Column Chromatography Detection Options: UV, ELSD, MS — When to Use Which and Setup Notes
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Detection Evolution and Objectives
Column chromatography detection has evolved significantly since its inception in the early 20th century. Initially limited to visual observation of colored compounds, the field has progressed through several technological revolutions that have dramatically enhanced sensitivity, selectivity, and applicability across scientific disciplines.
The 1950s marked the introduction of UV detection, representing the first major instrumental advancement in chromatographic detection. This technology enabled scientists to detect compounds based on their absorption of ultraviolet light, significantly expanding the range of detectable analytes beyond visibly colored substances.
The 1970s witnessed the emergence of Evaporative Light Scattering Detection (ELSD), addressing a critical limitation of UV detection by enabling the analysis of compounds lacking chromophores. This development was particularly valuable for lipid research, carbohydrate analysis, and pharmaceutical development where many target compounds are UV-transparent.
The 1980s and 1990s brought mass spectrometry (MS) into mainstream chromatographic detection, revolutionizing the field with unprecedented sensitivity and structural information capabilities. The coupling of chromatography with MS represented a paradigm shift, allowing for both quantification and identification of compounds in complex mixtures.
Recent decades have seen remarkable refinements in all three technologies. UV detectors have evolved from single-wavelength to diode array systems capable of collecting full spectra in real-time. ELSD has improved in sensitivity and reproducibility through advanced nebulization and evaporation technologies. MS has undergone perhaps the most dramatic transformation, with innovations in ionization techniques (ESI, APCI, MALDI) and mass analyzers (quadrupole, time-of-flight, orbitrap) expanding its application across virtually all scientific disciplines.
The primary objective of modern chromatographic detection is to achieve optimal analytical performance characterized by high sensitivity, selectivity, reproducibility, and broad applicability. Additional goals include increased throughput, reduced sample requirements, enhanced robustness, and improved integration with data processing systems.
Future development aims to address remaining challenges, including improved detection of isomeric compounds, enhanced compatibility with green chemistry principles, miniaturization for point-of-care applications, and seamless integration with artificial intelligence for automated method development and data interpretation. The field continues to evolve toward more comprehensive, sensitive, and accessible analytical solutions across pharmaceutical, environmental, clinical, and industrial applications.
The 1950s marked the introduction of UV detection, representing the first major instrumental advancement in chromatographic detection. This technology enabled scientists to detect compounds based on their absorption of ultraviolet light, significantly expanding the range of detectable analytes beyond visibly colored substances.
The 1970s witnessed the emergence of Evaporative Light Scattering Detection (ELSD), addressing a critical limitation of UV detection by enabling the analysis of compounds lacking chromophores. This development was particularly valuable for lipid research, carbohydrate analysis, and pharmaceutical development where many target compounds are UV-transparent.
The 1980s and 1990s brought mass spectrometry (MS) into mainstream chromatographic detection, revolutionizing the field with unprecedented sensitivity and structural information capabilities. The coupling of chromatography with MS represented a paradigm shift, allowing for both quantification and identification of compounds in complex mixtures.
Recent decades have seen remarkable refinements in all three technologies. UV detectors have evolved from single-wavelength to diode array systems capable of collecting full spectra in real-time. ELSD has improved in sensitivity and reproducibility through advanced nebulization and evaporation technologies. MS has undergone perhaps the most dramatic transformation, with innovations in ionization techniques (ESI, APCI, MALDI) and mass analyzers (quadrupole, time-of-flight, orbitrap) expanding its application across virtually all scientific disciplines.
The primary objective of modern chromatographic detection is to achieve optimal analytical performance characterized by high sensitivity, selectivity, reproducibility, and broad applicability. Additional goals include increased throughput, reduced sample requirements, enhanced robustness, and improved integration with data processing systems.
Future development aims to address remaining challenges, including improved detection of isomeric compounds, enhanced compatibility with green chemistry principles, miniaturization for point-of-care applications, and seamless integration with artificial intelligence for automated method development and data interpretation. The field continues to evolve toward more comprehensive, sensitive, and accessible analytical solutions across pharmaceutical, environmental, clinical, and industrial applications.
