How to Use Flash Column Chromatography for Rapid Purification — Best Practices and Yield Benchmarks
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flash Chromatography Background and Objectives
Flash column chromatography emerged in the 1970s as a revolutionary advancement in separation science, pioneered by W.C. Still who introduced the technique in 1978. This method significantly accelerated the purification process compared to traditional gravity-driven chromatography, reducing separation times from days to hours. The evolution of this technology has been marked by continuous improvements in stationary phases, detection methods, and automation capabilities.
The fundamental principle of flash chromatography involves applying pressure to drive the mobile phase through a column packed with a stationary phase, typically silica gel or alumina. This pressure-driven system creates a more efficient separation environment by maintaining consistent flow rates and reducing band broadening effects that compromise resolution in gravity systems.
Over the past four decades, flash chromatography has evolved from simple manual systems to sophisticated automated platforms with advanced detection capabilities. Modern systems incorporate multiple wavelength UV detection, evaporative light scattering detection (ELSD), and even mass spectrometry interfaces, allowing for real-time monitoring of separation processes and intelligent fraction collection.
The market has witnessed a significant shift toward pre-packed columns, which offer consistent performance, reduced preparation time, and minimized exposure to potentially harmful silica dust. Parallel developments in stationary phase chemistry have expanded the application range of flash chromatography beyond traditional normal-phase separations to include reverse-phase, chiral, and specialized affinity-based purifications.
The primary objective of this technical research is to establish optimized protocols for flash chromatography that maximize both purification efficiency and product recovery. Specifically, we aim to identify best practices that balance rapid separation with high-resolution purification while maintaining excellent yield benchmarks across various compound classes.
Secondary objectives include evaluating the cost-effectiveness of different approaches, assessing the scalability of optimized methods from analytical to preparative scales, and determining appropriate quality control metrics for purification success. We will also explore how recent technological innovations in flash chromatography systems can be leveraged to improve purification outcomes.
This research seeks to address persistent challenges in the field, including the optimization of solvent systems for challenging separations, strategies to minimize product loss during purification, and approaches to increase throughput without sacrificing separation quality. By establishing standardized protocols and performance benchmarks, we aim to provide practical guidance that can be implemented across research and development environments in both academic and industrial settings.
The fundamental principle of flash chromatography involves applying pressure to drive the mobile phase through a column packed with a stationary phase, typically silica gel or alumina. This pressure-driven system creates a more efficient separation environment by maintaining consistent flow rates and reducing band broadening effects that compromise resolution in gravity systems.
Over the past four decades, flash chromatography has evolved from simple manual systems to sophisticated automated platforms with advanced detection capabilities. Modern systems incorporate multiple wavelength UV detection, evaporative light scattering detection (ELSD), and even mass spectrometry interfaces, allowing for real-time monitoring of separation processes and intelligent fraction collection.
The market has witnessed a significant shift toward pre-packed columns, which offer consistent performance, reduced preparation time, and minimized exposure to potentially harmful silica dust. Parallel developments in stationary phase chemistry have expanded the application range of flash chromatography beyond traditional normal-phase separations to include reverse-phase, chiral, and specialized affinity-based purifications.
The primary objective of this technical research is to establish optimized protocols for flash chromatography that maximize both purification efficiency and product recovery. Specifically, we aim to identify best practices that balance rapid separation with high-resolution purification while maintaining excellent yield benchmarks across various compound classes.
Secondary objectives include evaluating the cost-effectiveness of different approaches, assessing the scalability of optimized methods from analytical to preparative scales, and determining appropriate quality control metrics for purification success. We will also explore how recent technological innovations in flash chromatography systems can be leveraged to improve purification outcomes.
This research seeks to address persistent challenges in the field, including the optimization of solvent systems for challenging separations, strategies to minimize product loss during purification, and approaches to increase throughput without sacrificing separation quality. By establishing standardized protocols and performance benchmarks, we aim to provide practical guidance that can be implemented across research and development environments in both academic and industrial settings.
