Column Reuse and Regeneration: Lifetime, Cleaning Protocols and QC Checks
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Column Reuse Background and Objectives
Chromatography columns represent a significant investment in analytical and preparative processes across pharmaceutical, biotechnology, and chemical industries. The evolution of column technology has progressed from single-use applications to the current emphasis on reusability and regeneration, driven by economic pressures and sustainability concerns. This technological progression has been marked by innovations in stationary phase chemistry, column packing techniques, and material science advancements that enhance durability and chemical resistance.
The historical trajectory of chromatography column reuse began in the 1970s with rudimentary cleaning protocols for silica-based columns. By the 1990s, more robust polymeric and hybrid materials emerged, significantly extending column lifetimes. The past decade has witnessed remarkable improvements in column engineering, with modern columns capable of withstanding hundreds of injection cycles when properly maintained.
Current industry trends indicate a growing focus on maximizing column lifetime while maintaining separation efficiency and reproducibility. This trend is particularly pronounced in biopharmaceutical manufacturing, where chromatography represents up to 60% of downstream processing costs. The economic implications of column reuse are substantial, with potential savings of 30-70% compared to single-use approaches, depending on application complexity and cleaning requirements.
The primary objective of this technical research is to comprehensively evaluate the factors affecting chromatography column lifetime, establish evidence-based cleaning and regeneration protocols, and develop robust quality control metrics to ensure consistent performance across multiple uses. This includes investigating the chemical and physical mechanisms of column degradation, identifying optimal cleaning agents for various contaminants, and establishing quantitative parameters for column performance assessment.
Additionally, this research aims to explore the relationship between column chemistry, operating conditions, and reusability potential. Understanding these correlations will enable the development of predictive models for column lifetime estimation and facilitate informed decision-making regarding column replacement versus regeneration.
The ultimate goal is to establish standardized, application-specific guidelines for column reuse that balance analytical integrity with economic efficiency. These guidelines will address critical questions regarding the maximum number of recommended reuse cycles, appropriate cleaning methodologies for different sample matrices, and definitive quality control checks to validate column performance after regeneration procedures.
The historical trajectory of chromatography column reuse began in the 1970s with rudimentary cleaning protocols for silica-based columns. By the 1990s, more robust polymeric and hybrid materials emerged, significantly extending column lifetimes. The past decade has witnessed remarkable improvements in column engineering, with modern columns capable of withstanding hundreds of injection cycles when properly maintained.
Current industry trends indicate a growing focus on maximizing column lifetime while maintaining separation efficiency and reproducibility. This trend is particularly pronounced in biopharmaceutical manufacturing, where chromatography represents up to 60% of downstream processing costs. The economic implications of column reuse are substantial, with potential savings of 30-70% compared to single-use approaches, depending on application complexity and cleaning requirements.
The primary objective of this technical research is to comprehensively evaluate the factors affecting chromatography column lifetime, establish evidence-based cleaning and regeneration protocols, and develop robust quality control metrics to ensure consistent performance across multiple uses. This includes investigating the chemical and physical mechanisms of column degradation, identifying optimal cleaning agents for various contaminants, and establishing quantitative parameters for column performance assessment.
Additionally, this research aims to explore the relationship between column chemistry, operating conditions, and reusability potential. Understanding these correlations will enable the development of predictive models for column lifetime estimation and facilitate informed decision-making regarding column replacement versus regeneration.
The ultimate goal is to establish standardized, application-specific guidelines for column reuse that balance analytical integrity with economic efficiency. These guidelines will address critical questions regarding the maximum number of recommended reuse cycles, appropriate cleaning methodologies for different sample matrices, and definitive quality control checks to validate column performance after regeneration procedures.
Market Analysis of Column Regeneration Technologies
The column regeneration technology market has experienced significant growth over the past decade, driven primarily by increasing cost pressures in analytical laboratories and growing environmental concerns. The global market for chromatography columns was valued at approximately $2.3 billion in 2022, with the regeneration segment representing about 15% of this market. This segment is projected to grow at a CAGR of 5.8% through 2028, outpacing the overall chromatography column market growth of 4.2%.
