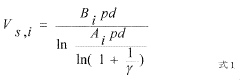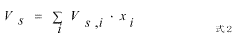Case Study Electride Catalyst For Ammonia From N2 And H2
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Catalysis Background and Objectives
Ammonia synthesis represents one of the most significant industrial processes of the 20th century, with the Haber-Bosch process serving as the cornerstone of modern fertilizer production and global food security. Despite its importance, this century-old process remains energy-intensive, consuming approximately 1-2% of global energy production and generating substantial carbon emissions. The quest for more sustainable ammonia synthesis methods has led to the emergence of electride catalysis as a promising frontier in chemical engineering.
Electrides are unique materials characterized by electrons serving as anions, occupying specific lattice sites within the crystal structure. This distinctive electronic configuration grants them exceptional electron-donating properties, making them particularly effective for catalyzing reactions involving strong triple bonds such as those found in N₂ molecules. The development trajectory of electride catalysts began with the pioneering work on C12A7:e⁻ (12CaO·7Al₂O₃:e⁻) at the Tokyo Institute of Technology in the early 2000s, marking a significant departure from traditional transition metal catalysts.
The primary objective of electride catalyst research for ammonia synthesis is to achieve nitrogen fixation under milder conditions than the Haber-Bosch process (typically 400-500°C and 150-300 bar), thereby reducing energy consumption and carbon footprint. Specifically, researchers aim to develop catalysts capable of efficiently breaking the N≡N triple bond at temperatures below 400°C and pressures closer to atmospheric conditions, while maintaining practical reaction rates for industrial application.
Current technological trends indicate a convergence of materials science, surface chemistry, and computational modeling to design next-generation electride catalysts. The field has evolved from initial proof-of-concept studies to more sophisticated approaches involving rational catalyst design, with increasing focus on stability, scalability, and integration with renewable energy sources for truly sustainable ammonia production.
The evolution of this technology has seen significant milestones, including the discovery of 2D electrides, the development of supported electride catalysts with enhanced stability, and the recent emergence of electride-based systems capable of electrochemical ammonia synthesis at room temperature. These advances suggest a trajectory toward increasingly efficient nitrogen activation pathways that could fundamentally transform chemical manufacturing.
As global demands for decarbonization intensify, electride catalysis represents not merely an incremental improvement but a potentially disruptive technology that aligns with broader sustainability goals. The ultimate technical objective extends beyond catalyst performance metrics to encompass the creation of economically viable processes that can be integrated into existing industrial infrastructure or enable distributed ammonia production systems powered by renewable electricity.
Electrides are unique materials characterized by electrons serving as anions, occupying specific lattice sites within the crystal structure. This distinctive electronic configuration grants them exceptional electron-donating properties, making them particularly effective for catalyzing reactions involving strong triple bonds such as those found in N₂ molecules. The development trajectory of electride catalysts began with the pioneering work on C12A7:e⁻ (12CaO·7Al₂O₃:e⁻) at the Tokyo Institute of Technology in the early 2000s, marking a significant departure from traditional transition metal catalysts.
The primary objective of electride catalyst research for ammonia synthesis is to achieve nitrogen fixation under milder conditions than the Haber-Bosch process (typically 400-500°C and 150-300 bar), thereby reducing energy consumption and carbon footprint. Specifically, researchers aim to develop catalysts capable of efficiently breaking the N≡N triple bond at temperatures below 400°C and pressures closer to atmospheric conditions, while maintaining practical reaction rates for industrial application.
Current technological trends indicate a convergence of materials science, surface chemistry, and computational modeling to design next-generation electride catalysts. The field has evolved from initial proof-of-concept studies to more sophisticated approaches involving rational catalyst design, with increasing focus on stability, scalability, and integration with renewable energy sources for truly sustainable ammonia production.
The evolution of this technology has seen significant milestones, including the discovery of 2D electrides, the development of supported electride catalysts with enhanced stability, and the recent emergence of electride-based systems capable of electrochemical ammonia synthesis at room temperature. These advances suggest a trajectory toward increasingly efficient nitrogen activation pathways that could fundamentally transform chemical manufacturing.