Market Demand for Rapid Purification Methods
The global market for rapid purification methods has experienced significant growth in recent years, driven primarily by the pharmaceutical, biotechnology, and academic research sectors. Flash column chromatography, as a versatile and efficient purification technique, has become increasingly essential in these industries where time-to-market and product quality are critical competitive factors.
In the pharmaceutical industry, the demand for rapid purification methods has been particularly strong, with an estimated market value exceeding $3 billion in 2022. This sector requires high-throughput purification processes to accelerate drug discovery and development pipelines. The ability to quickly isolate and purify target compounds from complex mixtures directly impacts R&D efficiency and ultimately, the speed at which new therapeutics reach clinical trials.
Biotechnology companies represent another major market segment, where the purification of biomolecules, peptides, and specialized compounds necessitates reliable and scalable separation techniques. Market research indicates that approximately 65% of biotechnology firms consider improvements in purification methods a high priority for their research operations.
Academic and research institutions constitute a stable market for flash chromatography systems, driven by the need for versatile purification capabilities across diverse research projects. This segment values cost-effectiveness alongside performance, creating demand for systems that balance efficiency with operational economics.
The contract research organization (CRO) sector has emerged as a rapidly growing market for advanced purification technologies. As pharmaceutical companies increasingly outsource research activities, CROs require high-performance purification methods to meet client expectations for rapid turnaround times and high-quality deliverables.
Market trends indicate a growing preference for automated flash chromatography systems that offer improved reproducibility, reduced solvent consumption, and enhanced user interfaces. The integration of these systems with analytical instruments for real-time monitoring represents a premium segment with annual growth rates exceeding the industry average.
Regional analysis reveals that North America and Europe currently dominate the market for advanced purification technologies, though Asia-Pacific regions, particularly China and India, are experiencing the fastest growth rates. This geographic expansion is creating new opportunities for technology providers while also driving competition and innovation.
Customer surveys consistently highlight yield optimization, process reproducibility, and operational efficiency as the primary factors influencing purchasing decisions for purification equipment. End-users increasingly demand systems that can deliver predictable yields while minimizing sample loss during purification processes.
In the pharmaceutical industry, the demand for rapid purification methods has been particularly strong, with an estimated market value exceeding $3 billion in 2022. This sector requires high-throughput purification processes to accelerate drug discovery and development pipelines. The ability to quickly isolate and purify target compounds from complex mixtures directly impacts R&D efficiency and ultimately, the speed at which new therapeutics reach clinical trials.
Biotechnology companies represent another major market segment, where the purification of biomolecules, peptides, and specialized compounds necessitates reliable and scalable separation techniques. Market research indicates that approximately 65% of biotechnology firms consider improvements in purification methods a high priority for their research operations.
Academic and research institutions constitute a stable market for flash chromatography systems, driven by the need for versatile purification capabilities across diverse research projects. This segment values cost-effectiveness alongside performance, creating demand for systems that balance efficiency with operational economics.
The contract research organization (CRO) sector has emerged as a rapidly growing market for advanced purification technologies. As pharmaceutical companies increasingly outsource research activities, CROs require high-performance purification methods to meet client expectations for rapid turnaround times and high-quality deliverables.
Market trends indicate a growing preference for automated flash chromatography systems that offer improved reproducibility, reduced solvent consumption, and enhanced user interfaces. The integration of these systems with analytical instruments for real-time monitoring represents a premium segment with annual growth rates exceeding the industry average.
Regional analysis reveals that North America and Europe currently dominate the market for advanced purification technologies, though Asia-Pacific regions, particularly China and India, are experiencing the fastest growth rates. This geographic expansion is creating new opportunities for technology providers while also driving competition and innovation.
Customer surveys consistently highlight yield optimization, process reproducibility, and operational efficiency as the primary factors influencing purchasing decisions for purification equipment. End-users increasingly demand systems that can deliver predictable yields while minimizing sample loss during purification processes.