Pharmaceutical and biotechnology industries dominate the demand for column regeneration technologies, accounting for nearly 60% of the market share. These sectors face intense pressure to reduce operational costs while maintaining analytical integrity, making column regeneration an attractive option. Academic and research institutions represent the second-largest market segment at 20%, followed by food and beverage testing laboratories at 12%.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to expanding pharmaceutical manufacturing and increasing adoption of advanced analytical techniques.
The market is segmented by column type, with reversed-phase columns representing the largest segment (45%), followed by ion-exchange columns (25%), size-exclusion columns (15%), and others (15%). This distribution reflects the widespread use of reversed-phase chromatography in pharmaceutical analysis and quality control.
Key market drivers include rising analytical testing costs, environmental regulations limiting solvent disposal, and increasing pressure on laboratory budgets. The average cost savings from column regeneration versus replacement ranges from 60-80%, providing a compelling economic incentive for laboratories. Additionally, sustainability initiatives within organizations are promoting resource conservation practices, including column reuse.
Market restraints include concerns about column performance after multiple regeneration cycles, lack of standardized regeneration protocols across different column types, and manufacturer warranties that may be voided by regeneration. These factors have created opportunities for specialized service providers offering validated regeneration protocols and quality assurance testing.
The competitive landscape features both column manufacturers offering proprietary regeneration solutions and independent service providers specializing in multi-brand column regeneration. Recent market trends indicate a shift toward automated regeneration systems and the development of more robust column materials specifically designed for extended lifecycles through multiple regeneration processes.
Pharmaceutical and biotechnology industries dominate the demand for column regeneration technologies, accounting for nearly 60% of the market share. These sectors face intense pressure to reduce operational costs while maintaining analytical integrity, making column regeneration an attractive option. Academic and research institutions represent the second-largest market segment at 20%, followed by food and beverage testing laboratories at 12%.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to expanding pharmaceutical manufacturing and increasing adoption of advanced analytical techniques.
The market is segmented by column type, with reversed-phase columns representing the largest segment (45%), followed by ion-exchange columns (25%), size-exclusion columns (15%), and others (15%). This distribution reflects the widespread use of reversed-phase chromatography in pharmaceutical analysis and quality control.
Key market drivers include rising analytical testing costs, environmental regulations limiting solvent disposal, and increasing pressure on laboratory budgets. The average cost savings from column regeneration versus replacement ranges from 60-80%, providing a compelling economic incentive for laboratories. Additionally, sustainability initiatives within organizations are promoting resource conservation practices, including column reuse.
Market restraints include concerns about column performance after multiple regeneration cycles, lack of standardized regeneration protocols across different column types, and manufacturer warranties that may be voided by regeneration. These factors have created opportunities for specialized service providers offering validated regeneration protocols and quality assurance testing.
The competitive landscape features both column manufacturers offering proprietary regeneration solutions and independent service providers specializing in multi-brand column regeneration. Recent market trends indicate a shift toward automated regeneration systems and the development of more robust column materials specifically designed for extended lifecycles through multiple regeneration processes.
Current Challenges in Column Lifetime Extension
Despite significant advancements in chromatography column technology, several persistent challenges continue to limit column lifetime extension efforts. The primary obstacle remains the irreversible degradation of stationary phases under routine operating conditions. Silica-based columns are particularly vulnerable to hydrolysis at extreme pH values, with gradual dissolution occurring at pH > 8, compromising column integrity over time. Similarly, polymeric columns face oxidative degradation when exposed to certain mobile phases containing reactive components or when operated at elevated temperatures.
Contamination management presents another significant challenge. Sample matrices containing strongly retained compounds, particulates, or biological materials can progressively foul column beds, leading to increased backpressure, peak distortion, and reduced separation efficiency. Current cleaning protocols often fail to completely remove these deeply embedded contaminants without simultaneously damaging the stationary phase.