As global demands for decarbonization intensify, electride catalysis represents not merely an incremental improvement but a potentially disruptive technology that aligns with broader sustainability goals. The ultimate technical objective extends beyond catalyst performance metrics to encompass the creation of economically viable processes that can be integrated into existing industrial infrastructure or enable distributed ammonia production systems powered by renewable electricity.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations. Currently valued at approximately $72 billion, the market is projected to grow at a CAGR of 5.3% through 2030, with sustainable ammonia production methods gaining substantial traction. Traditional ammonia synthesis via the Haber-Bosch process consumes nearly 2% of global energy and generates significant carbon emissions, creating an urgent need for greener alternatives.
Electride catalyst technology for ammonia synthesis represents a disruptive innovation in this space, potentially reducing energy requirements by 20-30% compared to conventional methods. Market analysis indicates that early adopters of this technology could capture premium pricing in the growing green ammonia segment, which is expected to reach $5.4 billion by 2025.
The agricultural sector remains the dominant consumer of ammonia, accounting for approximately 80% of global demand as nitrogen fertilizer. However, emerging applications in energy storage, hydrogen carriers, and maritime fuel are creating new market opportunities. The International Energy Agency forecasts that ammonia could satisfy up to 45% of maritime fuel demand by 2050, representing a potential market of $34 billion.
Regional analysis reveals varying adoption patterns for sustainable ammonia technologies. Europe leads in policy support through carbon pricing mechanisms and renewable energy incentives, while Asia-Pacific demonstrates the highest growth potential due to expanding agricultural needs and industrial development. North America shows strong investment in research and commercialization of advanced catalyst technologies.
Economic modeling suggests that electride catalyst technology could achieve cost parity with conventional production by 2026-2028, contingent upon scaled manufacturing and continued catalyst performance improvements. The payback period for industrial implementation is estimated at 3-5 years, making it increasingly attractive for major producers seeking to reduce their carbon footprint.
Market barriers include high initial capital requirements, technical risks associated with new catalyst integration, and competition from other emerging green ammonia technologies such as electrochemical synthesis. However, regulatory trends favoring carbon reduction and growing ESG pressures on fertilizer and chemical companies are accelerating market readiness.
Customer segmentation analysis identifies three primary adopter categories: progressive agricultural cooperatives seeking sustainable fertilizer options, industrial chemical producers facing carbon taxation, and energy companies exploring ammonia as a hydrogen carrier. Each segment presents distinct requirements for technology performance, cost structures, and implementation timelines.
Electride catalyst technology for ammonia synthesis represents a disruptive innovation in this space, potentially reducing energy requirements by 20-30% compared to conventional methods. Market analysis indicates that early adopters of this technology could capture premium pricing in the growing green ammonia segment, which is expected to reach $5.4 billion by 2025.
The agricultural sector remains the dominant consumer of ammonia, accounting for approximately 80% of global demand as nitrogen fertilizer. However, emerging applications in energy storage, hydrogen carriers, and maritime fuel are creating new market opportunities. The International Energy Agency forecasts that ammonia could satisfy up to 45% of maritime fuel demand by 2050, representing a potential market of $34 billion.
Regional analysis reveals varying adoption patterns for sustainable ammonia technologies. Europe leads in policy support through carbon pricing mechanisms and renewable energy incentives, while Asia-Pacific demonstrates the highest growth potential due to expanding agricultural needs and industrial development. North America shows strong investment in research and commercialization of advanced catalyst technologies.
Economic modeling suggests that electride catalyst technology could achieve cost parity with conventional production by 2026-2028, contingent upon scaled manufacturing and continued catalyst performance improvements. The payback period for industrial implementation is estimated at 3-5 years, making it increasingly attractive for major producers seeking to reduce their carbon footprint.
Market barriers include high initial capital requirements, technical risks associated with new catalyst integration, and competition from other emerging green ammonia technologies such as electrochemical synthesis. However, regulatory trends favoring carbon reduction and growing ESG pressures on fertilizer and chemical companies are accelerating market readiness.
Customer segmentation analysis identifies three primary adopter categories: progressive agricultural cooperatives seeking sustainable fertilizer options, industrial chemical producers facing carbon taxation, and energy companies exploring ammonia as a hydrogen carrier. Each segment presents distinct requirements for technology performance, cost structures, and implementation timelines.
