How to Scale Column Chromatography from Analytical to Prep — Column Sizing and Solvent Use Table
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Scale-Up Background and Objectives
Column chromatography has evolved significantly since its inception in the early 20th century, transforming from a purely analytical technique to an essential purification method in pharmaceutical, biotechnology, and chemical industries. The transition from analytical to preparative (prep) scale represents a critical advancement that has enabled industrial-scale production of high-purity compounds. This evolution has been driven by increasing demands for higher throughput, better resolution, and more cost-effective purification processes.
The fundamental principles of chromatography remain consistent across scales, involving the separation of compounds based on their differential interactions with stationary and mobile phases. However, the scale-up process introduces numerous complexities that must be carefully addressed to maintain separation efficiency while increasing productivity.
Recent technological developments have focused on improving column design, packing materials, and operational parameters to facilitate seamless scale-up. The introduction of high-performance liquid chromatography (HPLC) in the 1970s marked a significant milestone, followed by the development of ultra-high-performance liquid chromatography (UHPLC) in the early 2000s, which further enhanced separation capabilities.
Current industry trends indicate a growing emphasis on continuous chromatography processes, particularly in biopharmaceutical manufacturing, where the need for efficient purification of biologics has driven innovation. Simultaneously, there is increasing interest in sustainable chromatography practices, focusing on reducing solvent consumption and environmental impact during scale-up operations.
The primary objective of this technical research is to establish a comprehensive framework for scaling column chromatography from analytical to preparative scale, with particular emphasis on column sizing methodologies and solvent utilization optimization. This includes developing predictive models for scale-up parameters, identifying critical process variables, and establishing practical guidelines for maintaining separation efficiency across different scales.
Additionally, this research aims to address common challenges encountered during scale-up, such as increased back pressure, band broadening, and heat generation, which can significantly impact separation performance. By systematically analyzing these factors, we seek to provide solutions that enable more reliable and efficient scale-up processes.
The ultimate goal is to develop a standardized approach to chromatography scale-up that minimizes experimental iterations, reduces development time, and optimizes resource utilization while maintaining or improving separation quality. This would significantly benefit industries relying on chromatographic purification by reducing costs, increasing throughput, and enhancing product quality.
The fundamental principles of chromatography remain consistent across scales, involving the separation of compounds based on their differential interactions with stationary and mobile phases. However, the scale-up process introduces numerous complexities that must be carefully addressed to maintain separation efficiency while increasing productivity.
Recent technological developments have focused on improving column design, packing materials, and operational parameters to facilitate seamless scale-up. The introduction of high-performance liquid chromatography (HPLC) in the 1970s marked a significant milestone, followed by the development of ultra-high-performance liquid chromatography (UHPLC) in the early 2000s, which further enhanced separation capabilities.
Current industry trends indicate a growing emphasis on continuous chromatography processes, particularly in biopharmaceutical manufacturing, where the need for efficient purification of biologics has driven innovation. Simultaneously, there is increasing interest in sustainable chromatography practices, focusing on reducing solvent consumption and environmental impact during scale-up operations.
The primary objective of this technical research is to establish a comprehensive framework for scaling column chromatography from analytical to preparative scale, with particular emphasis on column sizing methodologies and solvent utilization optimization. This includes developing predictive models for scale-up parameters, identifying critical process variables, and establishing practical guidelines for maintaining separation efficiency across different scales.
Additionally, this research aims to address common challenges encountered during scale-up, such as increased back pressure, band broadening, and heat generation, which can significantly impact separation performance. By systematically analyzing these factors, we seek to provide solutions that enable more reliable and efficient scale-up processes.
The ultimate goal is to develop a standardized approach to chromatography scale-up that minimizes experimental iterations, reduces development time, and optimizes resource utilization while maintaining or improving separation quality. This would significantly benefit industries relying on chromatographic purification by reducing costs, increasing throughput, and enhancing product quality.
Market Analysis for Preparative Chromatography Solutions
The global preparative chromatography market has been experiencing robust growth, valued at approximately $1.2 billion in 2022 and projected to reach $1.8 billion by 2027, representing a compound annual growth rate (CAGR) of 8.5%. This growth is primarily driven by increasing demand from pharmaceutical and biotechnology sectors, where purification processes are critical for drug development and manufacturing.