Market Applications and Analytical Demands
The chromatography detection market is experiencing robust growth driven by increasing demands across pharmaceutical, biotechnology, food and beverage, environmental, and clinical sectors. The global chromatography instrumentation market is currently valued at over 10 billion USD, with detection technologies representing a significant portion of this value. Annual growth rates in this segment consistently exceed 5%, reflecting the expanding applications and technological advancements.
Pharmaceutical and biopharmaceutical industries remain the dominant consumers of chromatography detection technologies, accounting for approximately 60% of the market share. These sectors require increasingly sensitive and selective detection methods for drug development, quality control, and regulatory compliance. The rise of biologics and biosimilars has particularly accelerated demand for mass spectrometry detection due to its unparalleled capabilities in protein characterization.
Food safety and environmental monitoring represent rapidly growing application areas, with regulatory agencies worldwide implementing stricter testing requirements. These applications often demand multi-detection approaches, combining UV for routine screening with MS for confirmation of results, driving the market for integrated detection systems.
Clinical diagnostics represents another expanding market segment, where LC-MS systems are increasingly replacing traditional immunoassays for certain biomarker analyses due to superior specificity and sensitivity. This transition is creating new opportunities for manufacturers of specialized detection systems optimized for clinical workflows.
The analytical demands across these markets share common themes but with varying priorities. Pharmaceutical applications prioritize reproducibility, compliance with regulatory standards, and complete characterization capabilities. Environmental and food testing emphasize high-throughput screening capabilities with minimal false negatives. Clinical applications demand robust, user-friendly systems with rapid turnaround times.
Sensitivity requirements continue to increase across all sectors, with detection limits in the picogram to femtogram range now expected for many applications. This trend has accelerated the adoption of mass spectrometry, particularly triple quadrupole and high-resolution accurate mass systems, despite their higher cost compared to optical detection methods.
Cost-effectiveness remains a critical consideration, particularly for routine quality control applications where UV detection continues to dominate due to its reliability and lower operational costs. However, the growing need for universal detection capabilities is driving increased adoption of ELSD and CAD technologies as complementary or alternative approaches to UV detection for compounds lacking chromophores.
Data integration capabilities have emerged as a key market differentiator, with users increasingly demanding seamless integration between different detection technologies and laboratory information management systems. This trend is pushing manufacturers to develop more sophisticated software platforms capable of processing and interpreting multi-detector data streams.
Pharmaceutical and biopharmaceutical industries remain the dominant consumers of chromatography detection technologies, accounting for approximately 60% of the market share. These sectors require increasingly sensitive and selective detection methods for drug development, quality control, and regulatory compliance. The rise of biologics and biosimilars has particularly accelerated demand for mass spectrometry detection due to its unparalleled capabilities in protein characterization.
Food safety and environmental monitoring represent rapidly growing application areas, with regulatory agencies worldwide implementing stricter testing requirements. These applications often demand multi-detection approaches, combining UV for routine screening with MS for confirmation of results, driving the market for integrated detection systems.
Clinical diagnostics represents another expanding market segment, where LC-MS systems are increasingly replacing traditional immunoassays for certain biomarker analyses due to superior specificity and sensitivity. This transition is creating new opportunities for manufacturers of specialized detection systems optimized for clinical workflows.
The analytical demands across these markets share common themes but with varying priorities. Pharmaceutical applications prioritize reproducibility, compliance with regulatory standards, and complete characterization capabilities. Environmental and food testing emphasize high-throughput screening capabilities with minimal false negatives. Clinical applications demand robust, user-friendly systems with rapid turnaround times.
Sensitivity requirements continue to increase across all sectors, with detection limits in the picogram to femtogram range now expected for many applications. This trend has accelerated the adoption of mass spectrometry, particularly triple quadrupole and high-resolution accurate mass systems, despite their higher cost compared to optical detection methods.
Cost-effectiveness remains a critical consideration, particularly for routine quality control applications where UV detection continues to dominate due to its reliability and lower operational costs. However, the growing need for universal detection capabilities is driving increased adoption of ELSD and CAD technologies as complementary or alternative approaches to UV detection for compounds lacking chromophores.