The lack of standardized, non-destructive quality control methods for assessing column condition represents a critical gap in lifetime management strategies. While parameters such as theoretical plate count, asymmetry factor, and retention time monitoring provide valuable information, they often detect problems only after significant column deterioration has occurred. This reactive rather than predictive approach limits proactive maintenance opportunities.
Column regeneration techniques face efficiency limitations with each regeneration cycle typically resulting in some permanent loss of performance. Research indicates that most columns can undergo only 3-5 effective regeneration cycles before performance metrics fall below acceptable thresholds, regardless of cleaning protocol optimization.
The economic and operational trade-offs between column regeneration and replacement create decision-making challenges for laboratories. The labor costs, downtime, and validation requirements associated with regeneration procedures must be carefully weighed against the purchase price of new columns, particularly for regulated environments where requalification is mandatory.
Emerging analytical demands for higher throughput, greater sensitivity, and more complex separations place additional stress on columns, accelerating deterioration rates. The push toward ultra-high-pressure liquid chromatography (UHPLC) with sub-2μm particles has intensified mechanical stress factors, creating new failure modes that traditional lifetime extension approaches cannot adequately address.
Finally, the environmental sustainability concerns surrounding column disposal and replacement create tension between operational efficiency and corporate sustainability goals, driving the need for more effective regeneration technologies that can significantly extend useful column lifetimes while maintaining analytical performance.
Contamination management presents another significant challenge. Sample matrices containing strongly retained compounds, particulates, or biological materials can progressively foul column beds, leading to increased backpressure, peak distortion, and reduced separation efficiency. Current cleaning protocols often fail to completely remove these deeply embedded contaminants without simultaneously damaging the stationary phase.
The lack of standardized, non-destructive quality control methods for assessing column condition represents a critical gap in lifetime management strategies. While parameters such as theoretical plate count, asymmetry factor, and retention time monitoring provide valuable information, they often detect problems only after significant column deterioration has occurred. This reactive rather than predictive approach limits proactive maintenance opportunities.
Column regeneration techniques face efficiency limitations with each regeneration cycle typically resulting in some permanent loss of performance. Research indicates that most columns can undergo only 3-5 effective regeneration cycles before performance metrics fall below acceptable thresholds, regardless of cleaning protocol optimization.
The economic and operational trade-offs between column regeneration and replacement create decision-making challenges for laboratories. The labor costs, downtime, and validation requirements associated with regeneration procedures must be carefully weighed against the purchase price of new columns, particularly for regulated environments where requalification is mandatory.
Emerging analytical demands for higher throughput, greater sensitivity, and more complex separations place additional stress on columns, accelerating deterioration rates. The push toward ultra-high-pressure liquid chromatography (UHPLC) with sub-2μm particles has intensified mechanical stress factors, creating new failure modes that traditional lifetime extension approaches cannot adequately address.
Finally, the environmental sustainability concerns surrounding column disposal and replacement create tension between operational efficiency and corporate sustainability goals, driving the need for more effective regeneration technologies that can significantly extend useful column lifetimes while maintaining analytical performance.
Established Column Cleaning and Regeneration Protocols
01 Column lifetime extension methods
Various methods can be employed to extend the lifetime of chromatography columns, including proper storage conditions, regular maintenance, and appropriate operating parameters. Techniques such as controlled flow rates, temperature regulation, and pressure monitoring help prevent column degradation. Additionally, using pre-columns or guard columns can protect the main column from contaminants and particulates, thereby extending its useful life and maintaining separation efficiency over time.- Column cleaning methods for extended lifetime: Various cleaning methods can significantly extend the lifetime of chromatography columns. These include using specific cleaning solutions, automated cleaning protocols, and regeneration techniques that effectively remove contaminants without damaging the column matrix. Proper cleaning between runs prevents cross-contamination and maintains separation efficiency. Regular implementation of these cleaning procedures helps preserve column performance characteristics and extends operational lifespan.