Pharmaceutical companies remain the largest consumer segment, accounting for nearly 65% of the market share. The biotechnology sector follows at 20%, with academic research institutions and food testing laboratories comprising the remaining 15%. Geographically, North America dominates with 40% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (5%).
The demand for preparative chromatography solutions is increasingly influenced by the growing pipeline of biopharmaceuticals and biosimilars. With over 8,000 drugs in clinical development globally and biologics representing approximately 40% of this pipeline, the need for efficient purification technologies continues to expand. Additionally, the COVID-19 pandemic has accelerated investment in biopharmaceutical manufacturing infrastructure, creating new opportunities for preparative chromatography systems.
Key market drivers include the shift toward continuous manufacturing processes, increasing regulatory requirements for purity in pharmaceutical products, and growing adoption of single-use technologies. The trend toward process intensification has created demand for more efficient chromatography solutions that can handle higher throughput while maintaining resolution and recovery rates.
Customer pain points in scaling from analytical to preparative chromatography include high solvent consumption costs, which can represent 30-45% of operational expenses, complex method transfer processes, and challenges in maintaining separation efficiency at larger scales. Users are increasingly seeking integrated solutions that simplify scale-up calculations and reduce development time.
Market research indicates that customers prioritize three key factors when selecting preparative chromatography solutions: scalability (ease of method transfer from analytical to production scale), cost efficiency (particularly regarding solvent usage), and technical support for method development. Solutions addressing these needs, especially those offering predictive modeling for column sizing and solvent consumption, command premium pricing in the market.
Emerging market trends include increased adoption of supercritical fluid chromatography (SFC) as a greener alternative to traditional methods, integration of automation and digital tools for method development, and growing interest in multi-column continuous chromatography systems that optimize productivity and reduce solvent consumption by 40-60% compared to batch processes.
Pharmaceutical companies remain the largest consumer segment, accounting for nearly 65% of the market share. The biotechnology sector follows at 20%, with academic research institutions and food testing laboratories comprising the remaining 15%. Geographically, North America dominates with 40% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (5%).
The demand for preparative chromatography solutions is increasingly influenced by the growing pipeline of biopharmaceuticals and biosimilars. With over 8,000 drugs in clinical development globally and biologics representing approximately 40% of this pipeline, the need for efficient purification technologies continues to expand. Additionally, the COVID-19 pandemic has accelerated investment in biopharmaceutical manufacturing infrastructure, creating new opportunities for preparative chromatography systems.
Key market drivers include the shift toward continuous manufacturing processes, increasing regulatory requirements for purity in pharmaceutical products, and growing adoption of single-use technologies. The trend toward process intensification has created demand for more efficient chromatography solutions that can handle higher throughput while maintaining resolution and recovery rates.
Customer pain points in scaling from analytical to preparative chromatography include high solvent consumption costs, which can represent 30-45% of operational expenses, complex method transfer processes, and challenges in maintaining separation efficiency at larger scales. Users are increasingly seeking integrated solutions that simplify scale-up calculations and reduce development time.
Market research indicates that customers prioritize three key factors when selecting preparative chromatography solutions: scalability (ease of method transfer from analytical to production scale), cost efficiency (particularly regarding solvent usage), and technical support for method development. Solutions addressing these needs, especially those offering predictive modeling for column sizing and solvent consumption, command premium pricing in the market.
Emerging market trends include increased adoption of supercritical fluid chromatography (SFC) as a greener alternative to traditional methods, integration of automation and digital tools for method development, and growing interest in multi-column continuous chromatography systems that optimize productivity and reduce solvent consumption by 40-60% compared to batch processes.
Current Challenges in Analytical-to-Prep Scale Transition
The transition from analytical to preparative scale chromatography presents significant challenges that must be addressed to ensure successful scale-up operations. One of the primary obstacles is the non-linear relationship between column dimensions and separation performance. While analytical columns typically have internal diameters of 2-4.6mm, preparative columns can range from 10mm to several centimeters, requiring complex calculations to maintain resolution and efficiency.
Sample loading capacity represents another critical challenge. Analytical methods typically employ dilute samples with minimal column loading, whereas preparative applications demand maximum sample throughput. This fundamental difference necessitates careful optimization of sample concentration, injection volume, and loading techniques to prevent band broadening and resolution loss during scale-up.