Data integration capabilities have emerged as a key market differentiator, with users increasingly demanding seamless integration between different detection technologies and laboratory information management systems. This trend is pushing manufacturers to develop more sophisticated software platforms capable of processing and interpreting multi-detector data streams.
Current Detection Technologies and Limitations
Column chromatography detection technologies have evolved significantly over the past decades, with three primary detection methods dominating the analytical landscape: Ultraviolet-Visible (UV) detection, Evaporative Light Scattering Detection (ELSD), and Mass Spectrometry (MS). Each technology presents distinct capabilities and limitations that influence their application in various analytical scenarios.
UV detection remains the most widely implemented technique due to its relative simplicity, cost-effectiveness, and reliability. Operating on the principle of light absorption by analytes containing chromophores, UV detectors offer excellent sensitivity for compounds with conjugated systems. However, they demonstrate significant limitations when analyzing compounds lacking UV-absorbing functional groups, such as carbohydrates, lipids, and many natural products.
ELSD has emerged as a valuable alternative for detecting non-volatile compounds regardless of their spectroscopic properties. This technology functions by nebulizing the column effluent, evaporating the mobile phase, and measuring light scattering from residual analyte particles. While ELSD provides a more universal detection capability than UV, it suffers from lower sensitivity, potential thermal degradation of heat-sensitive compounds, and non-linear response characteristics that complicate quantitative analysis.
Mass spectrometry represents the most sophisticated detection option, offering unparalleled sensitivity, selectivity, and structural information. MS detectors ionize analytes and separate them based on their mass-to-charge ratios, enabling both qualitative and quantitative analysis with exceptional precision. Despite these advantages, MS systems present significant challenges including high acquisition and maintenance costs, complex method development, and potential matrix effects that can suppress ionization.
The interface between chromatographic systems and detectors introduces additional technical challenges. UV detectors require minimal modification to the chromatographic flow, but cell path length optimization remains critical for balancing sensitivity against peak broadening. ELSD systems demand careful temperature control across nebulization, evaporation, and detection stages to ensure reproducible results.
MS coupling presents the most complex integration challenges, requiring specialized interfaces such as electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) to convert liquid-phase analytes to gas-phase ions. These interfaces must effectively manage the transition from high-pressure liquid chromatography to the vacuum environment of the mass analyzer while minimizing analyte loss and maintaining chromatographic resolution.
Current technological limitations also include detector response variability across different compound classes, challenges in simultaneous multi-detector implementation, and data integration complexities when combining outputs from complementary detection methods. These limitations drive ongoing research into hybrid detection systems and advanced data processing algorithms to maximize analytical information while minimizing operational complexity.
UV detection remains the most widely implemented technique due to its relative simplicity, cost-effectiveness, and reliability. Operating on the principle of light absorption by analytes containing chromophores, UV detectors offer excellent sensitivity for compounds with conjugated systems. However, they demonstrate significant limitations when analyzing compounds lacking UV-absorbing functional groups, such as carbohydrates, lipids, and many natural products.
ELSD has emerged as a valuable alternative for detecting non-volatile compounds regardless of their spectroscopic properties. This technology functions by nebulizing the column effluent, evaporating the mobile phase, and measuring light scattering from residual analyte particles. While ELSD provides a more universal detection capability than UV, it suffers from lower sensitivity, potential thermal degradation of heat-sensitive compounds, and non-linear response characteristics that complicate quantitative analysis.
Mass spectrometry represents the most sophisticated detection option, offering unparalleled sensitivity, selectivity, and structural information. MS detectors ionize analytes and separate them based on their mass-to-charge ratios, enabling both qualitative and quantitative analysis with exceptional precision. Despite these advantages, MS systems present significant challenges including high acquisition and maintenance costs, complex method development, and potential matrix effects that can suppress ionization.
The interface between chromatographic systems and detectors introduces additional technical challenges. UV detectors require minimal modification to the chromatographic flow, but cell path length optimization remains critical for balancing sensitivity against peak broadening. ELSD systems demand careful temperature control across nebulization, evaporation, and detection stages to ensure reproducible results.