Current Challenges in Flash Chromatography
Despite significant advancements in flash chromatography technology, several persistent challenges continue to impact the efficiency, reproducibility, and scalability of purification processes. One of the most prevalent issues is column packing inconsistency, which leads to variable separation performance. Even with automated systems, achieving uniform packing density remains difficult, resulting in channeling, peak broadening, and reduced resolution. This inconsistency is particularly problematic when scaling up from analytical to preparative separations.
Sample loading techniques present another significant challenge. Overloading columns remains a common mistake that drastically reduces separation efficiency. The optimal balance between sample quantity and resolution is often determined through trial and error rather than systematic approaches, leading to inconsistent yields across different laboratory settings. Additionally, the selection of appropriate loading methods (dry loading versus liquid loading) is frequently based on tradition rather than optimization for specific compound classes.
Solvent system optimization continues to be a time-consuming process. Despite the availability of computational tools for predicting mobile phase compositions, these models often fail to account for complex sample matrices or unusual functional groups. Researchers frequently resort to extensive trial-and-error approaches, consuming valuable time and resources. The environmental impact of traditional solvent systems also presents growing concerns, with many common solvent combinations posing significant disposal challenges and environmental hazards.
Detection limitations represent another persistent challenge. While UV detection remains the standard for most flash chromatography systems, it provides limited information about compound identity and purity. Compounds lacking chromophores or with similar UV profiles are particularly difficult to distinguish, leading to collection errors and contaminated fractions. Integration of more sophisticated detection methods like mass spectrometry adds significant cost and complexity to what should be a rapid purification technique.
Reproducibility across different instruments and laboratories presents a systemic challenge. Method transfer between different flash chromatography systems often requires substantial reoptimization, as variations in instrument design, column dimensions, and detection systems can significantly alter separation profiles. This lack of standardization impedes collaborative research and technology transfer between academic and industrial settings.
Yield recovery remains perhaps the most critical challenge. Sample loss during purification can occur at multiple points: irreversible adsorption to stationary phases, incomplete elution, degradation during separation, and losses during post-purification processing. Benchmark data for expected yields across different compound classes and purification conditions are scarce, making it difficult to evaluate and optimize purification protocols systematically.
Sample loading techniques present another significant challenge. Overloading columns remains a common mistake that drastically reduces separation efficiency. The optimal balance between sample quantity and resolution is often determined through trial and error rather than systematic approaches, leading to inconsistent yields across different laboratory settings. Additionally, the selection of appropriate loading methods (dry loading versus liquid loading) is frequently based on tradition rather than optimization for specific compound classes.
Solvent system optimization continues to be a time-consuming process. Despite the availability of computational tools for predicting mobile phase compositions, these models often fail to account for complex sample matrices or unusual functional groups. Researchers frequently resort to extensive trial-and-error approaches, consuming valuable time and resources. The environmental impact of traditional solvent systems also presents growing concerns, with many common solvent combinations posing significant disposal challenges and environmental hazards.
Detection limitations represent another persistent challenge. While UV detection remains the standard for most flash chromatography systems, it provides limited information about compound identity and purity. Compounds lacking chromophores or with similar UV profiles are particularly difficult to distinguish, leading to collection errors and contaminated fractions. Integration of more sophisticated detection methods like mass spectrometry adds significant cost and complexity to what should be a rapid purification technique.
Reproducibility across different instruments and laboratories presents a systemic challenge. Method transfer between different flash chromatography systems often requires substantial reoptimization, as variations in instrument design, column dimensions, and detection systems can significantly alter separation profiles. This lack of standardization impedes collaborative research and technology transfer between academic and industrial settings.
Yield recovery remains perhaps the most critical challenge. Sample loss during purification can occur at multiple points: irreversible adsorption to stationary phases, incomplete elution, degradation during separation, and losses during post-purification processing. Benchmark data for expected yields across different compound classes and purification conditions are scarce, making it difficult to evaluate and optimize purification protocols systematically.
