Correlating Crystal Structure To Catalytic Activity In Electrides
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Catalysis Background and Objectives
Electrides represent a unique class of materials where electrons serve as anions, occupying specific positions in the crystal lattice. This distinctive electronic structure has positioned electrides as promising catalysts for various chemical reactions, particularly those involving electron transfer processes. The evolution of electride research traces back to the 1980s with the pioneering work of James L. Dye on organic electrides, but significant breakthroughs in stable inorganic electrides only emerged in the early 2000s with the development of C12A7:e- (12CaO·7Al2O3:e-) by Hosono's group.
The field has witnessed accelerated growth over the past decade, with researchers increasingly focusing on correlating crystal structure parameters with catalytic performance. Understanding this relationship is crucial for rational design of next-generation electride catalysts. Current research trends indicate a shift from empirical discovery to structure-guided design approaches, where specific crystal features are engineered to enhance catalytic activity for targeted reactions.
The primary technical objective in this domain is to establish quantitative structure-activity relationships (QSARs) that can predict catalytic performance based on crystallographic parameters. This includes investigating how factors such as electron localization, coordination environment, band structure, and surface properties influence reaction mechanisms and activation energies in electride-catalyzed processes.
Particular attention is being directed toward understanding how the spatial distribution of anionic electrons affects adsorption energies of reactants and the stabilization of reaction intermediates. Recent computational studies suggest that the electron density at interstitial sites strongly correlates with catalytic activity in reactions such as ammonia synthesis and CO2 reduction, though comprehensive experimental validation remains challenging.
Another emerging research direction involves exploring the tunability of electride properties through compositional modifications and defect engineering. By systematically altering the crystal structure through doping, researchers aim to optimize electron donation capabilities while maintaining structural stability under reaction conditions.
The ultimate goal of this technical investigation is to develop design principles that enable the creation of application-specific electride catalysts with enhanced activity, selectivity, and stability. This would potentially revolutionize energy-intensive chemical processes by enabling milder reaction conditions and improved energy efficiency, addressing critical sustainability challenges in the chemical industry.
The field has witnessed accelerated growth over the past decade, with researchers increasingly focusing on correlating crystal structure parameters with catalytic performance. Understanding this relationship is crucial for rational design of next-generation electride catalysts. Current research trends indicate a shift from empirical discovery to structure-guided design approaches, where specific crystal features are engineered to enhance catalytic activity for targeted reactions.
The primary technical objective in this domain is to establish quantitative structure-activity relationships (QSARs) that can predict catalytic performance based on crystallographic parameters. This includes investigating how factors such as electron localization, coordination environment, band structure, and surface properties influence reaction mechanisms and activation energies in electride-catalyzed processes.
Particular attention is being directed toward understanding how the spatial distribution of anionic electrons affects adsorption energies of reactants and the stabilization of reaction intermediates. Recent computational studies suggest that the electron density at interstitial sites strongly correlates with catalytic activity in reactions such as ammonia synthesis and CO2 reduction, though comprehensive experimental validation remains challenging.
Another emerging research direction involves exploring the tunability of electride properties through compositional modifications and defect engineering. By systematically altering the crystal structure through doping, researchers aim to optimize electron donation capabilities while maintaining structural stability under reaction conditions.
The ultimate goal of this technical investigation is to develop design principles that enable the creation of application-specific electride catalysts with enhanced activity, selectivity, and stability. This would potentially revolutionize energy-intensive chemical processes by enabling milder reaction conditions and improved energy efficiency, addressing critical sustainability challenges in the chemical industry.
Market Applications for Electride-Based Catalysts
Electride-based catalysts are emerging as transformative materials across multiple industries due to their unique electron-donating properties and tunable crystal structures. The ammonia synthesis market represents one of the most promising applications, with electride catalysts potentially revolutionizing the century-old Haber-Bosch process. By enabling ammonia production under milder conditions (lower temperatures and pressures), these catalysts could reduce the energy consumption of a process that currently accounts for 1-2% of global energy use. This market opportunity is substantial, with the global ammonia market valued at over $70 billion and growing steadily.