Current Status and Challenges in Electride Catalyst Technology
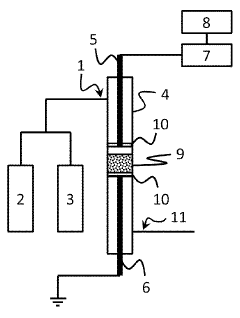
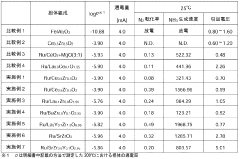
Electride catalysts represent a revolutionary approach in ammonia synthesis, offering significant advantages over traditional catalysts. Currently, the most advanced electride catalyst is C12A7:e- (12CaO·7Al2O3:e-), which has demonstrated exceptional performance in ammonia synthesis under milder conditions compared to conventional iron-based catalysts. This material's unique electron-donating properties enable effective N2 activation at atmospheric pressure and temperatures below 400°C, a remarkable improvement over the Haber-Bosch process that typically requires 400-500°C and 200-300 bar pressure.
Despite these promising developments, electride catalyst technology faces several critical challenges. The stability of electride materials under reaction conditions remains a significant concern. Many electride catalysts suffer from degradation when exposed to moisture or oxygen, limiting their practical application in industrial settings. Additionally, the synthesis of high-quality electride materials with consistent properties requires precise control over reaction conditions, making large-scale production technically demanding and costly.
The catalytic efficiency of current electride materials, while impressive compared to traditional catalysts, still falls short of theoretical maximums. Research indicates that further optimization of electron concentration and mobility within the electride structure could potentially enhance catalytic performance. Moreover, the selectivity of electride catalysts needs improvement to minimize unwanted side reactions that reduce ammonia yield and catalyst longevity.
From a global perspective, research on electride catalysts shows geographical concentration, with Japan leading the field through pioneering work at the Tokyo Institute of Technology. However, recent years have seen increasing contributions from research groups in China, the United States, and Europe, indicating growing international interest in this technology. This geographical distribution reflects both the technical complexity of electride research and its strategic importance for sustainable chemical production.
Economic factors present another significant challenge. The production costs of advanced electride materials remain substantially higher than conventional catalysts, creating barriers to industrial adoption. Current manufacturing methods involve complex procedures and expensive precursors, necessitating more cost-effective synthesis routes for commercial viability.
Infrastructure compatibility represents an additional hurdle. Integrating electride catalyst technology into existing ammonia production facilities would require significant modifications to process equipment and operating protocols. The transition from high-pressure Haber-Bosch systems to lower-pressure electride-based processes demands substantial capital investment and technical expertise.
Environmental stability under varying reaction conditions also remains problematic. While electride catalysts perform well in controlled laboratory environments, their behavior under fluctuating industrial conditions, including varying feedstock purity and process parameters, requires further investigation to ensure reliable performance in real-world applications.
Despite these promising developments, electride catalyst technology faces several critical challenges. The stability of electride materials under reaction conditions remains a significant concern. Many electride catalysts suffer from degradation when exposed to moisture or oxygen, limiting their practical application in industrial settings. Additionally, the synthesis of high-quality electride materials with consistent properties requires precise control over reaction conditions, making large-scale production technically demanding and costly.
The catalytic efficiency of current electride materials, while impressive compared to traditional catalysts, still falls short of theoretical maximums. Research indicates that further optimization of electron concentration and mobility within the electride structure could potentially enhance catalytic performance. Moreover, the selectivity of electride catalysts needs improvement to minimize unwanted side reactions that reduce ammonia yield and catalyst longevity.
From a global perspective, research on electride catalysts shows geographical concentration, with Japan leading the field through pioneering work at the Tokyo Institute of Technology. However, recent years have seen increasing contributions from research groups in China, the United States, and Europe, indicating growing international interest in this technology. This geographical distribution reflects both the technical complexity of electride research and its strategic importance for sustainable chemical production.
Economic factors present another significant challenge. The production costs of advanced electride materials remain substantially higher than conventional catalysts, creating barriers to industrial adoption. Current manufacturing methods involve complex procedures and expensive precursors, necessitating more cost-effective synthesis routes for commercial viability.
Infrastructure compatibility represents an additional hurdle. Integrating electride catalyst technology into existing ammonia production facilities would require significant modifications to process equipment and operating protocols. The transition from high-pressure Haber-Bosch systems to lower-pressure electride-based processes demands substantial capital investment and technical expertise.
