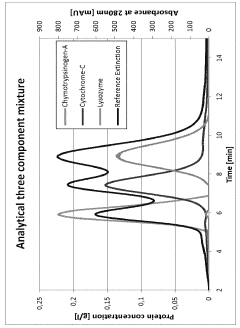
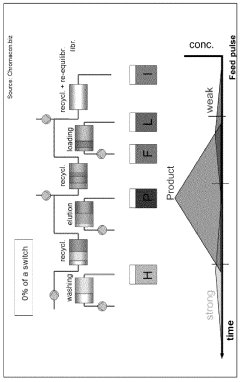
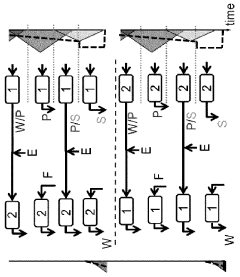
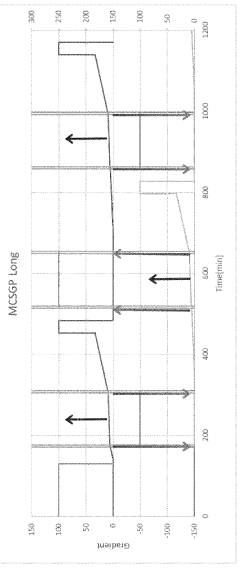
How to Design a Gradient Program to Separate Closely Eluting Impurities — Step-by-Step Example
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Background and Separation Goals
Chromatography has evolved significantly since its inception in the early 20th century, transforming from a simple separation technique into a sophisticated analytical methodology essential across pharmaceutical, environmental, and food industries. High-Performance Liquid Chromatography (HPLC) emerged in the 1970s as a revolutionary advancement, offering superior resolution and efficiency compared to traditional column chromatography. The subsequent development of Ultra-High Performance Liquid Chromatography (UHPLC) in the early 2000s further enhanced separation capabilities with sub-2μm particles and pressures exceeding 15,000 psi.
The separation of closely eluting impurities represents one of the most challenging aspects in modern chromatographic analysis, particularly in pharmaceutical quality control where regulatory requirements demand identification and quantification of impurities at increasingly lower levels. Current industry standards, guided by ICH Q3A and Q3B guidelines, require detection and characterization of impurities at thresholds as low as 0.05% relative to the active pharmaceutical ingredient.
Gradient elution has become the methodology of choice for complex separations, offering significant advantages over isocratic methods when dealing with samples containing components with widely varying retention characteristics. The fundamental principle involves changing the mobile phase composition during the separation, typically increasing the stronger solvent proportion over time, which allows manipulation of analyte retention behavior in real-time.
The technical evolution trajectory points toward increasingly sophisticated gradient programming capabilities, with modern systems offering multi-step gradients, concave and convex profiles, and even computer-optimized gradient shapes. Recent innovations include multi-dimensional chromatography approaches that combine orthogonal separation mechanisms to resolve previously inseparable compounds.
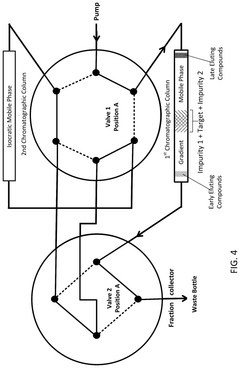
The primary technical goal in gradient development for closely eluting impurities is achieving baseline resolution (Rs ≥ 1.5) while maintaining reasonable analysis times and robust method performance. This requires systematic optimization of multiple parameters including initial and final mobile phase compositions, gradient slope, temperature, and pH conditions.
Secondary objectives typically include ensuring method transferability across different instruments and laboratories, minimizing solvent consumption through efficient gradient design, and developing methods capable of handling sample matrix variations without compromising separation quality. The ultimate aim is creating a "quality by design" approach where the method's design space is thoroughly understood, allowing for predictable performance across varying conditions.
Current technological trends indicate movement toward automated method development platforms that utilize algorithm-driven optimization strategies, machine learning for prediction of separation behavior, and in-silico modeling tools that can simulate chromatographic separations before laboratory implementation. These advancements promise to significantly reduce the time and resources required for developing robust gradient methods for challenging separations.
The separation of closely eluting impurities represents one of the most challenging aspects in modern chromatographic analysis, particularly in pharmaceutical quality control where regulatory requirements demand identification and quantification of impurities at increasingly lower levels. Current industry standards, guided by ICH Q3A and Q3B guidelines, require detection and characterization of impurities at thresholds as low as 0.05% relative to the active pharmaceutical ingredient.
