integration of chromatography with mass spectrometry provides superior detection capabilities; specialized column chemistries designed specifically for MS compatibility; intelligent software for peak tracking and collection. Weaknesses: Higher system complexity requires greater expertise to operate effectively; MS-compatible mobile phases may sometimes compromise separation performance; higher initial investment compared to conventional chromatography systems.
Critical Patents and Innovations in Column Efficiency
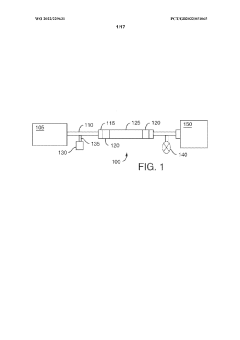
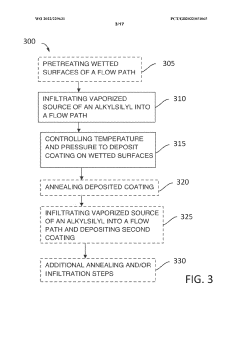
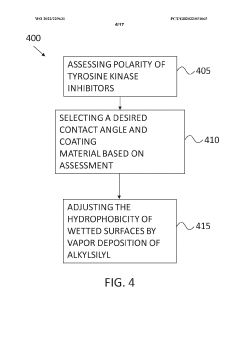
Use of low-bind surface coatings for analysis of tyrosine kinase inhibitors
PatentWO2022229631A1
Innovation
- Implementing a low-bind surface coating, such as an alkylsilyl coating, on metallic flow paths within chromatographic systems to minimize interactions between TKIs and metal surfaces, thereby reducing secondary chromatographic interactions and improving separation and detection accuracy.
A method for identification of polypeptide variants
PatentInactiveIN4703CHE2013A
Innovation
- Coupling reverse phase chromatography with mass spectrometry to identify and quantify polypeptide variants, including N-terminal pyroglutamate, lysine, and glyco-variants, through a method involving sample preparation, reverse phase chromatography, and mass spectrometry analysis, enabling precise and fast characterization of immunoglobulin compositions.
Solvent and Mobile Phase Optimization Strategies
Solvent selection and mobile phase composition represent critical factors in achieving optimal separation efficiency and recovery in column chromatography for small molecule purification. The choice of solvent system directly impacts analyte solubility, retention behavior, and peak shape. When addressing issues of low recovery and peak tailing, a systematic approach to mobile phase optimization can yield significant improvements in chromatographic performance.
Primary considerations for solvent selection include polarity matching with target compounds, solubility parameters, and compatibility with detection methods. For small molecules exhibiting peak tailing, adjusting the pH of the mobile phase can dramatically improve peak symmetry by controlling the ionization state of both the analyte and stationary phase. Generally, analytes should be maintained in a single ionic form throughout the separation process, either fully ionized or non-ionized.
Buffer selection and concentration play equally important roles in mobile phase optimization. For reversed-phase separations of basic compounds, the addition of triethylamine (0.1-0.5%) can effectively mask silanol interactions that contribute to peak tailing. Conversely, acidic modifiers such as formic acid or trifluoroacetic acid (0.05-0.1%) can improve peak shape for basic compounds by suppressing ionization.
Gradient optimization represents another powerful strategy for addressing recovery and tailing issues. Shallow gradients typically provide better resolution but may exacerbate peak broadening, while steeper gradients can improve peak shape and recovery of strongly retained compounds. Implementation of step gradients or segmented gradients can be particularly effective for samples containing compounds with widely varying retention characteristics.
Temperature control offers an often-overlooked parameter for mobile phase optimization. Elevated temperatures (30-60°C) can reduce mobile phase viscosity, improving mass transfer kinetics and potentially resolving peak tailing issues. Additionally, temperature adjustments can modify selectivity in a manner complementary to changes in organic modifier concentration.
For particularly challenging separations, the incorporation of additives such as ion-pairing reagents can transform separation selectivity. Hexanesulfonic acid or tetrabutylammonium salts (5-10 mM) can dramatically improve the chromatography of ionizable compounds by forming neutral complexes that interact more predictably with the stationary phase.
When troubleshooting persistent recovery issues, consideration should be given to potential on-column degradation or irreversible adsorption. In such cases, the addition of competing agents or protective additives like ascorbic acid for oxidation-sensitive compounds can significantly improve recovery without compromising separation efficiency.
Primary considerations for solvent selection include polarity matching with target compounds, solubility parameters, and compatibility with detection methods. For small molecules exhibiting peak tailing, adjusting the pH of the mobile phase can dramatically improve peak symmetry by controlling the ionization state of both the analyte and stationary phase. Generally, analytes should be maintained in a single ionic form throughout the separation process, either fully ionized or non-ionized.
Buffer selection and concentration play equally important roles in mobile phase optimization. For reversed-phase separations of basic compounds, the addition of triethylamine (0.1-0.5%) can effectively mask silanol interactions that contribute to peak tailing. Conversely, acidic modifiers such as formic acid or trifluoroacetic acid (0.05-0.1%) can improve peak shape for basic compounds by suppressing ionization.