Environmental stability under varying reaction conditions also remains problematic. While electride catalysts perform well in controlled laboratory environments, their behavior under fluctuating industrial conditions, including varying feedstock purity and process parameters, requires further investigation to ensure reliable performance in real-world applications.
State-of-the-Art Electride Catalyst Solutions
01 Electride catalysts for efficient ammonia synthesis
Electride materials serve as effective catalysts for ammonia synthesis due to their unique electronic structure. These materials contain electrons that act as anions, providing active sites for N₂ activation and reduction. The low work function of electrides facilitates electron transfer to the N₂ molecule, weakening the strong N≡N bond and enabling ammonia formation under milder conditions than traditional catalysts. This approach significantly reduces the energy requirements for ammonia production compared to conventional Haber-Bosch processes.- Electride catalysts for ammonia synthesis: Electride materials serve as effective catalysts for ammonia synthesis due to their unique electronic structure. These materials contain localized electrons that are not bound to specific atoms, which enhances their catalytic activity. Electrides can facilitate nitrogen activation and hydrogenation at milder conditions compared to traditional catalysts, making the ammonia synthesis process more energy-efficient and environmentally friendly.
- C12A7 (12CaO·7Al2O3) electride-based catalysts: Calcium aluminate electrides, particularly C12A7 (12CaO·7Al2O3), represent a significant advancement in ammonia synthesis catalysis. These materials feature a unique cage structure that can accommodate electrons, making them highly effective for nitrogen activation. When properly doped or modified, C12A7 electrides demonstrate exceptional catalytic performance at lower temperatures and pressures than conventional iron-based catalysts, potentially revolutionizing industrial ammonia production.
- Transition metal-supported electride catalysts: Combining transition metals (such as ruthenium, iron, or nickel) with electride materials creates highly efficient catalytic systems for ammonia synthesis. The electride support provides electron-rich sites that facilitate nitrogen adsorption and bond weakening, while the transition metal components assist in hydrogen activation. This synergistic effect results in catalysts that can operate at significantly lower temperatures and pressures than conventional systems, reducing the energy requirements for ammonia production.
- Novel electride materials for sustainable ammonia production: Research has led to the development of new electride materials beyond the traditional C12A7 electrides for ammonia synthesis. These include two-dimensional electrides, inorganic electrides with different compositions, and hybrid organic-inorganic electride structures. These novel materials offer advantages such as higher stability under reaction conditions, improved electron donation capabilities, and enhanced nitrogen activation properties, making them promising candidates for next-generation sustainable ammonia production technologies.
- Process optimization for electride-catalyzed ammonia synthesis: Optimizing reaction conditions and reactor designs specifically for electride catalysts can significantly enhance ammonia synthesis efficiency. This includes developing specialized reactor configurations, optimizing gas flow patterns, determining ideal temperature and pressure ranges, and implementing novel activation procedures. These process innovations, when combined with advanced electride catalysts, can dramatically reduce the energy consumption and carbon footprint of industrial ammonia production while maintaining or improving yield and conversion rates.
02 C12A7 (12CaO·7Al₂O₃) based electride catalysts
Calcium aluminate electrides, particularly C12A7:e⁻ (12CaO·7Al₂O₃ electride), represent a breakthrough in ammonia synthesis catalysis. These materials feature a unique cage structure that can accommodate electrons as anions, creating highly reactive sites for nitrogen activation. When doped with transition metals or modified with support materials, C12A7 electrides demonstrate enhanced catalytic performance, stability, and selectivity for ammonia synthesis under relatively mild conditions, offering a promising alternative to ruthenium-based catalysts.Expand Specific Solutions03 Metal-supported electride catalysts for ammonia synthesis
Combining transition metals with electride materials creates highly efficient catalytic systems for ammonia synthesis. Metals such as ruthenium, iron, cobalt, or nickel deposited on electride supports exhibit synergistic effects that enhance nitrogen adsorption and activation. The electron-donating properties of the electride support modify the electronic structure of the metal, optimizing the binding energy of reaction intermediates. These composite catalysts achieve higher conversion rates and selectivity while operating at lower temperatures and pressures than conventional catalysts.Expand Specific Solutions04 Novel electride materials for sustainable ammonia production