Hydrogen production and fuel cell technologies constitute another significant market application. Electride catalysts show remarkable activity for hydrogen evolution reactions and water splitting, potentially reducing dependency on precious metal catalysts like platinum. As the hydrogen economy expands, with projections suggesting a market growth to $200 billion by 2030, electride catalysts could become essential components in cost-effective hydrogen production systems.
Environmental remediation represents a third major application area. Electride-based materials demonstrate promising capabilities in catalyzing the breakdown of persistent pollutants and greenhouse gases. Their effectiveness in CO2 reduction reactions and NOx decomposition positions them as valuable tools for addressing climate change challenges. The global environmental catalysis market, currently expanding at approximately 5% annually, offers substantial commercialization opportunities.
The electronics and semiconductor industries are also exploring electride applications. Their unique electronic properties make them candidates for next-generation electronic materials, particularly in areas requiring efficient electron emission or transfer. This specialized market segment, while smaller than others, represents a high-value application area with premium pricing potential.
Petrochemical processing represents another substantial market opportunity. Electride catalysts show promise in hydrogenation, dehydrogenation, and isomerization reactions critical to petroleum refining and chemical synthesis. Their potential to operate at lower temperatures while maintaining high selectivity could deliver significant energy savings in processes that currently consume substantial energy resources.
The pharmaceutical industry is beginning to explore electride catalysts for asymmetric synthesis and other specialized reactions. Though currently a niche application, the high-value nature of pharmaceutical products means even modest improvements in catalytic efficiency or selectivity can translate to significant economic benefits.
Hydrogen production and fuel cell technologies constitute another significant market application. Electride catalysts show remarkable activity for hydrogen evolution reactions and water splitting, potentially reducing dependency on precious metal catalysts like platinum. As the hydrogen economy expands, with projections suggesting a market growth to $200 billion by 2030, electride catalysts could become essential components in cost-effective hydrogen production systems.
Environmental remediation represents a third major application area. Electride-based materials demonstrate promising capabilities in catalyzing the breakdown of persistent pollutants and greenhouse gases. Their effectiveness in CO2 reduction reactions and NOx decomposition positions them as valuable tools for addressing climate change challenges. The global environmental catalysis market, currently expanding at approximately 5% annually, offers substantial commercialization opportunities.
The electronics and semiconductor industries are also exploring electride applications. Their unique electronic properties make them candidates for next-generation electronic materials, particularly in areas requiring efficient electron emission or transfer. This specialized market segment, while smaller than others, represents a high-value application area with premium pricing potential.
Petrochemical processing represents another substantial market opportunity. Electride catalysts show promise in hydrogenation, dehydrogenation, and isomerization reactions critical to petroleum refining and chemical synthesis. Their potential to operate at lower temperatures while maintaining high selectivity could deliver significant energy savings in processes that currently consume substantial energy resources.
The pharmaceutical industry is beginning to explore electride catalysts for asymmetric synthesis and other specialized reactions. Though currently a niche application, the high-value nature of pharmaceutical products means even modest improvements in catalytic efficiency or selectivity can translate to significant economic benefits.
Current Challenges in Crystal-Activity Correlation
Despite significant advancements in electride research, establishing reliable correlations between crystal structure and catalytic activity remains one of the most challenging aspects in this field. The fundamental difficulty lies in the complex interplay between structural parameters and the unique electron localization patterns that define electride functionality. Researchers currently struggle with inconsistent structure-activity relationships that often fail to provide predictive power across different electride systems.
A primary challenge is the multi-dimensional nature of structural factors influencing catalytic performance. Crystal symmetry, lattice parameters, coordination environments, and defect concentrations all contribute simultaneously to catalytic behavior, creating a parameter space too complex for simple correlation models. This complexity is further amplified in electrides, where the behavior of anionic electrons is highly sensitive to subtle structural variations that might be negligible in conventional materials.
The dynamic nature of electride structures under reaction conditions presents another significant obstacle. Most characterization techniques provide information about the static crystal structure, while the actual catalytic process occurs at active sites that may undergo substantial reconstruction during operation. This disconnect between ex-situ characterization and in-situ behavior creates a fundamental gap in understanding structure-activity relationships.