- Quality control parameters and testing procedures: Quality control for chromatography columns involves monitoring key performance parameters such as theoretical plate count, peak symmetry, resolution, and pressure drop. Standardized testing procedures include efficiency tests, pressure tests, and chemical stability assessments. Regular performance verification using standard analytes helps detect early signs of column deterioration. Documentation of column history and performance trends enables predictive maintenance and ensures consistent analytical results.
- Column packing techniques and materials: Advanced packing techniques and materials significantly impact chromatography column performance and longevity. Uniform particle distribution and proper compression of stationary phases reduce void formation and channeling. Novel packing materials with enhanced chemical stability resist degradation during repeated use and cleaning cycles. Specialized packing equipment and protocols ensure reproducible column preparation, which directly influences separation efficiency and column lifetime.
- Automated monitoring systems for column performance: Automated systems for real-time monitoring of chromatography column performance help maintain quality control and extend column lifetime. These systems track parameters such as back pressure, peak shape, and retention time stability during operation. Integrated software can detect deviations from established performance baselines and alert operators before significant degradation occurs. Continuous monitoring enables timely intervention, optimized cleaning schedules, and data-driven decisions about column replacement.
- Novel column protection strategies: Innovative protection strategies help extend chromatography column lifetime and maintain separation efficiency. These include the use of guard columns to prevent contaminant entry, in-line filters to remove particulates, and specialized pre-treatment protocols for complex samples. Temperature control systems prevent thermal degradation of column materials. Optimized mobile phase composition and pH control minimize chemical stress on the stationary phase, while controlled flow rates prevent physical damage to the column bed.
02 Cleaning protocols and efficiency
Effective cleaning protocols are essential for maintaining chromatography column performance. These include reverse flow washing, solvent gradients, and specialized cleaning solutions designed to remove specific contaminants. The efficiency of cleaning procedures can be evaluated through pressure drop measurements, baseline stability, and resolution testing. Automated cleaning systems can provide consistent results while minimizing manual handling and reducing the risk of damage to column materials during the cleaning process.Expand Specific Solutions03 Quality control and performance monitoring
Quality control for chromatography columns involves systematic testing and monitoring of key performance indicators. This includes regular evaluation of theoretical plate count, peak symmetry, resolution, and retention time reproducibility. Implementing standardized test mixtures allows for consistent performance assessment and early detection of column deterioration. Advanced monitoring systems can track column performance trends over time, enabling predictive maintenance rather than reactive replacement.Expand Specific Solutions04 Novel column materials and designs
Innovations in column materials and designs have significantly improved chromatography column durability and performance. These include monolithic structures, hybrid particle technologies, and surface-modified stationary phases that resist degradation. Columns with improved pH stability, temperature resistance, and mechanical strength can withstand more aggressive cleaning procedures while maintaining separation efficiency. Some designs incorporate self-cleaning mechanisms or modular components that can be individually replaced when performance deteriorates.Expand Specific Solutions05 Automated systems for column management
Automated systems for chromatography column management integrate monitoring, cleaning, and quality control functions. These systems can automatically detect performance issues, implement appropriate cleaning protocols, and validate column performance after maintenance. Real-time monitoring capabilities allow for immediate intervention when parameters deviate from acceptable ranges. Some systems incorporate machine learning algorithms to optimize cleaning procedures based on column history and contaminant profiles, maximizing column lifetime while ensuring consistent separation quality.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The column reuse and regeneration market is in a mature growth phase, with increasing demand driven by cost-efficiency requirements in pharmaceutical and biotechnology sectors. The global chromatography columns market is estimated at $3.5-4 billion, growing at 5-7% annually. Leading players demonstrate varying levels of technical sophistication: Cytiva (formerly GE Healthcare) and Roche lead with advanced regeneration protocols and quality control systems, while YMC, Bio-Rad, and Amgen have developed proprietary cleaning technologies. Emerging players like ChromaCon are introducing innovative continuous chromatography solutions that extend column lifetime. The competitive landscape shows a clear stratification between established pharmaceutical companies focusing on internal applications and specialized equipment manufacturers developing commercial solutions for broader markets.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive column reuse strategy for chromatography processes in biopharmaceutical manufacturing. Their approach includes a systematic lifetime assessment protocol that tracks column performance across multiple cycles using critical quality attributes (CQAs) such as binding capacity, elution profile, and pressure drop. Roche implements a risk-based cleaning validation system with tiered cleaning protocols based on product characteristics and contamination risk. Their cleaning procedures typically involve sequential washes with buffer solutions at varying pH levels, followed by sanitization with sodium hydroxide solutions (0.1-1.0M) and storage in bacteriostatic solutions containing 20% ethanol. For quality control, Roche employs height equivalent to theoretical plate (HETP) measurements, asymmetry factor analysis, and dynamic binding capacity tests to determine column performance deterioration. Their regeneration protocols are product-specific and include the use of chaotropic agents like guanidine hydrochloride for protein A resins and specialized cleaning agents for mixed-mode chromatography.