Solvent consumption increases exponentially with column diameter, creating economic and environmental concerns. A typical analytical HPLC method might consume milliliters of solvent per run, while preparative separations can require liters or even hundreds of liters for industrial-scale operations. This dramatic increase necessitates consideration of solvent recycling systems and more efficient gradient profiles to minimize waste.
Heat dissipation becomes increasingly problematic at larger scales. The friction generated as mobile phase passes through the stationary phase creates heat that can be readily dissipated in analytical columns but becomes significant in preparative columns. Temperature gradients within larger columns can lead to inconsistent separation performance and reduced column lifetime.
Instrumentation limitations present additional barriers. Many laboratories possess sophisticated analytical HPLC systems but lack preparative capabilities with appropriate pumps, injection systems, and detectors. The substantial investment required for dedicated preparative systems often forces compromises in equipment selection.
Detection sensitivity decreases proportionally with dilution factors in larger columns. While analytical methods benefit from concentrated analyte bands and sensitive detectors, preparative separations produce more dilute fractions that challenge detection systems, particularly for minor components or impurities.
Reproducibility and robustness concerns intensify during scale-up. Small variations in packing quality, flow distribution, or wall effects that might be negligible in analytical columns can significantly impact separation performance in preparative columns. These effects become more pronounced as column diameter increases, requiring specialized packing techniques and equipment.
Regulatory and validation requirements add complexity for pharmaceutical and biopharmaceutical applications. Method transfer from analytical to preparative scale must demonstrate equivalent separation performance while accommodating the inherent differences between scales, creating documentation and validation challenges that extend beyond purely technical considerations.
Sample loading capacity represents another critical challenge. Analytical methods typically employ dilute samples with minimal column loading, whereas preparative applications demand maximum sample throughput. This fundamental difference necessitates careful optimization of sample concentration, injection volume, and loading techniques to prevent band broadening and resolution loss during scale-up.
Solvent consumption increases exponentially with column diameter, creating economic and environmental concerns. A typical analytical HPLC method might consume milliliters of solvent per run, while preparative separations can require liters or even hundreds of liters for industrial-scale operations. This dramatic increase necessitates consideration of solvent recycling systems and more efficient gradient profiles to minimize waste.
Heat dissipation becomes increasingly problematic at larger scales. The friction generated as mobile phase passes through the stationary phase creates heat that can be readily dissipated in analytical columns but becomes significant in preparative columns. Temperature gradients within larger columns can lead to inconsistent separation performance and reduced column lifetime.
Instrumentation limitations present additional barriers. Many laboratories possess sophisticated analytical HPLC systems but lack preparative capabilities with appropriate pumps, injection systems, and detectors. The substantial investment required for dedicated preparative systems often forces compromises in equipment selection.
Detection sensitivity decreases proportionally with dilution factors in larger columns. While analytical methods benefit from concentrated analyte bands and sensitive detectors, preparative separations produce more dilute fractions that challenge detection systems, particularly for minor components or impurities.
Reproducibility and robustness concerns intensify during scale-up. Small variations in packing quality, flow distribution, or wall effects that might be negligible in analytical columns can significantly impact separation performance in preparative columns. These effects become more pronounced as column diameter increases, requiring specialized packing techniques and equipment.
Regulatory and validation requirements add complexity for pharmaceutical and biopharmaceutical applications. Method transfer from analytical to preparative scale must demonstrate equivalent separation performance while accommodating the inherent differences between scales, creating documentation and validation challenges that extend beyond purely technical considerations.
Established Column Sizing and Solvent Optimization Techniques
01 Column sizing parameters for chromatography
Column sizing is critical for effective chromatographic separation. The dimensions of the column, including diameter and length, directly impact separation efficiency, resolution, and sample capacity. Proper sizing considerations include the sample volume, particle size of the stationary phase, and the desired flow rate. Optimizing these parameters ensures efficient separation while minimizing solvent consumption and analysis time.- Column sizing parameters for chromatography: The dimensions of chromatography columns significantly impact separation efficiency. Key parameters include column diameter, length, and particle size of the stationary phase. Proper sizing ensures optimal flow rates and resolution while minimizing pressure drops. Column dimensions should be selected based on sample volume, complexity, and the specific separation requirements. Smaller diameter columns are typically used for analytical purposes, while larger diameter columns are preferred for preparative separations.