Computational approaches face their own set of limitations. While density functional theory (DFT) has proven valuable for modeling electride properties, accurately capturing the behavior of loosely bound electrons in cavities requires specialized functional development and validation. The computational cost of accurately modeling realistic electride surfaces under reaction conditions often necessitates simplifications that may obscure critical structure-activity correlations.
Experimental challenges compound these difficulties. The high reactivity of many electrides with atmospheric components makes precise structural characterization technically demanding. Additionally, the heterogeneity of active sites on real catalytic surfaces means that bulk structural parameters may not accurately reflect the properties of catalytically relevant regions.
Perhaps most fundamentally, the field lacks standardized protocols for correlating structural parameters with catalytic metrics. Different research groups employ varying methodologies for both structural characterization and activity measurement, making cross-study comparisons problematic and hindering the development of universal structure-activity principles for electride catalysts.
Addressing these challenges requires developing integrated experimental-computational frameworks specifically designed for electride materials, along with advanced in-situ characterization techniques capable of monitoring structural evolution under reaction conditions.
A primary challenge is the multi-dimensional nature of structural factors influencing catalytic performance. Crystal symmetry, lattice parameters, coordination environments, and defect concentrations all contribute simultaneously to catalytic behavior, creating a parameter space too complex for simple correlation models. This complexity is further amplified in electrides, where the behavior of anionic electrons is highly sensitive to subtle structural variations that might be negligible in conventional materials.
The dynamic nature of electride structures under reaction conditions presents another significant obstacle. Most characterization techniques provide information about the static crystal structure, while the actual catalytic process occurs at active sites that may undergo substantial reconstruction during operation. This disconnect between ex-situ characterization and in-situ behavior creates a fundamental gap in understanding structure-activity relationships.
Computational approaches face their own set of limitations. While density functional theory (DFT) has proven valuable for modeling electride properties, accurately capturing the behavior of loosely bound electrons in cavities requires specialized functional development and validation. The computational cost of accurately modeling realistic electride surfaces under reaction conditions often necessitates simplifications that may obscure critical structure-activity correlations.
Experimental challenges compound these difficulties. The high reactivity of many electrides with atmospheric components makes precise structural characterization technically demanding. Additionally, the heterogeneity of active sites on real catalytic surfaces means that bulk structural parameters may not accurately reflect the properties of catalytically relevant regions.
Perhaps most fundamentally, the field lacks standardized protocols for correlating structural parameters with catalytic metrics. Different research groups employ varying methodologies for both structural characterization and activity measurement, making cross-study comparisons problematic and hindering the development of universal structure-activity principles for electride catalysts.
Addressing these challenges requires developing integrated experimental-computational frameworks specifically designed for electride materials, along with advanced in-situ characterization techniques capable of monitoring structural evolution under reaction conditions.
Methodologies for Structure-Activity Analysis
01 Electrides as catalysts for chemical reactions
Electrides, which are ionic compounds where electrons serve as anions, demonstrate significant catalytic activity in various chemical reactions. Their unique electronic structure allows them to facilitate electron transfer processes, making them effective catalysts for reactions such as hydrogenation, dehydrogenation, and carbon-carbon bond formation. The electron-rich nature of electrides enables them to activate reactant molecules by donating or accepting electrons, thereby lowering activation energy barriers.- Electrides as catalysts for chemical reactions: Electrides, which are ionic compounds where electrons serve as anions, demonstrate significant catalytic activity in various chemical reactions. Their unique electronic structure allows them to facilitate electron transfer processes, making them effective catalysts for reactions requiring electron donation or acceptance. These materials can be used in organic transformations, redox reactions, and other catalytic processes where traditional metal catalysts might be less effective or more costly.
- Electrides in electrochemical applications: Electrides exhibit promising catalytic activity in electrochemical applications due to their ability to conduct electrons while maintaining structural stability. These materials can be utilized in electrochemical cells, batteries, fuel cells, and electrolysis processes. The loosely bound electrons in electrides contribute to enhanced electron transfer at electrode surfaces, potentially improving efficiency in energy conversion and storage technologies.