Strengths: Robust data-driven approach to column lifetime prediction; comprehensive cleaning validation system that meets regulatory requirements; advanced analytical methods for performance monitoring. Weaknesses: Complex protocols require significant analytical resources; some cleaning agents may affect certain resin types over extended use; system may be overly conservative in some applications, leading to premature column retirement.
Cytiva Sweden AB
Technical Solution: Cytiva has pioneered an integrated approach to chromatography column management called "Smart Column Management" that maximizes resin lifetime while ensuring consistent purification performance. Their technology incorporates real-time monitoring systems that track pressure-flow relationships, UV absorbance patterns, and conductivity profiles to detect early signs of column deterioration. Cytiva's cleaning protocols are tailored to specific resin chemistries, with specialized procedures for protein A, ion exchange, and hydrophobic interaction media. For protein A resins, they've developed a proprietary cleaning cocktail that removes protein aggregates and lipids while preserving ligand activity, allowing up to 200 reuse cycles in some applications. Their regeneration process includes a patented "pulse cleaning" technique that applies alternating flow directions and buffer compositions to dislodge contaminants from difficult-to-reach areas within the packed bed. Quality control is maintained through automated testing stations that perform plate count, asymmetry, and dynamic binding capacity tests after predetermined cycle numbers. Cytiva also employs predictive algorithms that analyze trending data to forecast remaining column lifetime and schedule preventative maintenance.
Strengths: Highly automated monitoring systems reduce manual testing requirements; specialized cleaning cocktails extend column lifetime beyond industry standards; predictive maintenance reduces unexpected column failures. Weaknesses: Proprietary cleaning solutions increase operational costs; complex monitoring systems require specialized training; some automated protocols may be overly standardized for unique purification challenges.
Critical Patents and Innovations in Column Reuse
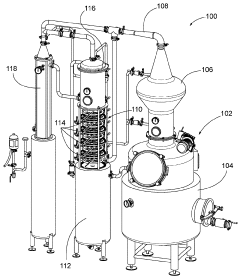
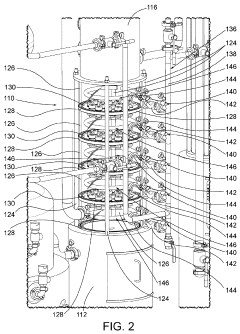
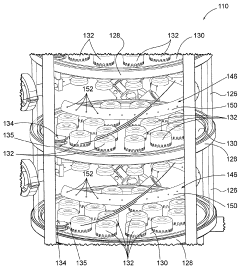
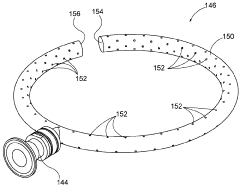
Systems and methods for cleaning a rectification column
PatentActiveUS10005003B1
Innovation
- A fluid distribution system with a ring having a plurality of apertures that propels pressurized cleaning fluid in a 360-degree trajectory, ensuring effective cleaning of deposits between plates and other hard-to-reach areas within the rectification column.