- Solvent selection and optimization: Solvent selection is critical for effective chromatographic separation. The choice of mobile phase affects selectivity, resolution, and sample retention. Factors to consider include solvent polarity, viscosity, and compatibility with both the sample and detection method. Gradient elution techniques using varying solvent compositions can improve separation of complex mixtures. Optimization of solvent systems can enhance separation efficiency while reducing analysis time and solvent consumption.
- Automated column chromatography systems: Automated systems for column chromatography improve reproducibility and efficiency. These systems incorporate precise control of flow rates, pressure, and solvent composition. Automation allows for unattended operation, reducing labor requirements and human error. Advanced systems may include features such as fraction collection, real-time monitoring, and software-controlled method development. Integration with detection systems enables immediate analysis of separated components.
- Scale-up and preparative chromatography: Scaling up chromatographic processes from analytical to preparative scale requires careful consideration of column dimensions and operating parameters. Challenges include maintaining separation efficiency while increasing throughput. Factors to consider include linear velocity, sample loading capacity, and pressure limitations. Proper scale-up methodologies can predict performance at larger scales based on small-scale experiments. Optimization of loading conditions and flow rates is essential for efficient large-scale separations.
- Novel stationary phases and column technologies: Innovations in stationary phase materials and column designs enhance separation performance. Advanced materials include monolithic columns, superficially porous particles, and functionalized silica. These technologies offer improved mass transfer, reduced back pressure, and enhanced selectivity. Specialized columns for specific applications, such as chiral separations or biomolecule purification, provide targeted separation capabilities. Novel column technologies can reduce solvent consumption and analysis time while improving resolution.
02 Solvent selection and optimization
The choice of solvent system significantly affects chromatographic performance. Solvent polarity, viscosity, and compatibility with both the stationary phase and analytes must be considered. Mobile phase composition can be optimized through gradient elution or isocratic conditions to enhance separation efficiency. Proper solvent selection reduces analysis time, improves resolution, and minimizes environmental impact through reduced solvent consumption.Expand Specific Solutions03 Automated column chromatography systems
Automated systems for column chromatography improve reproducibility and efficiency while reducing manual handling. These systems incorporate precise control of flow rates, pressure, and fraction collection. Advanced automation features include real-time monitoring of separation parameters, automated column equilibration, and programmable gradient profiles. Such systems optimize solvent usage through precise delivery mechanisms and can be integrated with analytical instruments for immediate analysis of fractions.Expand Specific Solutions04 Scale-up and industrial applications
Scaling up chromatographic processes from laboratory to industrial scale requires careful consideration of column dimensions, flow dynamics, and heat distribution. Proper scaling maintains separation efficiency while increasing throughput. Industrial applications often employ specialized column designs that optimize solvent consumption and pressure distribution. Considerations for scale-up include maintaining the ratio of column diameter to particle size, adjusting flow rates proportionally, and ensuring uniform sample distribution across the column cross-section.Expand Specific Solutions05 Novel column designs and materials
Innovations in column design and packing materials enhance separation efficiency and reduce solvent consumption. These include monolithic columns, core-shell particles, and columns with modified surface chemistries. Novel designs may incorporate temperature control systems, pressure-resistant housings, or specialized flow distributors. Advanced materials offer improved mechanical stability, higher surface area, and enhanced selectivity, allowing for faster separations with reduced solvent volumes and improved resolution.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Chromatography
The column chromatography scaling market is currently in a growth phase, characterized by increasing demand for efficient purification technologies in pharmaceutical and biotechnology sectors. The global market size for chromatography equipment is expanding steadily, driven by biopharmaceutical development and research applications. Technologically, the field shows moderate maturity with established principles but ongoing innovation in scaling methodologies. Leading players include Waters Technology Corp., Bio-Rad Laboratories, and Cytiva, who offer comprehensive scaling solutions; pharmaceutical companies like Amgen, Regeneron, and Takeda drive application development; while specialized firms such as Biotage AB and PureHoney Technologies focus on niche innovations. Academic institutions including Fudan University and McMaster University contribute fundamental research advancing scaling techniques and efficiency improvements.
Waters Technology Corp.