MS coupling presents the most complex integration challenges, requiring specialized interfaces such as electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) to convert liquid-phase analytes to gas-phase ions. These interfaces must effectively manage the transition from high-pressure liquid chromatography to the vacuum environment of the mass analyzer while minimizing analyte loss and maintaining chromatographic resolution.
Current technological limitations also include detector response variability across different compound classes, challenges in simultaneous multi-detector implementation, and data integration complexities when combining outputs from complementary detection methods. These limitations drive ongoing research into hybrid detection systems and advanced data processing algorithms to maximize analytical information while minimizing operational complexity.
Comparative Analysis of UV, ELSD and MS Detection Methods
01 UV Detection Methods in Column Chromatography
UV detection is widely used in column chromatography for compounds with chromophores. It offers good sensitivity for aromatic and conjugated compounds, with detection limits typically in the nanogram range. UV detectors are relatively inexpensive, easy to operate, and provide linear response over a wide concentration range. However, they are limited to compounds that absorb UV light and may have reduced selectivity in complex matrices.- UV Detection Methods in Column Chromatography: Ultraviolet (UV) detection is widely used in column chromatography for compounds that absorb UV light. It offers good sensitivity for aromatic compounds and those with conjugated double bonds. UV detectors are relatively inexpensive, easy to operate, and provide reliable quantitative analysis. However, they have limitations for compounds lacking chromophores. Modern UV detectors often feature variable wavelength capabilities to optimize detection for specific analytes.
- Mass Spectrometry (MS) Detection in Chromatography: Mass spectrometry detection provides high sensitivity and selectivity in column chromatography by measuring the mass-to-charge ratio of ionized molecules. MS detectors can identify and quantify compounds based on their molecular weight and fragmentation patterns, making them valuable for complex mixture analysis. They offer structural information about analytes and can detect compounds at very low concentrations. MS is particularly useful for bioanalytical applications and metabolite identification studies.
- Evaporative Light Scattering Detection (ELSD) Applications: ELSD is particularly valuable for detecting compounds that lack chromophores and cannot be easily detected by UV methods. It works by nebulizing the column effluent, evaporating the mobile phase, and detecting the light scattered by analyte particles. ELSD offers near-universal detection capabilities and is especially useful for lipids, carbohydrates, and natural products analysis. It provides better response consistency across different compound classes compared to other universal detectors.
- Comparative Analysis of Detection Methods: Different detection methods offer varying levels of sensitivity, selectivity, and applicability in column chromatography. UV detection provides good sensitivity for compounds with chromophores but limited universality. MS offers excellent sensitivity and selectivity but at higher cost and complexity. ELSD provides good universality but moderate sensitivity. The choice of detection method depends on the analyte properties, required sensitivity, and specific application requirements. Combining multiple detection methods can provide complementary information for comprehensive analysis.
- Advanced Detection Technologies and Hyphenated Techniques: Recent advances in chromatography detection include the development of hyphenated techniques that combine multiple detection methods. These include LC-MS/MS, GC-MS, and LC-UV-MS systems that provide enhanced analytical capabilities. Other innovations include charged aerosol detection (CAD), multi-wavelength UV detection, and improved data processing algorithms. These technologies offer improved sensitivity, selectivity, and applicability across a wider range of analytes and can overcome limitations of individual detection methods.