Regulatory Compliance for Reused Chromatography Columns
Regulatory compliance represents a critical consideration for biopharmaceutical manufacturers implementing chromatography column reuse strategies. The FDA, EMA, and other global regulatory bodies have established stringent guidelines that govern the validation, documentation, and quality control of reused chromatography columns to ensure product safety and efficacy.
The regulatory framework for column reuse primarily focuses on demonstrating that the regeneration process consistently yields columns that perform within established specifications. ICH Q7 guidelines specifically address equipment cleaning validation, requiring manufacturers to establish scientifically sound acceptance criteria for residual contaminants and demonstrate that cleaning procedures consistently meet these criteria.
FDA's Process Validation Guidance emphasizes a lifecycle approach that applies directly to column reuse protocols. Manufacturers must validate that columns maintain consistent performance characteristics throughout multiple use cycles, with particular attention to potential leachables, carryover contamination, and ligand stability. This validation typically requires extensive documentation of cleaning effectiveness through at least three consecutive successful cleaning cycles.
EMA guidelines further stipulate that column reuse validation must include worst-case scenario testing, where columns are deliberately challenged with high product loads or difficult-to-clean substances before undergoing standard cleaning protocols. This approach helps establish the robustness of cleaning procedures under challenging conditions.
Regulatory bodies also require comprehensive change control procedures for any modifications to established column cleaning or regeneration protocols. These changes must be supported by appropriate risk assessments and validation data demonstrating that column performance remains within acceptable parameters following the modified procedures.
Documentation requirements represent another significant regulatory consideration. Manufacturers must maintain detailed records of each column's usage history, including products processed, cleaning cycles completed, storage conditions, and quality control test results. This documentation serves as evidence of compliance during regulatory inspections and is essential for investigating any product quality deviations.
Regulatory agencies increasingly expect manufacturers to implement continuous monitoring programs that track column performance trends over time. These programs should include defined action limits and investigation procedures when performance metrics approach or exceed established thresholds, allowing for proactive column replacement before product quality is compromised.
The regulatory landscape continues to evolve, with increasing emphasis on risk-based approaches to column reuse validation. Recent guidance documents suggest that validation efforts should be proportional to the risk level associated with specific column applications, with higher-risk applications (such as final purification steps) requiring more extensive validation than lower-risk applications.
The regulatory framework for column reuse primarily focuses on demonstrating that the regeneration process consistently yields columns that perform within established specifications. ICH Q7 guidelines specifically address equipment cleaning validation, requiring manufacturers to establish scientifically sound acceptance criteria for residual contaminants and demonstrate that cleaning procedures consistently meet these criteria.
FDA's Process Validation Guidance emphasizes a lifecycle approach that applies directly to column reuse protocols. Manufacturers must validate that columns maintain consistent performance characteristics throughout multiple use cycles, with particular attention to potential leachables, carryover contamination, and ligand stability. This validation typically requires extensive documentation of cleaning effectiveness through at least three consecutive successful cleaning cycles.
EMA guidelines further stipulate that column reuse validation must include worst-case scenario testing, where columns are deliberately challenged with high product loads or difficult-to-clean substances before undergoing standard cleaning protocols. This approach helps establish the robustness of cleaning procedures under challenging conditions.
Regulatory bodies also require comprehensive change control procedures for any modifications to established column cleaning or regeneration protocols. These changes must be supported by appropriate risk assessments and validation data demonstrating that column performance remains within acceptable parameters following the modified procedures.
Documentation requirements represent another significant regulatory consideration. Manufacturers must maintain detailed records of each column's usage history, including products processed, cleaning cycles completed, storage conditions, and quality control test results. This documentation serves as evidence of compliance during regulatory inspections and is essential for investigating any product quality deviations.
Regulatory agencies increasingly expect manufacturers to implement continuous monitoring programs that track column performance trends over time. These programs should include defined action limits and investigation procedures when performance metrics approach or exceed established thresholds, allowing for proactive column replacement before product quality is compromised.
The regulatory landscape continues to evolve, with increasing emphasis on risk-based approaches to column reuse validation. Recent guidance documents suggest that validation efforts should be proportional to the risk level associated with specific column applications, with higher-risk applications (such as final purification steps) requiring more extensive validation than lower-risk applications.