Technical Solution: Waters Technology has developed a comprehensive scale-up methodology for column chromatography that focuses on maintaining chromatographic resolution while increasing throughput. Their approach utilizes the concept of geometric scaling, where the ratio of column length to diameter remains constant during scale-up. Waters' ACQUITY UPLC and ACQUITY Arc systems incorporate software tools that automatically calculate the appropriate column dimensions and flow rates when transitioning from analytical to preparative scale. Their technology employs particle size optimization, with smaller particles (sub-2μm) for analytical work and larger particles (5-10μm) for preparative applications, maintaining separation efficiency while reducing backpressure. Waters' Prep Calculator tool provides precise solvent consumption estimates based on column dimensions, helping researchers optimize mobile phase usage and reduce waste. Additionally, their columns feature proprietary surface technologies that enhance chemical stability and reduce secondary interactions, allowing for consistent performance across scales.
Strengths: Integrated software tools provide automated scaling calculations, reducing human error. Their column technology offers exceptional chemical stability across a wide pH range, enabling more flexible method development. Weaknesses: Higher initial investment compared to some competitors. Their proprietary column chemistries may require method adjustments when transferring from other vendors' analytical methods.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad's approach to column chromatography scale-up centers on their NGC™ Chromatography System platform, which offers modular configurations suitable for both analytical and preparative applications. Their methodology emphasizes maintaining the linear velocity of the mobile phase while increasing column diameter, preserving separation characteristics across scales. Bio-Rad has developed specialized software tools that calculate optimal flow rates and injection volumes based on column dimensions and particle characteristics. Their CHT™ Ceramic Hydroxyapatite media technology provides unique selectivity for biomolecule separations and maintains consistent performance from analytical to process scale. Bio-Rad's column technology incorporates uniform particle size distribution and optimized bed packing procedures to ensure consistent column efficiency across different scales. They provide detailed solvent usage tables that account for column void volumes, gradient delay volumes, and system hold-up volumes, enabling precise estimation of buffer requirements for preparative runs. Their scale-up protocols include recommendations for sample loading capacity based on empirical data rather than theoretical calculations alone.
Strengths: Modular system design allows gradual scale-up without complete platform changes. Their ceramic media offers exceptional chemical and physical stability, withstanding harsh cleaning conditions. Weaknesses: Some of their specialized media types have higher costs compared to conventional silica-based materials. Their systems may require more extensive user training due to the high degree of customization options.
Critical Parameters and Mathematical Models for Scale-Up
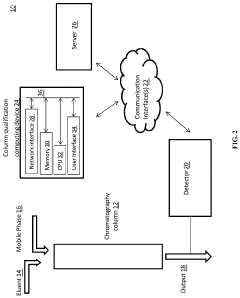
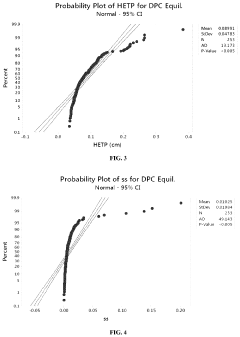
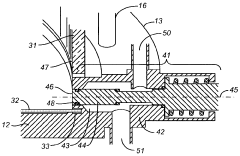
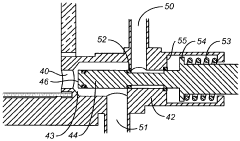
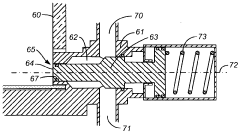
Chromatography column qualification in manufacturing methods for producing anti-TNF antibody compositions
PatentActiveUS12018075B2
Innovation
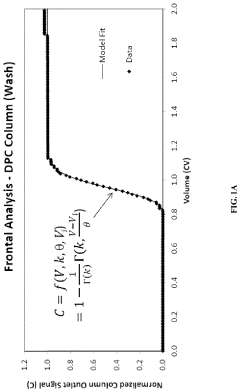
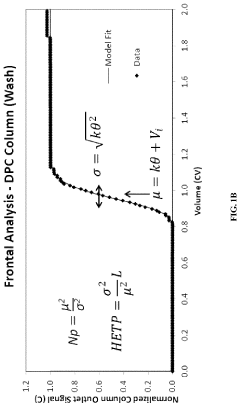
- A method using Gamma Distribution Transition Analysis (GDTA) is introduced, which involves collecting column outlet signals and accumulated flow parameters during mobile phase transitions, fitting a gamma cumulative distribution curve, and calculating HETP values to assess column quality, allowing for more sensitive and accurate monitoring of column performance.