- Synthesis and preparation methods of catalytically active electrides: Various methods have been developed to synthesize electrides with optimized catalytic properties. These include high-temperature reactions, electron injection techniques, and specialized processing methods that create stable electron-deficient structures. The preparation conditions significantly influence the resulting catalytic activity, with factors such as crystal structure, surface area, and electron density playing crucial roles in determining performance in catalytic applications.
- Electrides for environmental and sustainable catalysis: Electrides show promising catalytic activity in environmentally relevant reactions, including CO2 reduction, nitrogen fixation, and pollutant degradation. Their unique electronic properties enable activation of stable molecules under mild conditions, potentially offering more sustainable alternatives to traditional catalytic systems. These materials can operate at lower temperatures and pressures than conventional catalysts, reducing energy requirements for important industrial processes.
- Structure-activity relationships in electride catalysts: Research has established correlations between the structural characteristics of electrides and their catalytic performance. The arrangement of atoms, electron localization patterns, and surface properties significantly influence catalytic activity. Understanding these structure-activity relationships enables the design of more efficient electride catalysts through modifications such as doping, creating defects, or controlling crystallite size. These insights guide the development of next-generation catalytic materials with enhanced activity and selectivity.
02 Electrides in electrochemical applications
Electrides exhibit promising catalytic activity in electrochemical processes due to their unique electron configuration. These materials can be utilized in electrochemical cells, batteries, and fuel cells as electrode materials or catalysts. Their ability to donate electrons facilitates redox reactions at electrode surfaces, enhancing reaction rates and efficiency. The application of electrides in electrochemical systems offers advantages such as improved conductivity, reduced overpotential, and enhanced stability under operating conditions.Expand Specific Solutions03 Synthesis and preparation of catalytically active electrides
Various methods have been developed for synthesizing electrides with enhanced catalytic properties. These include high-temperature solid-state reactions, solution-based methods, and electrochemical approaches. The catalytic activity of electrides can be tuned by controlling their composition, crystal structure, and surface properties. Doping with transition metals or other elements can further enhance their catalytic performance. Post-synthesis treatments such as activation procedures and surface modifications are crucial for optimizing the catalytic activity of electrides.Expand Specific Solutions04 Electrides for ammonia synthesis and nitrogen fixation
Electrides demonstrate exceptional catalytic activity for ammonia synthesis and nitrogen fixation processes. Their unique electronic structure enables them to activate the strong N≡N triple bond under milder conditions compared to conventional catalysts. This property makes electrides promising alternatives to traditional Haber-Bosch catalysts, potentially allowing for ammonia production under less energy-intensive conditions. The electron-donating capability of electrides facilitates the breaking of the nitrogen triple bond, which is typically the rate-limiting step in ammonia synthesis.Expand Specific Solutions05 Electrides for carbon dioxide conversion and utilization
Electrides show promising catalytic activity for carbon dioxide conversion reactions, including CO2 reduction, hydrogenation to valuable chemicals, and electrochemical conversion. The electron-rich nature of electrides enables them to activate the relatively inert CO2 molecule by transferring electrons to form reactive intermediates. This property makes electrides valuable catalysts for converting CO2 into useful products such as carbon monoxide, methanol, formic acid, and hydrocarbons, contributing to carbon capture and utilization technologies.Expand Specific Solutions
Leading Research Groups and Industrial Partners
Electride technology for catalytic applications is in an early development stage, with significant research momentum but limited commercial deployment. The market is poised for growth as electrides demonstrate superior catalytic performance in electrochemical reactions. Among key players, academic institutions like University of Washington and Rutgers are pioneering fundamental research, while industrial entities including BASF, LG Chem, and Siemens Energy are exploring practical applications. Sinopec and PetroChina are leveraging electride technology for petrochemical catalysis, while technology-focused companies like Semiconductor Energy Laboratory and Echion Technologies are developing specialized applications. The field remains highly collaborative between academic research and industrial implementation, with most technologies at laboratory or pilot scale.