02 ELSD (Evaporative Light Scattering Detection) Methods
ELSD is a universal detection method suitable for non-volatile compounds regardless of their optical properties. It offers moderate sensitivity (typically microgram range) and is particularly valuable for compounds lacking chromophores that cannot be detected by UV. ELSD provides consistent response factors across different compound classes, making quantification more straightforward. The technique involves nebulization, evaporation, and light scattering measurement, making it compatible with gradient elution methods.Expand Specific Solutions03 Mass Spectrometry (MS) Detection in Chromatography
MS detection provides exceptional sensitivity and selectivity for column chromatography applications. It offers structural information and can detect compounds at picogram to femtogram levels. Various ionization techniques (ESI, APCI, MALDI) expand its applicability to different compound classes. MS detection enables identification of unknown compounds and can distinguish between co-eluting substances with different mass-to-charge ratios. The high specificity makes it particularly valuable for complex biological samples and trace analysis.Expand Specific Solutions04 Comparative Analysis of Detection Methods
Different detection methods offer complementary advantages for column chromatography. UV detection provides good sensitivity for compounds with chromophores but lacks universality. ELSD offers universal detection for non-volatile compounds but with moderate sensitivity. MS provides the highest sensitivity and selectivity but at higher cost and complexity. The choice depends on the analyte properties, required sensitivity, and available resources. Combined detection approaches can provide comprehensive analysis by leveraging the strengths of multiple detection methods.Expand Specific Solutions05 Novel and Hybrid Detection Technologies
Recent advances in column chromatography detection include hybrid and novel detection technologies. These include coupling multiple detection methods in series (UV-ELSD-MS), developing new ionization techniques for MS, and creating specialized detectors for specific applications. Miniaturization and automation have improved detection sensitivity and throughput. Machine learning algorithms are increasingly applied to process complex detection data. These innovations expand the applicability of column chromatography to challenging analytes and complex matrices.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Column chromatography detection technologies are evolving rapidly, with the market currently in a growth phase characterized by increasing adoption across pharmaceutical, chemical, and biotechnology sectors. The global analytical instrumentation market for these technologies exceeds $5 billion, with UV detection representing the mature baseline technology, while ELSD and MS detection systems reflect the industry's technological advancement trajectory. Waters Technology, Shimadzu, and Micromass UK (Waters subsidiary) lead in MS detection innovation, offering high-sensitivity solutions for complex analytes. AstraZeneca, Bayer, and Boehringer Ingelheim drive application development through implementation in drug discovery workflows. The technology landscape shows varying maturity levels: UV detection is fully commercialized and standardized, ELSD occupies the mid-maturity range with growing applications, while MS detection continues rapid development with significant R&D investment from major instrument manufacturers.
AstraZeneca AB
Technical Solution: AstraZeneca has developed proprietary column chromatography detection methodologies optimized for pharmaceutical development workflows. Their approach integrates multiple detection technologies within unified analytical platforms tailored to drug discovery and development needs. For UV detection, AstraZeneca employs custom wavelength optimization strategies based on chromophore characteristics of target compounds, enhancing sensitivity while minimizing interference. Their MS-based detection protocols incorporate rapid polarity switching and scheduled MRM (Multiple Reaction Monitoring) for simultaneous quantification of diverse compound classes in complex biological matrices. AstraZeneca has pioneered the implementation of charged aerosol detection (CAD) as a complementary technique to ELSD, offering improved linearity and sensitivity for compounds lacking chromophores. Their integrated data processing workflows enable automated method selection based on compound physicochemical properties, streamlining analytical decision-making. AstraZeneca has also developed novel column-detector coupling technologies that minimize band broadening and optimize detection sensitivity across diverse chromatographic conditions.
Strengths: Highly optimized workflows for pharmaceutical applications; seamless integration with drug development processes; advanced data management systems enabling cross-platform method transfer; extensive validation protocols ensuring regulatory compliance. Weaknesses: Solutions primarily optimized for pharmaceutical applications may have limited versatility in other fields; proprietary methodologies may require significant adaptation for non-standard applications; systems designed for regulatory compliance may sacrifice some flexibility.
Waters Technology Corp.
Technical Solution: Waters Technology Corp. has developed comprehensive column chromatography detection solutions integrating UV, ELSD, and MS technologies. Their ACQUITY UPLC systems feature advanced UV detectors with wavelength ranges from 190-800 nm and variable path length options for enhanced sensitivity. Their ACQUITY QDa Mass Detector provides accessible MS detection with simplified operation for routine analysis. Waters' integrated approach includes the ACQUITY UPLC H-Class PLUS system that allows seamless switching between detection methods based on analyte properties. Their patented sub-2-μm particle technology enhances chromatographic resolution while maintaining backpressure compatibility with conventional HPLC systems. Waters also offers the novel ACQUITY UPLC ELSD detector with improved sensitivity for non-volatile compounds lacking chromophores, featuring temperature-controlled nebulization and evaporation for consistent response across diverse mobile phases.