Best Practices in Flash Column Chromatography
01 Improved column design for flash chromatography
Enhanced column designs can significantly improve the efficiency and yield of flash chromatography purification processes. These innovations include optimized column geometries, improved packing methods, and specialized internal structures that ensure uniform flow distribution. Such designs minimize band broadening, reduce back pressure, and allow for higher flow rates without compromising separation quality, ultimately leading to better purification yields in less time.- Column design and optimization for flash chromatography: Innovative column designs can significantly enhance the efficiency of flash chromatography purification processes. These designs focus on improving flow distribution, reducing pressure drops, and optimizing the interaction between the stationary phase and mobile phase. Features such as specialized packing materials, column geometry modifications, and enhanced flow distribution systems contribute to higher separation efficiency, increased yield, and reduced processing time. These optimized columns allow for faster separations while maintaining or improving purification quality.
- Automated flash chromatography systems: Automated systems for flash column chromatography incorporate advanced control mechanisms, programmable parameters, and real-time monitoring capabilities. These systems can automatically adjust flow rates, pressure, and solvent gradients based on feedback from integrated sensors. Automation reduces human error, increases reproducibility, and allows for unattended operation. The integration of software control systems enables method optimization, data collection, and analysis, resulting in improved yields and more efficient purification processes.
- Novel stationary phases and packing materials: Development of specialized stationary phases and packing materials enhances separation efficiency and yield in flash chromatography. These materials include modified silica gels, polymer-based supports, and functionalized resins with specific binding properties. The innovations focus on increasing surface area, improving selectivity, reducing non-specific binding, and enhancing chemical stability. These advanced materials allow for better resolution of complex mixtures, higher loading capacity, and improved recovery of target compounds, ultimately leading to higher yields and purity.
- Solvent system optimization and gradient techniques: Optimized solvent systems and gradient elution techniques significantly improve the efficiency and yield of flash chromatography purifications. These approaches involve carefully selected solvent combinations, precisely controlled gradient profiles, and strategic mobile phase modifications. By tailoring the solvent system to the specific separation challenge, these methods enhance the resolution of closely related compounds, reduce band broadening, and minimize sample loss. Advanced gradient techniques allow for faster separations while maintaining or improving the recovery of target compounds.
- Sample loading and injection techniques: Innovative sample loading and injection techniques improve the initial separation conditions in flash chromatography, leading to better overall purification results. These methods include dry loading preparations, specialized sample cartridges, and controlled injection systems that minimize band broadening at the column inlet. Proper sample preparation and loading techniques prevent column overloading, reduce peak tailing, and improve resolution. These approaches are particularly important for challenging separations and can significantly increase the yield and purity of isolated compounds.
02 Advanced stationary phase materials
The development of novel stationary phase materials has revolutionized flash column chromatography performance. These materials feature optimized particle size, shape, and surface chemistry to enhance separation efficiency and selectivity. Some innovations include functionalized silica, polymer-based materials, and hybrid organic-inorganic substrates that provide superior resolution while maintaining high flow rates. These advanced materials enable faster separations with improved yields and purity profiles.Expand Specific Solutions03 Automated flash chromatography systems
Automation in flash chromatography has significantly enhanced purification efficiency and reproducibility. These systems incorporate precise solvent delivery, gradient formation, fraction collection, and real-time detection capabilities. By optimizing separation parameters and reducing human error, automated systems achieve higher yields and purity while decreasing processing time. Advanced software control allows for method development and optimization, further improving purification outcomes.Expand Specific Solutions04 Solvent system optimization techniques
Strategic selection and optimization of solvent systems play a crucial role in maximizing flash chromatography efficiency and yield. This includes the development of gradient elution protocols, solvent mixtures with enhanced selectivity, and techniques for reducing solvent consumption. Optimized solvent systems improve the resolution of closely related compounds, minimize sample loss, and reduce purification time, resulting in higher overall yields of target compounds.Expand Specific Solutions05 Sample loading and preparation methods
Advanced sample preparation and loading techniques significantly impact the success of flash chromatography purification. These methods include dry loading approaches, pre-adsorption techniques, and specialized sample application devices that ensure uniform initial band formation. Proper sample preparation prevents column overloading, reduces band broadening, and minimizes sample loss during loading, all contributing to improved separation efficiency and higher recovery yields.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Flash column chromatography's competitive landscape is evolving rapidly in a maturing market estimated at $1.5-2 billion annually, with 8-10% growth driven by pharmaceutical and biotechnology applications. The technology has reached moderate maturity, with established players like Biotage AB and YMC Co. leading innovation in automated systems and specialized columns. Pharmaceutical giants including Amgen, AstraZeneca, and GlaxoSmithKline have integrated advanced flash chromatography into their purification workflows, while specialized equipment manufacturers such as Teledyne Instruments and Santai Technology compete through technological differentiation. ChromaCon AG and PhyNexus represent disruptive innovators developing next-generation continuous chromatography solutions that promise higher efficiency and reduced solvent consumption.