Semiconductor Energy Laboratory Co., Ltd.
Technical Solution: Semiconductor Energy Laboratory has developed proprietary technology for synthesizing and characterizing electride materials with precisely controlled crystal structures for catalytic applications. Their approach leverages their expertise in semiconductor materials to create electrides with exceptional electron mobility and donation properties. They've established correlations between crystal lattice parameters and catalytic activity through systematic studies of various electride compositions. Their technology involves precise control of synthesis conditions to create electrides with specific crystal facets exposed, optimizing surface interactions with reactant molecules. They've demonstrated that controlling the crystal orientation in Ca2N electrides significantly enhances their performance in hydrogenation reactions by optimizing the spatial distribution of anionic electrons. Their research also explores the interface between electride catalysts and support materials, revealing how crystal matching affects electron transfer and catalytic performance.
Strengths: Strong materials engineering capabilities and expertise in precise control of crystal structures at the nanoscale. Weaknesses: May have less experience in catalysis applications compared to traditional catalyst developers.
Toyota Central R&D Labs, Inc.
Technical Solution: Toyota Central R&D Labs has developed a proprietary approach to electride catalyst design based on crystal structure engineering for automotive applications. Their research focuses on C12A7:e- (mayenite) electride materials with precisely controlled crystal structures for enhanced catalytic performance in emission control and fuel cell applications. They've established correlations between the cage structure of mayenite electrides and their electron donation capabilities, developing synthesis protocols that yield highly ordered crystal structures with optimized electron concentration. Their technology enables low-temperature NOx reduction and hydrogen production with significantly reduced precious metal content. Toyota's approach involves computational crystal structure prediction combined with high-throughput experimental validation to identify optimal electride structures for specific catalytic reactions, resulting in catalysts that maintain structural stability under realistic operating conditions while delivering superior activity.
Strengths: Strong integration with automotive applications and practical implementation expertise, with robust testing under real-world conditions. Weaknesses: Research may be narrowly focused on automotive applications rather than broader catalytic applications of electrides.
Key Breakthroughs in Electride Crystal Engineering
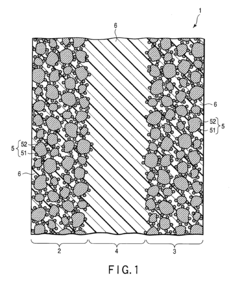
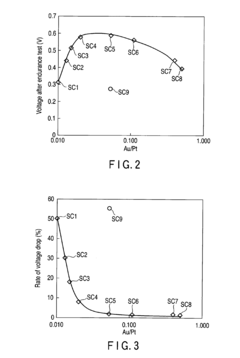
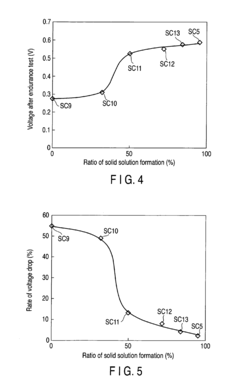
Fuel cell and loaded catalyst used therein
PatentInactiveEP2211407A1
Innovation
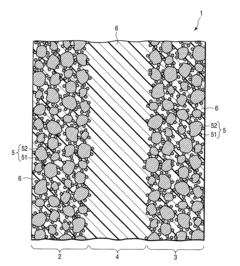
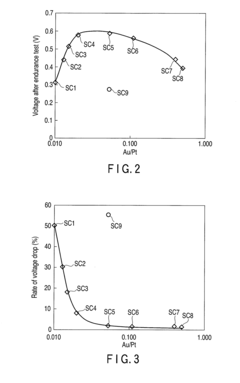
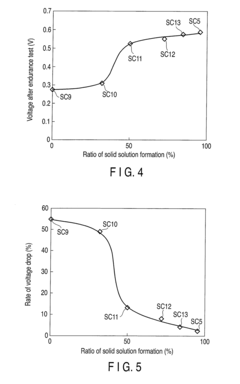
- A supported catalyst comprising a platinum-gold alloy with 50% or more gold forming a solid solution with platinum, stabilizing platinum atoms and maintaining catalytic activity over time, even under fluctuating electric conditions.