Gradient elution has become the methodology of choice for complex separations, offering significant advantages over isocratic methods when dealing with samples containing components with widely varying retention characteristics. The fundamental principle involves changing the mobile phase composition during the separation, typically increasing the stronger solvent proportion over time, which allows manipulation of analyte retention behavior in real-time.
The technical evolution trajectory points toward increasingly sophisticated gradient programming capabilities, with modern systems offering multi-step gradients, concave and convex profiles, and even computer-optimized gradient shapes. Recent innovations include multi-dimensional chromatography approaches that combine orthogonal separation mechanisms to resolve previously inseparable compounds.
The primary technical goal in gradient development for closely eluting impurities is achieving baseline resolution (Rs ≥ 1.5) while maintaining reasonable analysis times and robust method performance. This requires systematic optimization of multiple parameters including initial and final mobile phase compositions, gradient slope, temperature, and pH conditions.
Secondary objectives typically include ensuring method transferability across different instruments and laboratories, minimizing solvent consumption through efficient gradient design, and developing methods capable of handling sample matrix variations without compromising separation quality. The ultimate aim is creating a "quality by design" approach where the method's design space is thoroughly understood, allowing for predictable performance across varying conditions.
Current technological trends indicate movement toward automated method development platforms that utilize algorithm-driven optimization strategies, machine learning for prediction of separation behavior, and in-silico modeling tools that can simulate chromatographic separations before laboratory implementation. These advancements promise to significantly reduce the time and resources required for developing robust gradient methods for challenging separations.
Market Demand for High-Resolution Impurity Analysis
The pharmaceutical industry is experiencing a significant surge in demand for high-resolution impurity analysis techniques, driven primarily by increasingly stringent regulatory requirements. The FDA, EMA, and other global regulatory bodies have established progressively lower thresholds for impurity identification and quantification, requiring pharmaceutical manufacturers to detect and characterize impurities at levels as low as 0.05% of the active pharmaceutical ingredient (API).
This regulatory landscape has created a substantial market for advanced chromatographic methods capable of separating closely eluting impurities. The global pharmaceutical analytical testing outsourcing market, which includes impurity analysis services, was valued at $6.1 billion in 2022 and is projected to grow at a CAGR of 8.4% through 2030, according to Grand View Research.
Particularly notable is the demand from generic drug manufacturers, who face significant challenges in demonstrating bioequivalence and impurity profiles comparable to reference products. These companies require robust analytical methods that can reliably separate and identify structural analogs and process-related impurities with similar physicochemical properties to the API.
Biopharmaceutical companies represent another major market segment, with the complexity of biological products necessitating sophisticated impurity profiling techniques. The biologics market, valued at approximately $366 billion in 2022, demands highly specialized analytical approaches for detecting and characterizing product-related impurities and variants.
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) have responded to this market demand by expanding their analytical capabilities, with many investing in advanced HPLC and UHPLC systems specifically optimized for gradient elution techniques that can resolve closely eluting compounds.
The market is further driven by the pharmaceutical industry's focus on quality by design (QbD) principles, which emphasize thorough understanding of product and process variables, including impurity profiles. This approach requires robust analytical methods capable of detecting and separating even trace impurities throughout the drug development lifecycle.
Geographically, North America dominates the market for high-resolution impurity analysis, followed by Europe and Asia-Pacific. However, the fastest growth is observed in emerging markets, particularly India and China, where expanding pharmaceutical manufacturing capabilities and increasing regulatory scrutiny are creating substantial demand for advanced analytical techniques.
This regulatory landscape has created a substantial market for advanced chromatographic methods capable of separating closely eluting impurities. The global pharmaceutical analytical testing outsourcing market, which includes impurity analysis services, was valued at $6.1 billion in 2022 and is projected to grow at a CAGR of 8.4% through 2030, according to Grand View Research.
Particularly notable is the demand from generic drug manufacturers, who face significant challenges in demonstrating bioequivalence and impurity profiles comparable to reference products. These companies require robust analytical methods that can reliably separate and identify structural analogs and process-related impurities with similar physicochemical properties to the API.