Economic Impact and Sustainability Benefits
The economic implications of effective column reuse and regeneration strategies in chromatography extend far beyond simple cost savings. Laboratories implementing robust column management protocols can achieve significant reductions in operational expenses, with potential savings of 30-50% on annual column budgets. This economic advantage stems primarily from extending column lifetimes from typical single-use scenarios to multiple applications spanning months or even years of service.
Financial analysis reveals that high-performance columns, often costing between $500-$2,500 each, represent a substantial capital investment for analytical laboratories. By implementing systematic cleaning protocols and regeneration procedures, organizations can amortize this investment across hundreds of sample analyses, dramatically reducing the cost per analysis. Case studies from pharmaceutical quality control laboratories demonstrate that proper column maintenance can decrease column-related expenses by up to 65% over a three-year operational period.
The sustainability benefits of column reuse strategies align perfectly with growing corporate environmental responsibility initiatives. Each regenerated column represents a reduction in manufacturing resource consumption, packaging materials, and transportation-related carbon emissions. Quantitative environmental impact assessments indicate that extending column life by just three times can reduce the carbon footprint associated with chromatographic analysis by approximately 40-60%.
Waste reduction constitutes another significant sustainability advantage. Chromatography columns contain specialized silica materials, polymeric components, and in some cases, precious metals that present disposal challenges. Effective regeneration protocols minimize hazardous waste generation, reducing disposal costs and environmental impact. Organizations implementing comprehensive column reuse programs report hazardous waste reductions of 25-35% from their analytical departments.
The economic and sustainability benefits extend to operational efficiency as well. Laboratories with established column regeneration workflows experience fewer delays related to column replacement and equilibration, resulting in improved analytical throughput. This operational continuity translates to enhanced productivity, with some facilities reporting 15-20% increases in sample processing capacity without additional capital investment.
As regulatory and market pressures for sustainable laboratory practices intensify, column reuse strategies represent a compelling opportunity to simultaneously reduce costs, enhance environmental performance, and improve operational resilience. The return on investment for implementing comprehensive column management systems typically materializes within 6-12 months, making this approach both economically and environmentally advantageous.
Financial analysis reveals that high-performance columns, often costing between $500-$2,500 each, represent a substantial capital investment for analytical laboratories. By implementing systematic cleaning protocols and regeneration procedures, organizations can amortize this investment across hundreds of sample analyses, dramatically reducing the cost per analysis. Case studies from pharmaceutical quality control laboratories demonstrate that proper column maintenance can decrease column-related expenses by up to 65% over a three-year operational period.
The sustainability benefits of column reuse strategies align perfectly with growing corporate environmental responsibility initiatives. Each regenerated column represents a reduction in manufacturing resource consumption, packaging materials, and transportation-related carbon emissions. Quantitative environmental impact assessments indicate that extending column life by just three times can reduce the carbon footprint associated with chromatographic analysis by approximately 40-60%.
Waste reduction constitutes another significant sustainability advantage. Chromatography columns contain specialized silica materials, polymeric components, and in some cases, precious metals that present disposal challenges. Effective regeneration protocols minimize hazardous waste generation, reducing disposal costs and environmental impact. Organizations implementing comprehensive column reuse programs report hazardous waste reductions of 25-35% from their analytical departments.
The economic and sustainability benefits extend to operational efficiency as well. Laboratories with established column regeneration workflows experience fewer delays related to column replacement and equilibration, resulting in improved analytical throughput. This operational continuity translates to enhanced productivity, with some facilities reporting 15-20% increases in sample processing capacity without additional capital investment.
As regulatory and market pressures for sustainable laboratory practices intensify, column reuse strategies represent a compelling opportunity to simultaneously reduce costs, enhance environmental performance, and improve operational resilience. The return on investment for implementing comprehensive column management systems typically materializes within 6-12 months, making this approach both economically and environmentally advantageous.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!