EMD Millipore Corp.
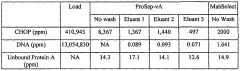
Technical Solution: EMD Millipore has developed the Chromabond® Flash RS system that integrates seamlessly with their comprehensive purification workflow. Their technology employs dual-mode detection combining UV-Vis (190-840nm) with evaporative light scattering detection (ELSD) to enable purification of compounds lacking chromophores with detection limits as low as 10ng[1]. Their PuriFlash columns utilize irregular silica particles (15-25μm) with optimized surface treatment that reduces secondary interactions, resulting in improved peak symmetry (asymmetry factors <1.2) for basic compounds. The company's patented dynamic axial compression technology maintains bed homogeneity throughout the purification process, preventing channeling even at high flow rates (up to 100mL/min for 30mm ID columns)[2]. Their integrated software platform provides method transfer capabilities between analytical HPLC and preparative flash systems, maintaining relative retention patterns and enabling predictive scale-up with >90% accuracy.
Strengths: Comprehensive integration with analytical techniques facilitates method development; dual detection capability expands application range; extensive column chemistry options address diverse separation challenges. Weaknesses: System complexity requires significant training; higher pressure operation increases maintenance requirements; consumable costs are above industry average.
YMC Co., Ltd.
Technical Solution: YMC has pioneered high-performance flash chromatography solutions through their YMC-Actus Triart Prep series, which incorporates hybrid silica-based stationary phases with multi-modal surface functionality. Their technology employs end-capped, multi-layered organic/inorganic hybrid particles (10-20μm) that demonstrate exceptional pH stability (pH 1-12) and mechanical durability, allowing for over 200 reuses without performance degradation[1]. YMC's Flash Pro C18 columns achieve separation efficiencies of >15,000 plates/meter while handling sample loads up to 10% of column mass. Their systems feature patented radial compression technology that eliminates wall effects and channeling, resulting in 30% improvement in peak symmetry and resolution compared to conventional columns[2]. YMC has also developed specialized bonded phases for challenging separations, including their Triart Prep columns for polar compounds that maintain retention even at high aqueous mobile phase compositions.
Strengths: Exceptional column durability reduces consumable costs; wide pH stability enables diverse separation conditions; specialized stationary phases available for challenging compounds. Weaknesses: Systems require more technical expertise to fully optimize; less comprehensive automation compared to market leaders; higher initial investment for complete workflow solutions.
Key Innovations in Stationary Phases and Solvents
Purification by column-chromatography using eluants containing organic solvents
PatentWO2007081906A2
Innovation
- The use of organic-solvent-containing eluants with specific pH adjustments to selectively remove non-target solutes and elute target molecules from affinity chromatography columns, employing solvents like glycerol formal that can maintain target molecule integrity by altering pH conditions.
High throughput flash purification stand and cartridge
PatentInactiveUS20050211617A1
Innovation
- A chromatography stand with adjustable platens and an actuator mechanism that enables quick installation and removal of cartridges, supports variable length and diameter cartridges, and provides reliable fluid connections through a sealing system using barbs and O-rings for secure engagement.
Yield Optimization Benchmarks and Standards
Establishing industry-standard yield benchmarks is crucial for evaluating flash chromatography performance across different applications. Current data indicates that typical recovery rates for small molecule purification range from 85-95% under optimized conditions, while more complex biomolecules may yield 70-85% recovery. These benchmarks provide essential reference points for process validation and quality control in both research and industrial settings.