Biopharmaceutical companies represent another major market segment, with the complexity of biological products necessitating sophisticated impurity profiling techniques. The biologics market, valued at approximately $366 billion in 2022, demands highly specialized analytical approaches for detecting and characterizing product-related impurities and variants.
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) have responded to this market demand by expanding their analytical capabilities, with many investing in advanced HPLC and UHPLC systems specifically optimized for gradient elution techniques that can resolve closely eluting compounds.
The market is further driven by the pharmaceutical industry's focus on quality by design (QbD) principles, which emphasize thorough understanding of product and process variables, including impurity profiles. This approach requires robust analytical methods capable of detecting and separating even trace impurities throughout the drug development lifecycle.
Geographically, North America dominates the market for high-resolution impurity analysis, followed by Europe and Asia-Pacific. However, the fastest growth is observed in emerging markets, particularly India and China, where expanding pharmaceutical manufacturing capabilities and increasing regulatory scrutiny are creating substantial demand for advanced analytical techniques.
Current Challenges in Closely Eluting Impurities Separation
The separation of closely eluting impurities presents significant challenges in modern analytical chemistry, particularly in pharmaceutical quality control and development. Current high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC) methods often struggle to achieve adequate resolution between structurally similar compounds that exhibit nearly identical retention behaviors.
One of the primary technical obstacles is the inherent physicochemical similarity between target analytes and their related impurities. These compounds frequently share comparable functional groups, molecular weights, and polarity profiles, resulting in minimal differences in their interaction with stationary phases. This similarity creates narrow elution windows where multiple compounds co-elute, making accurate quantification and identification extremely difficult.
Traditional isocratic methods have proven largely inadequate for these complex separation challenges. Even when using highly specialized columns with enhanced selectivity, isocratic conditions often fail to provide sufficient resolution for closely eluting peaks. This limitation has driven the industry toward more sophisticated gradient approaches, which themselves introduce additional variables and complexity to method development.
The optimization of gradient parameters represents another significant hurdle. Parameters including initial and final mobile phase composition, gradient slope, temperature, pH, and buffer concentration must be precisely controlled and systematically optimized. Small variations in any of these parameters can dramatically impact separation efficiency, creating reproducibility challenges across different instruments and laboratories.
Column technology limitations further compound these difficulties. While modern stationary phases offer improved selectivity, many still struggle with closely eluting compounds that differ only slightly in their chemical structure. The trade-off between resolution and analysis time remains a persistent challenge, particularly in high-throughput environments where rapid methods are essential.
Method robustness and transferability issues also plague current separation techniques. Methods that successfully separate closely eluting impurities in development laboratories often fail when transferred to quality control environments or different geographical locations due to subtle variations in instrumentation, reagent quality, or environmental conditions.
Detection sensitivity presents additional complications, especially when impurities are present at trace levels. As separation efficiency improves through gradient optimization, peak dilution can occur, potentially pushing impurity signals below detection limits. This necessitates a careful balance between chromatographic resolution and detection capability.
Regulatory requirements add another layer of complexity, with agencies demanding increasingly stringent separation of all potential impurities, including those that may co-elute under standard conditions. Meeting these requirements while maintaining practical, efficient analytical methods represents a significant ongoing challenge for analytical scientists across multiple industries.
One of the primary technical obstacles is the inherent physicochemical similarity between target analytes and their related impurities. These compounds frequently share comparable functional groups, molecular weights, and polarity profiles, resulting in minimal differences in their interaction with stationary phases. This similarity creates narrow elution windows where multiple compounds co-elute, making accurate quantification and identification extremely difficult.
Traditional isocratic methods have proven largely inadequate for these complex separation challenges. Even when using highly specialized columns with enhanced selectivity, isocratic conditions often fail to provide sufficient resolution for closely eluting peaks. This limitation has driven the industry toward more sophisticated gradient approaches, which themselves introduce additional variables and complexity to method development.
The optimization of gradient parameters represents another significant hurdle. Parameters including initial and final mobile phase composition, gradient slope, temperature, pH, and buffer concentration must be precisely controlled and systematically optimized. Small variations in any of these parameters can dramatically impact separation efficiency, creating reproducibility challenges across different instruments and laboratories.
Column technology limitations further compound these difficulties. While modern stationary phases offer improved selectivity, many still struggle with closely eluting compounds that differ only slightly in their chemical structure. The trade-off between resolution and analysis time remains a persistent challenge, particularly in high-throughput environments where rapid methods are essential.