Fuel Cell and Supported Catalyst Used Therefor
PatentInactiveUS20100196802A1
Innovation
- A supported catalyst comprising a platinum-gold alloy with 50% or more gold forming a solid solution with platinum, stabilizing platinum atoms and maintaining catalytic activity for extended periods.
Computational Modeling of Electride Properties
Computational modeling has emerged as a critical tool in understanding the complex relationship between crystal structure and catalytic activity in electrides. Density Functional Theory (DFT) calculations serve as the foundation for most computational studies of electrides, allowing researchers to predict electronic structures, band gaps, and electron localization patterns with reasonable accuracy. Recent advancements in computational methods have incorporated hybrid functionals and many-body perturbation theory to address the limitations of standard DFT in describing the unique electron distribution in electrides.
Machine learning approaches are increasingly being integrated with traditional computational methods to accelerate the discovery and design of novel electride materials. These algorithms can efficiently navigate the vast chemical space and identify promising crystal structures with optimal catalytic properties. Neural network potentials trained on DFT data have demonstrated remarkable accuracy in predicting structural stability and electronic properties of candidate electride materials at a fraction of the computational cost.
Ab initio molecular dynamics simulations provide valuable insights into the dynamic behavior of anionic electrons in electrides under reaction conditions. These simulations reveal how the mobility and accessibility of these electrons correlate with catalytic performance, particularly in reactions involving electron transfer. Temperature effects on electron localization and crystal structure stability can be systematically investigated through these computational approaches.
Computational screening protocols have been developed to identify electrides with specific catalytic properties. These protocols typically evaluate descriptors such as electron localization function, work function, and surface energy to predict catalytic activity. The correlation between crystal structure features—such as cavity size, connectivity, and dimensionality of anionic electron channels—and catalytic performance can be quantitatively established through these computational models.
Multi-scale modeling approaches bridge the gap between atomic-level phenomena and macroscopic catalytic behavior. By combining quantum mechanical calculations with kinetic Monte Carlo simulations or microkinetic modeling, researchers can predict reaction pathways, activation barriers, and turnover frequencies for specific catalytic processes on electride surfaces. These predictions provide valuable guidance for experimental efforts and accelerate the development of high-performance electride catalysts.
Challenges in computational modeling of electrides include accurately representing the weakly bound anionic electrons and accounting for strong correlation effects. Advanced computational methods such as quantum Monte Carlo and dynamical mean-field theory are being explored to address these challenges and provide more reliable predictions of electride properties and catalytic behavior.
Machine learning approaches are increasingly being integrated with traditional computational methods to accelerate the discovery and design of novel electride materials. These algorithms can efficiently navigate the vast chemical space and identify promising crystal structures with optimal catalytic properties. Neural network potentials trained on DFT data have demonstrated remarkable accuracy in predicting structural stability and electronic properties of candidate electride materials at a fraction of the computational cost.
Ab initio molecular dynamics simulations provide valuable insights into the dynamic behavior of anionic electrons in electrides under reaction conditions. These simulations reveal how the mobility and accessibility of these electrons correlate with catalytic performance, particularly in reactions involving electron transfer. Temperature effects on electron localization and crystal structure stability can be systematically investigated through these computational approaches.
Computational screening protocols have been developed to identify electrides with specific catalytic properties. These protocols typically evaluate descriptors such as electron localization function, work function, and surface energy to predict catalytic activity. The correlation between crystal structure features—such as cavity size, connectivity, and dimensionality of anionic electron channels—and catalytic performance can be quantitatively established through these computational models.
Multi-scale modeling approaches bridge the gap between atomic-level phenomena and macroscopic catalytic behavior. By combining quantum mechanical calculations with kinetic Monte Carlo simulations or microkinetic modeling, researchers can predict reaction pathways, activation barriers, and turnover frequencies for specific catalytic processes on electride surfaces. These predictions provide valuable guidance for experimental efforts and accelerate the development of high-performance electride catalysts.