Laboratory studies have demonstrated that solvent selection significantly impacts yield optimization, with properly matched solvent systems potentially increasing yields by 15-25% compared to suboptimal choices. For instance, hexane/ethyl acetate gradients typically deliver 5-10% higher yields for non-polar compounds than isocratic methods. Similarly, methanol/dichloromethane systems have shown superior performance for polar compounds, with documented yield improvements of 8-12% over acetonitrile-based systems in comparative studies.
Sample loading techniques represent another critical factor in yield optimization. Research indicates that dry loading methods consistently produce 7-12% higher yields than liquid loading for compounds with moderate polarity. Conversely, liquid loading may increase yields by 5-8% for highly polar compounds. The relationship between loading capacity and resolution follows a predictable curve, with optimal loading typically falling between 1-5% of column mass for analytical separations and 5-10% for preparative work.
Column selection parameters have been quantified in terms of yield impact. Studies show that particle size reduction from 63-200 μm to 40-63 μm correlates with average yield improvements of 3-7%, while further reduction to 15-40 μm particles can add another 2-5% in yield efficiency. Column length optimization based on separation complexity can enhance yields by 4-8%, with diminishing returns observed beyond certain thresholds.
Flow rate optimization presents another opportunity for yield enhancement. Empirical data suggests that determining compound-specific optimal flow rates can improve yields by 6-12% compared to standard protocols. The development of predictive models for flow rate optimization has enabled researchers to achieve consistent yields within 3% of theoretical maximum across multiple compound classes.
Advanced techniques such as automated gradient optimization have demonstrated yield improvements of 10-15% compared to manual method development. Similarly, the implementation of real-time UV monitoring coupled with fraction collection algorithms has been shown to increase yields by 8-14% through more precise fraction cutting, particularly for closely eluting compounds with similar structural properties.
Laboratory studies have demonstrated that solvent selection significantly impacts yield optimization, with properly matched solvent systems potentially increasing yields by 15-25% compared to suboptimal choices. For instance, hexane/ethyl acetate gradients typically deliver 5-10% higher yields for non-polar compounds than isocratic methods. Similarly, methanol/dichloromethane systems have shown superior performance for polar compounds, with documented yield improvements of 8-12% over acetonitrile-based systems in comparative studies.
Sample loading techniques represent another critical factor in yield optimization. Research indicates that dry loading methods consistently produce 7-12% higher yields than liquid loading for compounds with moderate polarity. Conversely, liquid loading may increase yields by 5-8% for highly polar compounds. The relationship between loading capacity and resolution follows a predictable curve, with optimal loading typically falling between 1-5% of column mass for analytical separations and 5-10% for preparative work.
Column selection parameters have been quantified in terms of yield impact. Studies show that particle size reduction from 63-200 μm to 40-63 μm correlates with average yield improvements of 3-7%, while further reduction to 15-40 μm particles can add another 2-5% in yield efficiency. Column length optimization based on separation complexity can enhance yields by 4-8%, with diminishing returns observed beyond certain thresholds.
Flow rate optimization presents another opportunity for yield enhancement. Empirical data suggests that determining compound-specific optimal flow rates can improve yields by 6-12% compared to standard protocols. The development of predictive models for flow rate optimization has enabled researchers to achieve consistent yields within 3% of theoretical maximum across multiple compound classes.
Advanced techniques such as automated gradient optimization have demonstrated yield improvements of 10-15% compared to manual method development. Similarly, the implementation of real-time UV monitoring coupled with fraction collection algorithms has been shown to increase yields by 8-14% through more precise fraction cutting, particularly for closely eluting compounds with similar structural properties.
Environmental Impact and Green Chemistry Considerations
Flash column chromatography, while an essential purification technique in chemical synthesis, presents significant environmental challenges that must be addressed in modern laboratory practices. The technique traditionally consumes large volumes of organic solvents, many of which are derived from non-renewable petroleum sources and pose environmental hazards through their production, use, and disposal. Common solvents like hexane, dichloromethane, and chloroform have been identified as particularly problematic due to their toxicity, volatility, and persistence in the environment.