Strengths: Seamless integration of multiple detection technologies in unified platforms; proprietary particle technology for enhanced resolution; user-friendly software interface allowing method development across detection modes. Weaknesses: Higher initial investment compared to single-detector systems; proprietary consumables may increase operational costs; complex systems require specialized training for optimal utilization.
Technical Principles and Innovations in Detection Systems
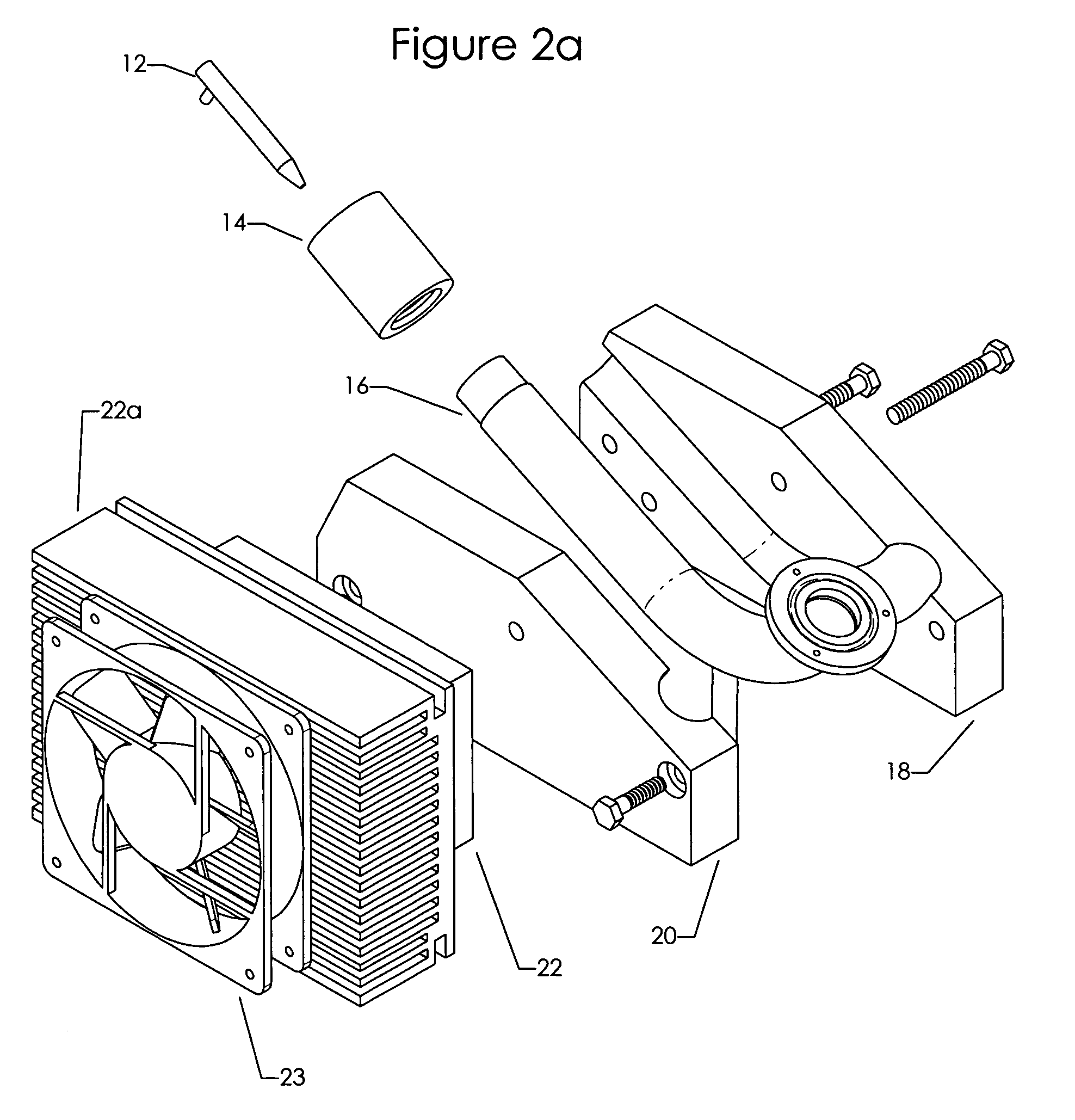
Aerosol splitter for ELSD
PatentInactiveUS7290723B1
Innovation
- A thermally controlled aerosol splitter using a combination of geometry and thermal techniques, where the split ratio is varied by changing the temperature of the Spray Chamber, allowing for smooth adjustment of aerosol diversion from 1% to 99% without mechanical components, enabling user-controlled optimization for diverse chromatography conditions.