Method robustness and transferability issues also plague current separation techniques. Methods that successfully separate closely eluting impurities in development laboratories often fail when transferred to quality control environments or different geographical locations due to subtle variations in instrumentation, reagent quality, or environmental conditions.
Detection sensitivity presents additional complications, especially when impurities are present at trace levels. As separation efficiency improves through gradient optimization, peak dilution can occur, potentially pushing impurity signals below detection limits. This necessitates a careful balance between chromatographic resolution and detection capability.
Regulatory requirements add another layer of complexity, with agencies demanding increasingly stringent separation of all potential impurities, including those that may co-elute under standard conditions. Meeting these requirements while maintaining practical, efficient analytical methods represents a significant ongoing challenge for analytical scientists across multiple industries.
Current Gradient Program Design Methodologies
01 Multi-step gradient elution programs
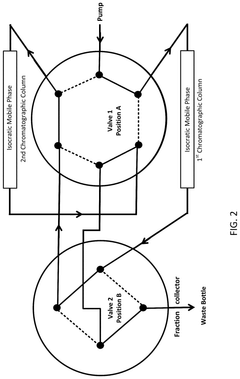
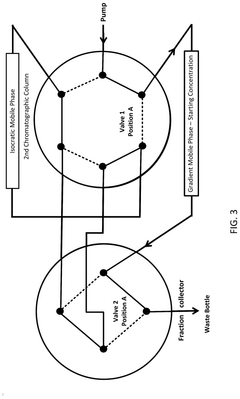
Multi-step gradient elution programs involve carefully designed changes in mobile phase composition over time to enhance separation of closely eluting impurities. These programs typically include sequential increases or decreases in organic solvent concentration at specific time intervals, allowing for improved resolution of compounds with similar retention characteristics. The gradient can be optimized by adjusting parameters such as slope, hold times at specific compositions, and transition rates between different mobile phase compositions.- Multi-step gradient elution programs: Multi-step gradient elution programs involve carefully designed changes in mobile phase composition over time to enhance separation of closely eluting impurities. These programs typically include sequential increases or decreases in the concentration of organic solvents or buffers at predetermined time intervals. By optimizing the gradient slope and step duration, chromatographers can achieve improved resolution between compounds with similar retention characteristics, allowing for better identification and quantification of impurities.
- Temperature-controlled gradient chromatography: Temperature control during gradient elution provides an additional parameter for optimizing the separation of closely eluting impurities. By precisely manipulating column temperature alongside solvent composition changes, chromatographers can exploit differences in thermodynamic properties between similar compounds. Temperature programming can be particularly effective for separating structurally related impurities that respond differently to thermal conditions, enhancing resolution in complex samples where traditional gradient approaches alone are insufficient.
- pH-modified gradient systems: pH-modified gradient systems utilize controlled changes in mobile phase pH alongside solvent composition changes to separate closely eluting impurities. This approach is particularly effective for ionizable compounds whose retention behavior is pH-dependent. By incorporating buffer systems with specific pH ranges and implementing gradients that modify both solvent strength and pH, chromatographers can achieve separation of impurities that would co-elute under constant pH conditions, significantly improving resolution of structurally similar compounds.
- Specialized stationary phases for gradient separation: Specialized stationary phases designed specifically for gradient elution can dramatically improve separation of closely eluting impurities. These phases feature modified surface chemistries, particle technologies, and pore structures that maintain consistent performance across changing mobile phase conditions. Some incorporate mixed-mode retention mechanisms or unique selectivity characteristics that can resolve structurally similar compounds even under challenging gradient conditions, providing enhanced separation power for complex pharmaceutical and biological samples.
- Advanced gradient optimization algorithms: Advanced computational methods and algorithms enable systematic optimization of gradient programs for separating closely eluting impurities. These approaches use mathematical modeling, machine learning, and statistical design of experiments to predict optimal gradient conditions. By analyzing multiple parameters simultaneously and simulating chromatographic behavior, these tools can identify ideal gradient profiles that maximize resolution between critical pairs of compounds while minimizing analysis time. This data-driven approach significantly reduces method development time and improves separation quality.