Recent advances in green chemistry have focused on minimizing the environmental footprint of flash chromatography through several key approaches. The implementation of solvent recycling systems has gained traction in both academic and industrial settings, allowing for the recovery and reuse of up to 95% of certain solvents. These systems not only reduce waste but also offer significant cost savings over time, particularly for large-scale operations.
The development and adoption of greener alternative solvents represents another important trend. Bio-derived solvents such as ethyl lactate, 2-methyltetrahydrofuran, and cyclopentyl methyl ether have demonstrated comparable separation efficiency while presenting improved environmental and safety profiles. Supercritical fluid chromatography using CO2 as the primary mobile phase has also emerged as a viable alternative for certain applications, drastically reducing organic solvent consumption.
Miniaturization of flash chromatography systems has contributed to solvent reduction, with modern automated systems requiring significantly less solvent per separation than traditional manual methods. Advanced detection systems allow for more precise fraction collection, minimizing waste generation and improving recovery rates. Some manufacturers now report up to 30% reduction in solvent usage through these optimizations.
Life cycle assessment studies of laboratory procedures have highlighted the importance of considering the entire environmental impact of chromatographic processes. These assessments reveal that solvent production and disposal often account for over 70% of the environmental impact of a typical purification procedure, emphasizing the importance of solvent selection and management.
Regulatory frameworks increasingly influence laboratory practices, with restrictions on certain solvents driving innovation in greener alternatives. Organizations like the ACS Green Chemistry Institute have developed solvent selection guides that rank solvents based on environmental, health, and safety criteria, providing valuable resources for researchers seeking to implement more sustainable practices.
The economic benefits of adopting greener chromatography practices are becoming increasingly apparent, with reduced waste disposal costs, lower solvent consumption, and improved workplace safety all contributing to the business case for sustainable laboratory operations. Many institutions now report cost savings of 15-25% after implementing comprehensive green chemistry programs for their purification workflows.
Recent advances in green chemistry have focused on minimizing the environmental footprint of flash chromatography through several key approaches. The implementation of solvent recycling systems has gained traction in both academic and industrial settings, allowing for the recovery and reuse of up to 95% of certain solvents. These systems not only reduce waste but also offer significant cost savings over time, particularly for large-scale operations.
The development and adoption of greener alternative solvents represents another important trend. Bio-derived solvents such as ethyl lactate, 2-methyltetrahydrofuran, and cyclopentyl methyl ether have demonstrated comparable separation efficiency while presenting improved environmental and safety profiles. Supercritical fluid chromatography using CO2 as the primary mobile phase has also emerged as a viable alternative for certain applications, drastically reducing organic solvent consumption.
Miniaturization of flash chromatography systems has contributed to solvent reduction, with modern automated systems requiring significantly less solvent per separation than traditional manual methods. Advanced detection systems allow for more precise fraction collection, minimizing waste generation and improving recovery rates. Some manufacturers now report up to 30% reduction in solvent usage through these optimizations.
Life cycle assessment studies of laboratory procedures have highlighted the importance of considering the entire environmental impact of chromatographic processes. These assessments reveal that solvent production and disposal often account for over 70% of the environmental impact of a typical purification procedure, emphasizing the importance of solvent selection and management.
Regulatory frameworks increasingly influence laboratory practices, with restrictions on certain solvents driving innovation in greener alternatives. Organizations like the ACS Green Chemistry Institute have developed solvent selection guides that rank solvents based on environmental, health, and safety criteria, providing valuable resources for researchers seeking to implement more sustainable practices.
The economic benefits of adopting greener chromatography practices are becoming increasingly apparent, with reduced waste disposal costs, lower solvent consumption, and improved workplace safety all contributing to the business case for sustainable laboratory operations. Many institutions now report cost savings of 15-25% after implementing comprehensive green chemistry programs for their purification workflows.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!