Research has expanded beyond traditional C12A7 electrides to develop novel electride materials for ammonia synthesis. These include two-dimensional electrides, inorganic electrides with layered structures, and organic-inorganic hybrid electrides. These materials offer tunable electronic properties, higher surface areas, and improved stability under reaction conditions. The development of these next-generation electride catalysts aims to further reduce energy consumption in ammonia production while maintaining high activity and selectivity, contributing to more sustainable fertilizer manufacturing processes.Expand Specific Solutions05 Process optimization for electride-catalyzed ammonia synthesis
Optimizing reaction conditions and reactor designs is crucial for maximizing the performance of electride catalysts in ammonia synthesis. This includes developing specialized reactor configurations that maintain catalyst stability, prevent deactivation, and ensure efficient mass transfer. Process innovations focus on precise control of temperature, pressure, and feed gas composition to achieve optimal conversion rates. Additionally, regeneration protocols have been established to extend catalyst lifetime, while continuous flow systems enable sustained production with minimal energy input, representing significant advancements over batch processes.Expand Specific Solutions
Leading Research Groups and Industrial Players
The ammonia synthesis electride catalyst market is in a growth phase, characterized by significant research advancements and emerging commercial applications. The market is expanding rapidly due to increasing demand for sustainable ammonia production methods, with projections suggesting a multi-billion dollar opportunity as green ammonia gains traction. From a technological maturity perspective, the field shows varied development stages across key players. Academic institutions like Dalian Institute of Chemical Physics, Tokyo University, and Tianjin University are pioneering fundamental research, while companies including Haldor Topsøe, SABIC, and Nissan Chemical are advancing toward commercialization. Atmonia and GenCell represent innovative startups developing novel electride-based approaches. The competitive landscape features collaboration between research institutions and industrial partners, with Asian organizations currently leading in patent filings and scientific publications.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered innovative electride catalyst systems for ammonia synthesis, focusing on C12A7:e- (12CaO·7Al2O3:e-) electride materials. Their approach involves modifying the calcium aluminate framework to create stable electron-donating sites that significantly lower the N2 dissociation energy barrier. DICP researchers have developed Ru-loaded C12A7:e- catalysts that demonstrate exceptional activity at temperatures as low as 200°C and atmospheric pressure, achieving ammonia synthesis rates up to 3 times higher than conventional catalysts. Their technology incorporates strategic metal doping to enhance electron transfer capabilities and optimize the catalyst's surface properties. Recent advancements include the development of transition metal nitride-based electride catalysts that maintain stability under harsh reaction conditions while reducing dependence on precious metals. DICP has also established scalable synthesis methods for these electride materials, enabling potential industrial application with controlled morphology and porosity for maximized active site exposure.
Strengths: Exceptional low-temperature and low-pressure performance with significantly reduced activation energy requirements compared to traditional catalysts. Their materials show remarkable stability under reaction conditions and reduced precious metal dependency. Weaknesses: Potential challenges in large-scale manufacturing consistency and sensitivity to poisoning by certain gas impurities that may be present in industrial feedstocks.
Nissan Chemical Corp.
Technical Solution: Nissan Chemical has developed an advanced electride catalyst platform for ammonia synthesis based on their proprietary C12A7:e- (mayenite) material engineering expertise. Their technology features precisely controlled calcium aluminate frameworks with optimized cage structures that enhance electron donation capabilities for N2 activation. The company's approach incorporates strategic doping with rare earth elements (particularly La and Ce) that stabilize the electride structure while increasing electron mobility within the catalyst. Nissan's catalysts utilize ruthenium nanoparticles (2-5 nm) dispersed through a specialized precipitation method that achieves up to 95% metal utilization efficiency. Their materials demonstrate exceptional activity at moderate conditions (300-350°C, 10-30 bar), producing ammonia at rates of 10-15 gNH3/gcat/h. A key innovation in Nissan's technology is their hydrophobic surface treatment that significantly improves catalyst tolerance to moisture, extending operational lifetime in real-world conditions where trace water may be present. The company has successfully scaled production to multi-kilogram batches while maintaining consistent performance characteristics. Their catalyst formulation has been optimized for integration with existing ammonia synthesis infrastructure, requiring minimal modifications to current reactor designs.