02 Temperature-controlled gradient chromatography
Temperature control during gradient elution provides an additional parameter for improving separation of closely eluting impurities. By precisely controlling column temperature or implementing temperature gradients alongside solvent gradients, chromatographers can enhance selectivity for compounds with similar chemical properties. Temperature adjustments can alter the interaction between analytes and stationary phase, changing elution patterns and improving resolution of critical pairs that are difficult to separate using solvent gradients alone.Expand Specific Solutions03 Specialized stationary phases for complex separations
Selection of specialized stationary phases combined with optimized gradient programs significantly improves separation of closely eluting impurities. Novel stationary phases with unique selectivity characteristics, such as mixed-mode phases, chiral columns, or those with specific functional groups, can provide enhanced resolution when conventional columns fail. These specialized phases, when used with carefully designed gradient programs, can resolve complex mixtures by exploiting subtle differences in analyte-stationary phase interactions.Expand Specific Solutions04 pH and buffer modulation in gradient programs
Strategic modulation of pH and buffer composition throughout the gradient program enhances separation of structurally similar compounds. By incorporating pH changes or buffer concentration gradients alongside solvent gradients, chromatographers can manipulate the ionization state of analytes during separation. This approach is particularly effective for compounds whose retention is highly influenced by their ionization state, allowing for resolution of impurities that co-elute under conventional gradient conditions.Expand Specific Solutions05 Advanced gradient optimization algorithms
Computer-assisted optimization algorithms help develop highly effective gradient programs for separating closely eluting impurities. These algorithms use experimental data to model chromatographic behavior and predict optimal gradient conditions, including segment durations, solvent composition changes, and flow rates. Machine learning approaches can analyze multiple parameters simultaneously to identify the most efficient gradient program for specific separation challenges, reducing method development time and improving resolution of difficult-to-separate compounds.Expand Specific Solutions
Leading Manufacturers and Research Groups in Chromatography
The gradient program design for separating closely eluting impurities is currently in a growth phase, with the global chromatography market expanding at approximately 8-9% annually. This technical challenge exists within a mature analytical chemistry sector valued at over $10 billion. From a technological maturity perspective, companies demonstrate varying capabilities: Serum Institute of India and Industrial Technology Research Institute lead with advanced separation technologies, while pharmaceutical players like Hybio Pharmaceutical and Astex Therapeutics focus on application-specific implementations. Applied Materials and Lam Research contribute hardware innovations, while academic institutions such as Shandong University of Technology and Institute of Process Engineering (CAS) drive fundamental research. The field is evolving toward automated, AI-assisted gradient optimization methods that enhance separation efficiency while reducing development time.
Serum Institute of India Pvt Ltd.
Technical Solution: Serum Institute has developed a comprehensive gradient elution HPLC methodology for separating closely eluting impurities in vaccine and biopharmaceutical production. Their approach utilizes a multi-stage gradient program with precise pH control and buffer composition adjustments. The method begins with an initial shallow gradient (0.5% change in organic modifier per minute) to maximize resolution in critical separation regions, followed by steeper gradients to reduce overall analysis time. They employ specialized C18 columns with sub-2μm particle size and controlled temperature environments (typically 30-35°C) to enhance selectivity. Their system incorporates real-time UV detection at multiple wavelengths (typically 214nm and 280nm) to identify co-eluting compounds with different spectral properties. For particularly challenging separations, they implement a "sandwich injection" technique where samples are bracketed between small volumes of specialized solvents to improve peak shape and resolution.
Strengths: Exceptional resolution of closely related protein impurities; validated for regulatory compliance; highly reproducible with RSD <1% for retention times. Weaknesses: Requires sophisticated equipment; method development is time-intensive; may require specialized columns that increase operational costs.
Hybio Pharmaceutical Co., Ltd.
Technical Solution: Hybio Pharmaceutical has engineered a proprietary gradient elution system specifically for peptide and protein therapeutics that employs a multi-dimensional approach to separate structurally similar impurities. Their technology combines traditional reversed-phase HPLC with ion-exchange mechanisms in a single method. The gradient program features a segmented approach with three distinct phases: an initial isocratic hold (2-3 minutes) to allow sample focusing, followed by a shallow gradient segment (0.2-0.5% change in acetonitrile per minute) where critical separations occur, and finally a steeper gradient to elute strongly retained compounds. Their method incorporates temperature programming alongside solvent gradients, with precise temperature control (±0.1°C) that changes during the separation to exploit thermodynamic selectivity differences between target molecules and impurities. The company has also developed specialized mobile phase additives that enhance selectivity for specific structural differences, such as deamidated variants or oxidized forms of peptides.