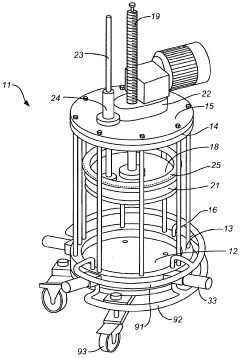
Chromatography column with pack, unpack, and clean-in-place features
PatentWO2008134413A1
Innovation
- A chromatography column design featuring a series of valves distributed around the column that allow for filling, emptying, and cleaning of the packed bed and supply/discharge lines through a common valve system, with a movable plug that maintains flow communication without obstructing the column interior, enabling unpacking and cleaning in place without disturbing the column contents.
Environmental Impact and Green Chemistry Considerations
The scaling of column chromatography from analytical to preparative scale presents significant environmental challenges that must be addressed through sustainable practices. Solvent usage in preparative chromatography can be substantial, with typical runs consuming liters of mobile phase that often contain environmentally harmful organic solvents such as acetonitrile, methanol, and hexane. These solvents contribute to air pollution, pose health risks to laboratory personnel, and create hazardous waste disposal issues.
Recent advancements in green chemistry have introduced alternative approaches to minimize environmental impact. Solvent recycling systems have become increasingly sophisticated, allowing for the recovery of up to 80% of mobile phase components in preparative chromatography. These systems employ distillation or membrane filtration techniques to purify used solvents, significantly reducing waste generation and operational costs.
The development of environmentally friendly mobile phases represents another important trend. Bio-derived solvents such as ethyl lactate and 2-methyltetrahydrofuran offer lower toxicity profiles while maintaining separation efficiency. Additionally, supercritical fluid chromatography using CO2 as the primary mobile phase component has gained traction as a greener alternative, reducing organic solvent consumption by up to 90% compared to traditional HPLC methods.
Water consumption in column chromatography also presents environmental concerns, particularly during column washing and equilibration steps. Optimized gradient profiles and reduced equilibration times can minimize water usage without compromising separation quality. Some modern systems incorporate water recycling capabilities, further reducing the environmental footprint of preparative chromatography operations.
Energy efficiency considerations are increasingly important when scaling up chromatographic processes. Larger columns and higher flow rates require substantial pumping power and temperature control systems. Energy-efficient HPLC instruments with optimized fluid dynamics and thermal management can reduce electricity consumption by 30-40% compared to older systems. Additionally, scheduling batch separations during off-peak hours can distribute energy demand more efficiently.
Waste management strategies specific to preparative chromatography must address both liquid and solid waste streams. Column packing materials eventually require disposal or regeneration, with silica-based materials presenting particular environmental challenges. Newer biodegradable stationary phases and regeneration protocols extend column lifetimes and reduce solid waste generation. For liquid waste, appropriate segregation and treatment before disposal are essential to prevent environmental contamination.
Recent advancements in green chemistry have introduced alternative approaches to minimize environmental impact. Solvent recycling systems have become increasingly sophisticated, allowing for the recovery of up to 80% of mobile phase components in preparative chromatography. These systems employ distillation or membrane filtration techniques to purify used solvents, significantly reducing waste generation and operational costs.
The development of environmentally friendly mobile phases represents another important trend. Bio-derived solvents such as ethyl lactate and 2-methyltetrahydrofuran offer lower toxicity profiles while maintaining separation efficiency. Additionally, supercritical fluid chromatography using CO2 as the primary mobile phase component has gained traction as a greener alternative, reducing organic solvent consumption by up to 90% compared to traditional HPLC methods.
Water consumption in column chromatography also presents environmental concerns, particularly during column washing and equilibration steps. Optimized gradient profiles and reduced equilibration times can minimize water usage without compromising separation quality. Some modern systems incorporate water recycling capabilities, further reducing the environmental footprint of preparative chromatography operations.
Energy efficiency considerations are increasingly important when scaling up chromatographic processes. Larger columns and higher flow rates require substantial pumping power and temperature control systems. Energy-efficient HPLC instruments with optimized fluid dynamics and thermal management can reduce electricity consumption by 30-40% compared to older systems. Additionally, scheduling batch separations during off-peak hours can distribute energy demand more efficiently.