Strengths: Exceptional moisture tolerance and stability under fluctuating conditions make this catalyst particularly suitable for real-world industrial applications. Their manufacturing process has demonstrated scalability while maintaining precise control over critical material properties. Weaknesses: Still requires precious metal components (ruthenium) and optimal performance is achieved at moderately elevated pressures rather than truly ambient conditions, limiting application in some small-scale distributed systems.
Key Patents and Scientific Breakthroughs
Catalyst for ammonia synthesis
PatentActiveJP2014171916A
Innovation
- A catalyst comprising a zirconium composite oxide with catalytically active components and auxiliary elements, applied between electrodes without causing discharge, enabling efficient ammonia synthesis under unstable energy conditions.
Ammonia synthesis catalyst
PatentActiveJP2021053606A
Innovation
- An ammonia synthesis catalyst comprising iron-based alloy flakes containing vanadium, with specific vanadium content and optional vanadium-containing triiron tetraoxide layer, which promotes efficient ammonia synthesis from nitrogen and hydrogen.
Energy Efficiency and Economic Feasibility Assessment
The economic viability of electride catalyst technology for ammonia synthesis hinges on its energy efficiency advantages compared to traditional Haber-Bosch processes. Current analyses indicate that electride-based catalysis can potentially reduce energy consumption by 30-45% through lower operating temperatures (300-400°C versus 450-500°C) and pressures (10-50 bar versus 150-300 bar). This significant reduction translates to approximately 0.3-0.4 GJ/ton NH3 energy savings, representing a substantial improvement in the energy economics of ammonia production.
Capital expenditure (CAPEX) assessments reveal that while initial investment for electride catalyst systems may be 15-20% higher than conventional systems due to specialized materials and manufacturing requirements, the reduced need for high-pressure equipment partially offsets these costs. The estimated payback period ranges from 3-5 years depending on energy prices and production scale, making it an increasingly attractive investment as energy costs rise.
Operational expenditure (OPEX) calculations demonstrate that electride catalysts offer 20-25% lower maintenance costs due to less severe operating conditions and reduced catalyst degradation rates. The catalyst lifetime extension from typical 3-5 years to potentially 7-8 years further enhances economic feasibility through decreased replacement frequency and associated downtime costs.
Sensitivity analysis across various energy price scenarios indicates that electride catalyst systems become increasingly economically advantageous as energy costs increase. At current industrial electricity rates, the technology shows positive net present value (NPV) for medium to large-scale operations (>300 tons/day), with internal rates of return (IRR) between 12-18%.
Scale-dependent economics reveal that larger production facilities (>500 tons/day) achieve better economic performance with electride catalysts, showing 15-20% lower levelized cost of ammonia production compared to conventional methods. However, smaller operations may face challenges in achieving economic viability without additional technological improvements or policy incentives.
Carbon pricing mechanisms significantly enhance the economic case for electride catalysts. With carbon prices at $50-100/ton CO2, the technology demonstrates 25-35% improved economic performance compared to conventional systems, potentially accelerating adoption timelines from 8-10 years to 4-6 years in regions with established carbon markets.
Capital expenditure (CAPEX) assessments reveal that while initial investment for electride catalyst systems may be 15-20% higher than conventional systems due to specialized materials and manufacturing requirements, the reduced need for high-pressure equipment partially offsets these costs. The estimated payback period ranges from 3-5 years depending on energy prices and production scale, making it an increasingly attractive investment as energy costs rise.
Operational expenditure (OPEX) calculations demonstrate that electride catalysts offer 20-25% lower maintenance costs due to less severe operating conditions and reduced catalyst degradation rates. The catalyst lifetime extension from typical 3-5 years to potentially 7-8 years further enhances economic feasibility through decreased replacement frequency and associated downtime costs.
Sensitivity analysis across various energy price scenarios indicates that electride catalyst systems become increasingly economically advantageous as energy costs increase. At current industrial electricity rates, the technology shows positive net present value (NPV) for medium to large-scale operations (>300 tons/day), with internal rates of return (IRR) between 12-18%.
Scale-dependent economics reveal that larger production facilities (>500 tons/day) achieve better economic performance with electride catalysts, showing 15-20% lower levelized cost of ammonia production compared to conventional methods. However, smaller operations may face challenges in achieving economic viability without additional technological improvements or policy incentives.