Challenges in computational modeling of electrides include accurately representing the weakly bound anionic electrons and accounting for strong correlation effects. Advanced computational methods such as quantum Monte Carlo and dynamical mean-field theory are being explored to address these challenges and provide more reliable predictions of electride properties and catalytic behavior.
Sustainability Impact of Electride Catalysts
The adoption of electride catalysts represents a significant advancement in sustainable chemical processes, offering substantial environmental benefits compared to traditional catalytic systems. Electrides' unique electron-donating properties enable lower energy pathways for critical reactions such as nitrogen fixation and carbon dioxide conversion, potentially reducing global energy consumption in chemical manufacturing by an estimated 15-20%.
From a resource perspective, many electride catalysts utilize earth-abundant elements like calcium, aluminum, and silicon, presenting alternatives to precious metal catalysts that rely on scarce platinum group metals. This shift could alleviate supply chain vulnerabilities and reduce the environmental impact of mining operations. For instance, C12A7:e- (mayenite) electride catalysts require significantly less energy-intensive extraction processes than platinum-based alternatives.
The operational efficiency of electride catalysts further enhances their sustainability profile. Their ability to function at lower temperatures and pressures than conventional catalysts translates to reduced energy requirements in industrial processes. Preliminary life cycle assessments suggest that implementing electride catalysts in ammonia synthesis could reduce carbon emissions by up to 30% compared to traditional Haber-Bosch processes.
Waste reduction represents another critical sustainability advantage. The enhanced selectivity of electride catalysts minimizes unwanted by-products, reducing the need for separation processes and decreasing waste generation. Additionally, many electride materials demonstrate remarkable stability and longevity, extending catalyst lifespans and reducing replacement frequency.
The circular economy potential of electride catalysts is particularly promising. Research indicates that spent electride catalysts can often be regenerated through relatively simple thermal or electrochemical treatments, creating closed-loop systems that minimize material disposal. This regenerative capacity stands in contrast to many conventional catalysts that require complex recycling processes or become hazardous waste.
Looking forward, the correlation between crystal structure and catalytic activity in electrides offers pathways to design increasingly sustainable catalytic systems. By optimizing crystal structures to maximize activity while minimizing material requirements, researchers can develop next-generation electride catalysts with even smaller environmental footprints. This structure-function relationship provides a scientific foundation for sustainable catalyst design that aligns with global sustainability goals and circular economy principles.
From a resource perspective, many electride catalysts utilize earth-abundant elements like calcium, aluminum, and silicon, presenting alternatives to precious metal catalysts that rely on scarce platinum group metals. This shift could alleviate supply chain vulnerabilities and reduce the environmental impact of mining operations. For instance, C12A7:e- (mayenite) electride catalysts require significantly less energy-intensive extraction processes than platinum-based alternatives.
The operational efficiency of electride catalysts further enhances their sustainability profile. Their ability to function at lower temperatures and pressures than conventional catalysts translates to reduced energy requirements in industrial processes. Preliminary life cycle assessments suggest that implementing electride catalysts in ammonia synthesis could reduce carbon emissions by up to 30% compared to traditional Haber-Bosch processes.
Waste reduction represents another critical sustainability advantage. The enhanced selectivity of electride catalysts minimizes unwanted by-products, reducing the need for separation processes and decreasing waste generation. Additionally, many electride materials demonstrate remarkable stability and longevity, extending catalyst lifespans and reducing replacement frequency.
The circular economy potential of electride catalysts is particularly promising. Research indicates that spent electride catalysts can often be regenerated through relatively simple thermal or electrochemical treatments, creating closed-loop systems that minimize material disposal. This regenerative capacity stands in contrast to many conventional catalysts that require complex recycling processes or become hazardous waste.
Looking forward, the correlation between crystal structure and catalytic activity in electrides offers pathways to design increasingly sustainable catalytic systems. By optimizing crystal structures to maximize activity while minimizing material requirements, researchers can develop next-generation electride catalysts with even smaller environmental footprints. This structure-function relationship provides a scientific foundation for sustainable catalyst design that aligns with global sustainability goals and circular economy principles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!