Strengths: Exceptional selectivity for peptide-related impurities; highly adaptable to different peptide families; compatible with mass spectrometry detection. Weaknesses: Complex method development requires specialized expertise; system requires precise temperature control hardware; method transfer between laboratories can be challenging.
Critical Parameters for Optimizing Impurity Separation
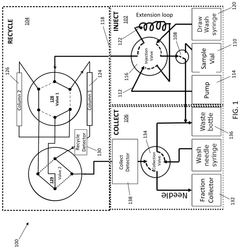
Automated semi-preparative gradient recycling liquid chromatography
PatentActiveUS12078621B2
Innovation
- The implementation of a twin column recycling liquid chromatography (TCRLC) system that uses a combination of gradient and isocratic mobile phases to separate early and late eluting impurities from the analyte band, allowing for the recycling of the analyte band between two chromatographic columns until baseline resolution is achieved, thereby overcoming the limitations of standard preparative chromatography.
Continuous gradient elution chromatographic fractionation
PatentWO2024056626A1
Innovation
- A continuous gradient elution chromatographic fractionation method that involves loading a feed solution onto a chromatography matrix, contacting it with an elution solution, collecting specific elution fractions, and reloading them onto the same or different matrices, allowing for repeated cycles with alternating chromatography matrices and controlled elution solution concentration to enhance product separation and purity.
Method Validation and Regulatory Compliance
Method validation is a critical component in the development of gradient programs for separating closely eluting impurities in pharmaceutical analysis. Regulatory bodies such as the FDA, EMA, and ICH have established stringent guidelines that must be adhered to when developing and validating analytical methods. For gradient elution techniques specifically, validation parameters including specificity, linearity, accuracy, precision, robustness, and system suitability must be thoroughly assessed and documented.
The ICH Q2(R1) guideline provides the framework for method validation, requiring demonstration that the gradient method can reliably separate and quantify target compounds from closely eluting impurities. When developing a gradient program for closely eluting impurities, validation protocols must verify that the method maintains resolution throughout the expected range of operating conditions and sample concentrations.
Specificity testing is particularly crucial for gradient methods separating closely eluting impurities, as it confirms the method's ability to unequivocally assess each analyte in the presence of components that may be expected to be present. This includes testing with forced degradation samples to ensure that degradation products do not co-elute with compounds of interest.
Robustness studies for gradient methods require additional considerations compared to isocratic methods. Parameters such as gradient slope, initial and final mobile phase compositions, column temperature fluctuations, and flow rate variations must be systematically evaluated to ensure consistent separation of closely eluting impurities under slightly different conditions.
System suitability tests (SSTs) serve as an ongoing verification that the gradient method performs as intended during routine use. For methods targeting closely eluting impurities, critical SST parameters include resolution between critical pairs, tailing factors, theoretical plates, and retention time reproducibility. These parameters should be established during validation and monitored during routine analysis.
Regulatory submissions require comprehensive documentation of the gradient method development process, including the scientific rationale for gradient profile selection, optimization experiments, and validation results. Authorities expect to see evidence that the method has been challenged appropriately to demonstrate its fitness for purpose in separating and quantifying closely eluting impurities.
Transfer of validated gradient methods between laboratories requires additional verification to ensure that the separation of closely eluting impurities is maintained across different instruments and laboratory environments. This often involves collaborative testing and statistical evaluation of results to confirm method reproducibility.
The ICH Q2(R1) guideline provides the framework for method validation, requiring demonstration that the gradient method can reliably separate and quantify target compounds from closely eluting impurities. When developing a gradient program for closely eluting impurities, validation protocols must verify that the method maintains resolution throughout the expected range of operating conditions and sample concentrations.
Specificity testing is particularly crucial for gradient methods separating closely eluting impurities, as it confirms the method's ability to unequivocally assess each analyte in the presence of components that may be expected to be present. This includes testing with forced degradation samples to ensure that degradation products do not co-elute with compounds of interest.
Robustness studies for gradient methods require additional considerations compared to isocratic methods. Parameters such as gradient slope, initial and final mobile phase compositions, column temperature fluctuations, and flow rate variations must be systematically evaluated to ensure consistent separation of closely eluting impurities under slightly different conditions.
System suitability tests (SSTs) serve as an ongoing verification that the gradient method performs as intended during routine use. For methods targeting closely eluting impurities, critical SST parameters include resolution between critical pairs, tailing factors, theoretical plates, and retention time reproducibility. These parameters should be established during validation and monitored during routine analysis.