Mass spectrometry for determining if a mutated variant of a target protein is present in a sample
PatentWO2016051191A1
Innovation
- A method using mass spectrometry that involves fragmenting target proteins to form ions, analyzing these ions to detect mass-to-charge ratio differences indicative of mutations, allowing for rapid identification of mutated variants without the need for DNA analysis or extensive protein sequencing.
Method Validation and Regulatory Compliance
Method validation is a critical component in chromatographic analysis, ensuring that detection methods—whether UV, ELSD, or MS—produce reliable, reproducible results that meet regulatory requirements. For pharmaceutical and clinical applications, validation protocols must adhere to guidelines established by regulatory bodies such as the FDA, EMA, and ICH.
The validation process for UV detection typically focuses on linearity, accuracy, precision, specificity, and robustness. UV methods generally require simpler validation approaches compared to MS, but must still demonstrate consistent performance across the analytical range. Calibration curves should exhibit linearity with R² values exceeding 0.995 for most applications.
ELSD validation presents unique challenges due to its non-linear response characteristics. Validation protocols must account for this non-linearity through appropriate mathematical models, often employing logarithmic or power function transformations. Particular attention must be paid to the lower limit of quantification (LLOQ) as ELSD sensitivity can vary significantly based on compound properties.
MS detection demands the most comprehensive validation approach. Method validation must address additional parameters including ion suppression effects, matrix effects, and fragmentation pattern consistency. For LC-MS/MS methods, regulatory bodies typically require validation of parameters such as carryover, recovery, and stability under various conditions. The FDA's Bioanalytical Method Validation Guidance provides specific requirements for MS-based methods in bioanalytical applications.
Regulatory compliance considerations vary by application field. For pharmaceutical quality control, methods must comply with ICH Q2(R1) guidelines, while clinical diagnostics require adherence to CLIA regulations in the US or equivalent standards internationally. Environmental testing follows EPA or equivalent protocols, with specific requirements for detection limits and quality control samples.
Documentation requirements represent another critical aspect of compliance. Validation reports must include detailed information on system suitability tests, calibration procedures, and quality control measures. For GMP environments, complete audit trails and electronic records must comply with 21 CFR Part 11 or equivalent regulations regarding electronic signatures and data integrity.
Risk assessment frameworks should be implemented to determine the appropriate validation strategy based on the intended use of the analytical method. Critical quality attributes should be identified and prioritized according to their impact on method performance and data reliability, allowing for a science-based approach to validation that satisfies regulatory requirements while optimizing resource allocation.
The validation process for UV detection typically focuses on linearity, accuracy, precision, specificity, and robustness. UV methods generally require simpler validation approaches compared to MS, but must still demonstrate consistent performance across the analytical range. Calibration curves should exhibit linearity with R² values exceeding 0.995 for most applications.
ELSD validation presents unique challenges due to its non-linear response characteristics. Validation protocols must account for this non-linearity through appropriate mathematical models, often employing logarithmic or power function transformations. Particular attention must be paid to the lower limit of quantification (LLOQ) as ELSD sensitivity can vary significantly based on compound properties.
MS detection demands the most comprehensive validation approach. Method validation must address additional parameters including ion suppression effects, matrix effects, and fragmentation pattern consistency. For LC-MS/MS methods, regulatory bodies typically require validation of parameters such as carryover, recovery, and stability under various conditions. The FDA's Bioanalytical Method Validation Guidance provides specific requirements for MS-based methods in bioanalytical applications.
Regulatory compliance considerations vary by application field. For pharmaceutical quality control, methods must comply with ICH Q2(R1) guidelines, while clinical diagnostics require adherence to CLIA regulations in the US or equivalent standards internationally. Environmental testing follows EPA or equivalent protocols, with specific requirements for detection limits and quality control samples.