Waste management strategies specific to preparative chromatography must address both liquid and solid waste streams. Column packing materials eventually require disposal or regeneration, with silica-based materials presenting particular environmental challenges. Newer biodegradable stationary phases and regeneration protocols extend column lifetimes and reduce solid waste generation. For liquid waste, appropriate segregation and treatment before disposal are essential to prevent environmental contamination.
Economic Analysis of Preparative Chromatography Implementation
The implementation of preparative chromatography represents a significant capital investment for pharmaceutical, biotechnology, and chemical companies. Initial equipment costs for preparative chromatography systems typically range from $100,000 to over $1 million, depending on scale, automation level, and detection capabilities. This substantial upfront investment necessitates thorough economic analysis to justify the expenditure.
Operating costs present another critical economic consideration. Solvent consumption increases exponentially when scaling from analytical to preparative chromatography. For instance, scaling a 4.6mm analytical column to a 50mm preparative column results in approximately 118 times greater solvent consumption. At industrial scale, this can translate to thousands of liters of solvent annually, with associated costs for purchase, handling, and disposal.
Labor costs vary significantly based on automation level. Manual preparative systems require constant operator attention, while fully automated systems can run with minimal supervision. A semi-automated system typically requires 0.5 FTE (full-time equivalent) personnel, representing $40,000-$70,000 annually in developed markets.
Return on investment calculations must consider throughput capabilities. A properly sized preparative chromatography system can process 10-100 times more material than analytical systems. For pharmaceutical applications, this translates to potential revenue generation of $10,000-$100,000 per batch of purified compound, depending on the compound's value.
Facility requirements add hidden costs often overlooked in initial analyses. Preparative chromatography requires dedicated space with proper ventilation, safety features, and sometimes explosion-proof designs for handling volatile solvents. These infrastructure modifications typically add 15-25% to the total implementation cost.
Maintenance expenses accumulate over the equipment lifecycle. Annual maintenance costs generally range from 5-15% of the initial capital investment. Column replacement represents a significant recurring expense, with preparative columns costing $5,000-$30,000 depending on dimensions and stationary phase.
Optimization opportunities can significantly improve economic outcomes. Proper method development can reduce solvent consumption by 20-40% compared to direct scale-up approaches. Similarly, recycling technologies can recover 50-80% of solvents, substantially reducing operational costs and environmental impact.
The economic analysis must also consider alternative purification technologies. While preparative chromatography offers superior resolution and purity, simpler techniques like crystallization or extraction may prove more economical for certain applications, achieving 80% of the purification need at 20% of the cost.
Operating costs present another critical economic consideration. Solvent consumption increases exponentially when scaling from analytical to preparative chromatography. For instance, scaling a 4.6mm analytical column to a 50mm preparative column results in approximately 118 times greater solvent consumption. At industrial scale, this can translate to thousands of liters of solvent annually, with associated costs for purchase, handling, and disposal.
Labor costs vary significantly based on automation level. Manual preparative systems require constant operator attention, while fully automated systems can run with minimal supervision. A semi-automated system typically requires 0.5 FTE (full-time equivalent) personnel, representing $40,000-$70,000 annually in developed markets.
Return on investment calculations must consider throughput capabilities. A properly sized preparative chromatography system can process 10-100 times more material than analytical systems. For pharmaceutical applications, this translates to potential revenue generation of $10,000-$100,000 per batch of purified compound, depending on the compound's value.
Facility requirements add hidden costs often overlooked in initial analyses. Preparative chromatography requires dedicated space with proper ventilation, safety features, and sometimes explosion-proof designs for handling volatile solvents. These infrastructure modifications typically add 15-25% to the total implementation cost.
Maintenance expenses accumulate over the equipment lifecycle. Annual maintenance costs generally range from 5-15% of the initial capital investment. Column replacement represents a significant recurring expense, with preparative columns costing $5,000-$30,000 depending on dimensions and stationary phase.
Optimization opportunities can significantly improve economic outcomes. Proper method development can reduce solvent consumption by 20-40% compared to direct scale-up approaches. Similarly, recycling technologies can recover 50-80% of solvents, substantially reducing operational costs and environmental impact.
The economic analysis must also consider alternative purification technologies. While preparative chromatography offers superior resolution and purity, simpler techniques like crystallization or extraction may prove more economical for certain applications, achieving 80% of the purification need at 20% of the cost.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!