Gradient optimization represents another powerful strategy for addressing recovery and tailing issues. Shallow gradients typically provide better resolution but may exacerbate peak broadening, while steeper gradients can improve peak shape and recovery of strongly retained compounds. Implementation of step gradients or segmented gradients can be particularly effective for samples containing compounds with widely varying retention characteristics.
Temperature control offers an often-overlooked parameter for mobile phase optimization. Elevated temperatures (30-60°C) can reduce mobile phase viscosity, improving mass transfer kinetics and potentially resolving peak tailing issues. Additionally, temperature adjustments can modify selectivity in a manner complementary to changes in organic modifier concentration.
For particularly challenging separations, the incorporation of additives such as ion-pairing reagents can transform separation selectivity. Hexanesulfonic acid or tetrabutylammonium salts (5-10 mM) can dramatically improve the chromatography of ionizable compounds by forming neutral complexes that interact more predictably with the stationary phase.
When troubleshooting persistent recovery issues, consideration should be given to potential on-column degradation or irreversible adsorption. In such cases, the addition of competing agents or protective additives like ascorbic acid for oxidation-sensitive compounds can significantly improve recovery without compromising separation efficiency.
Regulatory Compliance in Pharmaceutical Purification Processes
Regulatory compliance in pharmaceutical purification processes represents a critical framework that governs the development, implementation, and validation of column chromatography techniques for small molecule purification. The pharmaceutical industry operates under stringent regulatory oversight from authorities such as the FDA, EMA, and ICH, which establish comprehensive guidelines to ensure product safety, efficacy, and quality.
When addressing low recovery and peak tailing issues in column chromatography, compliance with Good Manufacturing Practices (GMP) becomes paramount. These regulations mandate thorough documentation of all purification processes, including detailed standard operating procedures (SOPs), validation protocols, and quality control measures that can help identify and mitigate recovery problems.
The FDA's Process Validation Guidance emphasizes a lifecycle approach to validation, requiring pharmaceutical companies to demonstrate consistent control over critical quality attributes during purification. This includes establishing acceptable limits for impurities and ensuring that chromatographic methods consistently deliver the desired purity profile despite challenges like peak tailing.
ICH Q7 guidelines specifically address the requirements for active pharmaceutical ingredient (API) manufacturing, including purification steps. These guidelines necessitate robust change control procedures when modifying chromatographic parameters to address recovery issues, ensuring that any adjustments maintain compliance while improving performance.
Equipment qualification represents another crucial regulatory aspect. Under 21 CFR Part 211, all chromatography systems must undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) to verify their suitability for intended use and ability to deliver consistent results without peak tailing or recovery losses.
Data integrity requirements, as outlined in FDA's Data Integrity and Compliance guidance, mandate that all chromatographic data be attributable, legible, contemporaneously recorded, original, and accurate (ALCOA principles). This becomes especially important when troubleshooting purification issues, as all experimental data and corrective actions must be thoroughly documented.
Method validation according to ICH Q2(R1) guidelines requires demonstration of specificity, linearity, accuracy, precision, range, and robustness—parameters that can be significantly impacted by peak tailing. Regulatory bodies expect companies to establish acceptance criteria that account for these challenges while maintaining product quality.
Environmental considerations also factor into regulatory compliance, with agencies increasingly scrutinizing solvent usage and waste management in chromatographic processes. Companies must balance purification efficiency with environmental regulations, often necessitating green chemistry approaches when redesigning methods to improve recovery.
When addressing low recovery and peak tailing issues in column chromatography, compliance with Good Manufacturing Practices (GMP) becomes paramount. These regulations mandate thorough documentation of all purification processes, including detailed standard operating procedures (SOPs), validation protocols, and quality control measures that can help identify and mitigate recovery problems.
The FDA's Process Validation Guidance emphasizes a lifecycle approach to validation, requiring pharmaceutical companies to demonstrate consistent control over critical quality attributes during purification. This includes establishing acceptable limits for impurities and ensuring that chromatographic methods consistently deliver the desired purity profile despite challenges like peak tailing.
ICH Q7 guidelines specifically address the requirements for active pharmaceutical ingredient (API) manufacturing, including purification steps. These guidelines necessitate robust change control procedures when modifying chromatographic parameters to address recovery issues, ensuring that any adjustments maintain compliance while improving performance.
Equipment qualification represents another crucial regulatory aspect. Under 21 CFR Part 211, all chromatography systems must undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) to verify their suitability for intended use and ability to deliver consistent results without peak tailing or recovery losses.
Data integrity requirements, as outlined in FDA's Data Integrity and Compliance guidance, mandate that all chromatographic data be attributable, legible, contemporaneously recorded, original, and accurate (ALCOA principles). This becomes especially important when troubleshooting purification issues, as all experimental data and corrective actions must be thoroughly documented.
Method validation according to ICH Q2(R1) guidelines requires demonstration of specificity, linearity, accuracy, precision, range, and robustness—parameters that can be significantly impacted by peak tailing. Regulatory bodies expect companies to establish acceptance criteria that account for these challenges while maintaining product quality.
Environmental considerations also factor into regulatory compliance, with agencies increasingly scrutinizing solvent usage and waste management in chromatographic processes. Companies must balance purification efficiency with environmental regulations, often necessitating green chemistry approaches when redesigning methods to improve recovery.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!