Regulatory submissions require comprehensive documentation of the gradient method development process, including the scientific rationale for gradient profile selection, optimization experiments, and validation results. Authorities expect to see evidence that the method has been challenged appropriately to demonstrate its fitness for purpose in separating and quantifying closely eluting impurities.
Transfer of validated gradient methods between laboratories requires additional verification to ensure that the separation of closely eluting impurities is maintained across different instruments and laboratory environments. This often involves collaborative testing and statistical evaluation of results to confirm method reproducibility.
Automation and AI in Gradient Program Optimization
The integration of automation and artificial intelligence into gradient program optimization represents a significant advancement in chromatographic separation techniques. Traditional manual optimization of gradient programs for separating closely eluting impurities is time-consuming and often relies heavily on analyst expertise. Modern AI-driven approaches are transforming this process by reducing development time while improving separation quality.
Machine learning algorithms now enable automated prediction of optimal gradient conditions based on molecular structures and physicochemical properties of target compounds. These systems can analyze vast datasets of previous separations to identify patterns that human analysts might miss, leading to more efficient method development workflows.
Recent developments in automated chromatography systems incorporate real-time decision-making capabilities. These systems can dynamically adjust gradient parameters during method development runs, responding to separation performance metrics without human intervention. This adaptive optimization approach has demonstrated particular effectiveness when dealing with complex samples containing multiple closely eluting impurities.
Deep learning models trained on comprehensive chromatographic datasets can now predict retention behavior under various gradient conditions with remarkable accuracy. These models consider multiple variables simultaneously, including mobile phase composition, temperature, pH, and column characteristics, to suggest optimal separation parameters that might require dozens of manual experiments to discover.
Cloud-based platforms for chromatographic method development have emerged, allowing for distributed computing resources to tackle complex optimization problems. These systems can perform thousands of in silico experiments to identify the most promising gradient conditions before any laboratory work begins, significantly reducing solvent consumption and instrument time.
The implementation of digital twins for chromatographic systems enables virtual testing of gradient programs. Analysts can simulate separation outcomes for closely eluting impurities under various conditions, visualizing predicted chromatograms and resolution metrics before physical experimentation. This capability dramatically accelerates the method development process while reducing resource requirements.
Autonomous laboratory systems that combine robotics with AI decision-making represent the cutting edge in this field. These systems can independently design experiments, prepare samples, execute runs, analyze results, and iteratively refine gradient programs without human supervision. Early implementations have demonstrated the ability to develop optimized methods for separating challenging impurity profiles in a fraction of the time required by traditional approaches.
Machine learning algorithms now enable automated prediction of optimal gradient conditions based on molecular structures and physicochemical properties of target compounds. These systems can analyze vast datasets of previous separations to identify patterns that human analysts might miss, leading to more efficient method development workflows.
Recent developments in automated chromatography systems incorporate real-time decision-making capabilities. These systems can dynamically adjust gradient parameters during method development runs, responding to separation performance metrics without human intervention. This adaptive optimization approach has demonstrated particular effectiveness when dealing with complex samples containing multiple closely eluting impurities.
Deep learning models trained on comprehensive chromatographic datasets can now predict retention behavior under various gradient conditions with remarkable accuracy. These models consider multiple variables simultaneously, including mobile phase composition, temperature, pH, and column characteristics, to suggest optimal separation parameters that might require dozens of manual experiments to discover.
Cloud-based platforms for chromatographic method development have emerged, allowing for distributed computing resources to tackle complex optimization problems. These systems can perform thousands of in silico experiments to identify the most promising gradient conditions before any laboratory work begins, significantly reducing solvent consumption and instrument time.
The implementation of digital twins for chromatographic systems enables virtual testing of gradient programs. Analysts can simulate separation outcomes for closely eluting impurities under various conditions, visualizing predicted chromatograms and resolution metrics before physical experimentation. This capability dramatically accelerates the method development process while reducing resource requirements.
Autonomous laboratory systems that combine robotics with AI decision-making represent the cutting edge in this field. These systems can independently design experiments, prepare samples, execute runs, analyze results, and iteratively refine gradient programs without human supervision. Early implementations have demonstrated the ability to develop optimized methods for separating challenging impurity profiles in a fraction of the time required by traditional approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!