Documentation requirements represent another critical aspect of compliance. Validation reports must include detailed information on system suitability tests, calibration procedures, and quality control measures. For GMP environments, complete audit trails and electronic records must comply with 21 CFR Part 11 or equivalent regulations regarding electronic signatures and data integrity.
Risk assessment frameworks should be implemented to determine the appropriate validation strategy based on the intended use of the analytical method. Critical quality attributes should be identified and prioritized according to their impact on method performance and data reliability, allowing for a science-based approach to validation that satisfies regulatory requirements while optimizing resource allocation.
Cost-Benefit Analysis and Implementation Strategies
When implementing chromatography detection systems, organizations must carefully evaluate the cost-benefit relationship of each detection option. UV detectors represent the most economical entry point, with initial investments ranging from $5,000 to $15,000 and minimal operational costs. Their low maintenance requirements and robust performance make them ideal for routine analyses where target compounds have chromophores.
ELSD systems occupy the middle tier with acquisition costs between $20,000 and $40,000. While more expensive than UV, they offer significant advantages for non-chromophoric compounds. The cost-benefit equation improves for laboratories analyzing diverse sample types, as ELSD eliminates the need for multiple detection systems. However, organizations must account for ongoing nitrogen gas consumption and more frequent maintenance intervals.
MS detectors represent the premium segment, with investments starting at $100,000 and potentially exceeding $500,000 for high-resolution systems. Despite the substantial capital expenditure, the cost-benefit analysis often favors MS implementation for research facilities and regulated industries where compound identification certainty and structural information are paramount.
Implementation strategies should follow a phased approach based on analytical needs and budget constraints. Organizations with diverse analytical requirements might begin with UV detection for chromophore-containing compounds while outsourcing more complex analyses. As analytical demands grow, ELSD capabilities can be added to expand the detection portfolio.
For maximum return on investment, cross-training staff across multiple detection technologies ensures operational flexibility and reduces downtime. Preventative maintenance schedules should be established according to usage patterns, with more frequent attention to MS systems due to their complexity and sensitivity.
Laboratories should also consider vendor-neutral data systems capable of integrating signals from different detector types, allowing for method transfer and comparison across platforms. This approach provides long-term flexibility as detection needs evolve and new technologies emerge.
Finally, organizations should evaluate subscription-based service contracts against pay-per-service models based on in-house expertise and instrument utilization rates. High-throughput facilities typically benefit from comprehensive service agreements, while occasional users may find better value in as-needed service arrangements.
ELSD systems occupy the middle tier with acquisition costs between $20,000 and $40,000. While more expensive than UV, they offer significant advantages for non-chromophoric compounds. The cost-benefit equation improves for laboratories analyzing diverse sample types, as ELSD eliminates the need for multiple detection systems. However, organizations must account for ongoing nitrogen gas consumption and more frequent maintenance intervals.
MS detectors represent the premium segment, with investments starting at $100,000 and potentially exceeding $500,000 for high-resolution systems. Despite the substantial capital expenditure, the cost-benefit analysis often favors MS implementation for research facilities and regulated industries where compound identification certainty and structural information are paramount.
Implementation strategies should follow a phased approach based on analytical needs and budget constraints. Organizations with diverse analytical requirements might begin with UV detection for chromophore-containing compounds while outsourcing more complex analyses. As analytical demands grow, ELSD capabilities can be added to expand the detection portfolio.
For maximum return on investment, cross-training staff across multiple detection technologies ensures operational flexibility and reduces downtime. Preventative maintenance schedules should be established according to usage patterns, with more frequent attention to MS systems due to their complexity and sensitivity.
Laboratories should also consider vendor-neutral data systems capable of integrating signals from different detector types, allowing for method transfer and comparison across platforms. This approach provides long-term flexibility as detection needs evolve and new technologies emerge.
Finally, organizations should evaluate subscription-based service contracts against pay-per-service models based on in-house expertise and instrument utilization rates. High-throughput facilities typically benefit from comprehensive service agreements, while occasional users may find better value in as-needed service arrangements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!