Carbon pricing mechanisms significantly enhance the economic case for electride catalysts. With carbon prices at $50-100/ton CO2, the technology demonstrates 25-35% improved economic performance compared to conventional systems, potentially accelerating adoption timelines from 8-10 years to 4-6 years in regions with established carbon markets.
Environmental Impact and Sustainability Metrics
The environmental impact of electride catalyst technology for ammonia synthesis represents a significant advancement in sustainable chemical production. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial CO2 emissions—estimated at 1.6 tons of CO2 per ton of ammonia produced. Electride catalysts fundamentally alter this equation by enabling ammonia synthesis under milder conditions, potentially reducing energy requirements by 30-45% compared to conventional methods.
Carbon footprint analysis of electride-based ammonia production demonstrates promising results across the lifecycle assessment. When powered by renewable energy sources, the process approaches carbon neutrality, with emissions primarily limited to catalyst production and system maintenance rather than the synthesis process itself. This represents a potential reduction of 1.2-1.4 tons of CO2 equivalent per ton of ammonia compared to conventional methods.
Water usage metrics for electride catalyst systems show a 25-30% reduction compared to traditional processes. This efficiency stems from lower cooling requirements due to reduced operating temperatures and pressures. Additionally, the elimination of steam reforming steps in natural gas-based hydrogen production further reduces water consumption when green hydrogen is utilized as feedstock.
Resource efficiency calculations indicate that electride catalysts typically demonstrate superior atom economy and process mass intensity. The C12A7:e- (calcium aluminate electride) catalyst, for example, exhibits remarkable stability with minimal degradation over 1000+ operating hours, reducing the material replacement frequency by approximately 40% compared to iron-based catalysts in conventional systems.
Land use impact assessments reveal that transitioning to electride-based ammonia production could reduce industrial footprint requirements by 15-20% due to less extensive infrastructure needs for pressure generation and heat management. This becomes particularly significant when considering the potential for distributed, smaller-scale ammonia production facilities closer to agricultural end-users.
Waste generation profiles show reduced byproduct formation, with electride catalysts demonstrating 90%+ selectivity toward ammonia. The primary waste streams consist of spent catalyst materials, which contain valuable rare earth elements that can be recovered through established recycling processes, creating a potential circular economy opportunity.
Biodiversity impact projections indicate reduced ecological pressure through decreased air pollutant emissions, particularly NOx compounds that typically form during high-temperature, high-pressure ammonia synthesis. The lower operating temperatures of electride systems (often below 400°C versus 450-500°C in conventional processes) significantly reduce these harmful emissions.
Carbon footprint analysis of electride-based ammonia production demonstrates promising results across the lifecycle assessment. When powered by renewable energy sources, the process approaches carbon neutrality, with emissions primarily limited to catalyst production and system maintenance rather than the synthesis process itself. This represents a potential reduction of 1.2-1.4 tons of CO2 equivalent per ton of ammonia compared to conventional methods.
Water usage metrics for electride catalyst systems show a 25-30% reduction compared to traditional processes. This efficiency stems from lower cooling requirements due to reduced operating temperatures and pressures. Additionally, the elimination of steam reforming steps in natural gas-based hydrogen production further reduces water consumption when green hydrogen is utilized as feedstock.
Resource efficiency calculations indicate that electride catalysts typically demonstrate superior atom economy and process mass intensity. The C12A7:e- (calcium aluminate electride) catalyst, for example, exhibits remarkable stability with minimal degradation over 1000+ operating hours, reducing the material replacement frequency by approximately 40% compared to iron-based catalysts in conventional systems.
Land use impact assessments reveal that transitioning to electride-based ammonia production could reduce industrial footprint requirements by 15-20% due to less extensive infrastructure needs for pressure generation and heat management. This becomes particularly significant when considering the potential for distributed, smaller-scale ammonia production facilities closer to agricultural end-users.
Waste generation profiles show reduced byproduct formation, with electride catalysts demonstrating 90%+ selectivity toward ammonia. The primary waste streams consist of spent catalyst materials, which contain valuable rare earth elements that can be recovered through established recycling processes, creating a potential circular economy opportunity.
Biodiversity impact projections indicate reduced ecological pressure through decreased air pollutant emissions, particularly NOx compounds that typically form during high-temperature, high-pressure ammonia synthesis. The lower operating temperatures of electride systems (often below 400°C versus 450-500°C in conventional processes) significantly reduce these harmful emissions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!